Severe Hemodynamic Instability in a Young Pregnant Woman with Massive Pericardial Effusion and Pulmonary Embolism Secondary to Primary Mediastinal Non-Hodgkin’s Lymphoma
Abstract
1. Introduction
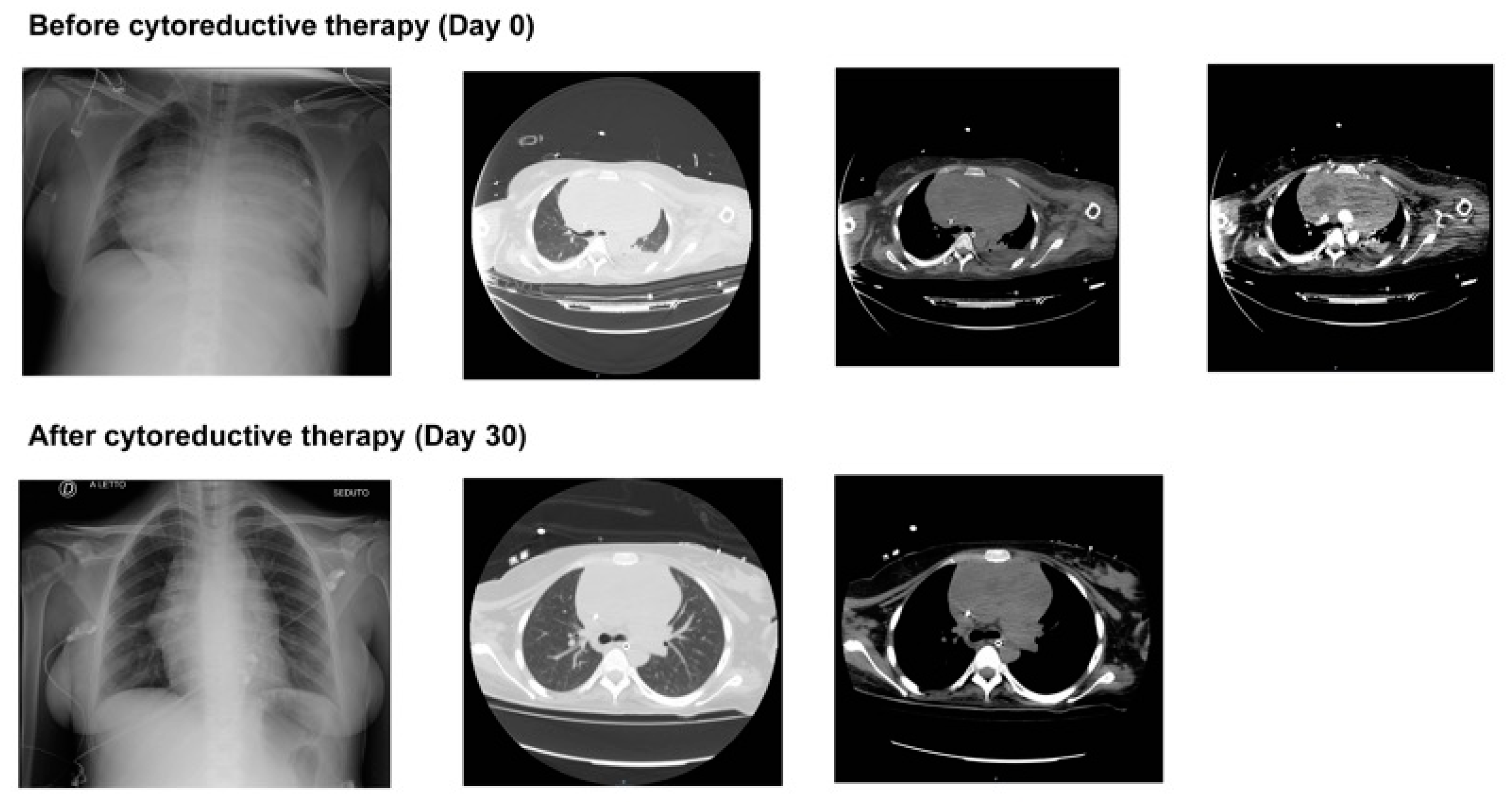
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pirosa, M.C.; Peccatori, F.A. Lymphomas in pregnancy. Hematol. Oncol. 2023, 41 (Suppl. S1), 70–74. [Google Scholar] [CrossRef]
- Pereg, D.; Koren, G.; Lishner, M. The treatment of hodgkin’s and non-hodgkin’s lymphoma in pregnancy. Haematologica 2007, 92, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Froesch, P.; Belisario-Filho, V.; Zucca, E. Hodgkin and non-hodgkin lymphomas during pregnancy. Recent Results Cancer Res. 2008, 178, 111–121. [Google Scholar] [PubMed]
- Inquilla Coyla, M.; Anchante Hernandez, H.; Medina Palomino, F. cardiovascular complications in pregnant woman with primary mediastinal b-cell lymphoma. Arch. Peru Cardiol. Cir. Cardiovasc. 2022, 3, 112–116. [Google Scholar] [PubMed]
- Pomp, E.R.; Lenselink, A.M.; Rosendaal, F.R.; Doggen, C.J. Pregnancy, the postpartum period and prothrombotic defects: Risk of venous thrombosis in the mega study. J. Thromb. Haemost. 2008, 6, 632–637. [Google Scholar] [CrossRef]
- Kearsley, R.; Stocks, G. Venous thromboembolism in pregnancy-diagnosis, management, and treatment. BJA Educ. 2021, 21, 117–123. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jimenez, D.; et al. 2019 esc guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the european respiratory society (ers). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Lorusso, R.; Shekar, K.; MacLaren, G.; Schmidt, M.; Pellegrino, V.; Meyns, B.; Haft, J.; Vercaemst, L.; Pappalardo, F.; Bermudez, C.; et al. Elso interim guidelines for venoarterial extracorporeal membrane oxygenation in adult cardiac patients. ASAIO J. 2021, 67, 827–844. [Google Scholar] [CrossRef]
- Garcia, D.A.; Baglin, T.P.; Weitz, J.I.; Samama, M.M. Parenteral anticoagulants: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012, 141, e24S–e43S. [Google Scholar] [CrossRef]
- Szold, O.; Khoury, W.; Biderman, P.; Klausner, J.M.; Halpern, P.; Weinbroum, A.A. Inhaled nitric oxide improves pulmonary functions following massive pulmonary embolism: A report of four patients and review of the literature. Lung 2006, 184, 1–5. [Google Scholar] [CrossRef]
- Capellier, G.; Jacques, T.; Balvay, P.; Blasco, G.; Belle, E.; Barale, F. Inhaled nitric oxide in patients with pulmonary embolism. Intensive Care Med. 1997, 23, 1089–1092. [Google Scholar] [CrossRef]
- Kearon, C.; Akl, E.A.; Comerota, A.J.; Prandoni, P.; Bounameaux, H.; Goldhaber, S.Z.; Nelson, M.E.; Wells, P.S.; Gould, M.K.; Dentali, F.; et al. Antithrombotic therapy for vte disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012, 141, e419S–e496S. [Google Scholar] [CrossRef]
- Cho, S.M.; Hwang, J.; Chiarini, G.; Amer, M.; Antonini, M.V.; Barrett, N.; Belohlavek, J.; Blatt, J.E.; Brodie, D.; Dalton, H.J.; et al. Neurological monitoring and management for adult extracorporeal membrane oxygenation patients: Extracorporeal life support organization consensus guidelines. ASAIO J. 2024, 70, e169–e181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Q.; Chang, H.; Kalra, A.; Humayun, M.; Rosenblatt, K.R.; Shah, V.A.; Geocadin, R.G.; Brown, C.H.; Kim, B.S.; Whitman, G.J.R.; et al. Continuous monitoring of cerebral autoregulation in adults supported by extracorporeal membrane oxygenation. Neurocrit. Care 2024, 41, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, R.; Barili, F.; Mauro, M.D.; Gelsomino, S.; Parise, O.; Rycus, P.T.; Maessen, J.; Mueller, T.; Muellenbach, R.; Belohlavek, J.; et al. In-hospital neurologic complications in adult patients undergoing venoarterial extracorporeal membrane oxygenation: Results from the extracorporeal life support organization registry. Crit. Care Med. 2016, 44, e964–e972. [Google Scholar] [CrossRef]
- van den Berg, M.J.W.; Heunks, L.; Doorduin, J. Advances in achieving lung and diaphragm-protective ventilation. Curr. Opin. Crit. Care 2025, 31, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Vetrugno, L.; Longhini, F. Lung ultrasound monitoring: Impact on economics and outcomes. Curr. Opin. Anaesthesiol. 2023, 36, 234–239. [Google Scholar] [CrossRef]
- Simonte, R.; Cammarota, G.; Vetrugno, L.; De Robertis, E.; Longhini, F.; Spadaro, S. Advanced respiratory monitoring during extracorporeal membrane oxygenation. J. Clin. Med. 2024, 13, 2541. [Google Scholar] [CrossRef]
- Cammarota, G.; Simonte, R.; Longhini, F.; Spadaro, S.; Vetrugno, L.; De Robertis, E. Advanced point-of-care bedside monitoring for acute respiratory failure. Anesthesiology 2023, 138, 317–334. [Google Scholar] [CrossRef]
- Alizadehasl, A.; Roudini, K.; Hesami, M.; Kosari, F.; Pouraliakbar, H.R.; Mohseni, M.; Dokhani, N. Mediastinal gray zone lymphoma in a pregnant woman presenting with cardiac tamponade. Cardiooncology 2023, 9, 27. [Google Scholar] [CrossRef]
- Garofalo, E.; Cammarota, G.; Neri, G.; Macheda, S.; Biamonte, E.; Pasqua, P.; Guzzo, M.L.; Longhini, F.; Bruni, A. Bivalirudin vs. Enoxaparin in intubated COVID-19 patients: A pilot multicenter randomized controlled trial. J. Clin. Med. 2022, 11, 5992. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Bruni, A.; Gulli, S.; Borrazzo, C.; Quirino, A.; Lionello, R.; Serapide, F.; Garofalo, E.; Serraino, R.; Romeo, F.; et al. Efficacy of cefiderocol- vs colistin-containing regimen for treatment of bacteraemic ventilator-associated pneumonia caused by carbapenem-resistant acinetobacter baumannii in patients with COVID-19. Int. J. Antimicrob Agents 2023, 62, 106825. [Google Scholar] [CrossRef]
- Gascon, P.; Awada, A.; Karihtala, P.; Lorenzen, S.; Minichsdorfer, C. Optimal use of granulocyte colony-stimulating factor prophylaxis to improve survival in cancer patients receiving treatment: An expert view. Wien Klin. Wochenschr. 2024, 136, 362–368. [Google Scholar] [CrossRef]
- Kepley, J.M.; Bates, K.; Mohiuddin, S.S. Physiology, maternal changes. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kumar, R.; Sinha, A.; Lin, M.J.; Uchino, R.; Butryn, T.; O’Mara, M.S.; Nanda, S.; Shirani, J.; Stawicki, S.P. Complications of pericardiocentesis: A clinical synopsis. Int. J. Crit. Illn. Inj. Sci. 2015, 5, 206–212. [Google Scholar]
- Silvestro, S.; Calcaterra, V.; Pelizzo, G.; Bramanti, P.; Mazzon, E. Prenatal hypoxia and placental oxidative stress: Insights from animal models to clinical evidences. Antioxidants 2020, 9, 414. [Google Scholar] [CrossRef]
- Gupta, S.; Naithani, U.; Madhanmohan, C.; Singh, A.; Reddy, P.; Gupta, A. Evaluation of decision-to-delivery interval in emergency cesarean section: A 1-year prospective audit in a tertiary care hospital. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 64–70. [Google Scholar] [CrossRef]
- Berger, D.S.; Garg, B.; Penfield, C.A.; Caughey, A.B. Respiratory distress syndrome is associated with increased morbidity and mortality in late preterm births. Am. J. Obstet. Gynecol. MFM 2024, 6, 101374. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; McClure, E.M. Appropriate use of antenatal corticosteroid prophylaxis. Obstet Gynecol 2015, 125, 285–287. [Google Scholar] [CrossRef]
- Ng, E.H.; Shah, V. Guidelines for surfactant replacement therapy in neonates. Paediatr. Child Health 2021, 26, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Longhini, F.; Jourdain, G.; Ammar, F.; Mokthari, M.; Boithias, C.; Romain, O.; Letamendia, E.; Tissieres, P.; Chabernaud, J.L.; De Luca, D. Outcomes of preterm neonates transferred between tertiary perinatal centers. Pediatr. Crit. Care Med. 2015, 16, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Cerrud-Rodriguez, R.C.; Rashid, S.M.I.; Shaqra, H.; Alkhalil, A.; Algodi, M.; Chudow, J.J.; Garcia, M.J.; Tauras, J.M.; Weisz, G. An overlap presentation of pericardial decompression syndrome and stress cardiomyopathy following therapeutic pericardiocentesis. JACC Case Rep. 2020, 2, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Regenbogen, T.; Chen, L.; Trinkaus, K.; Wang-Gillam, A.; Tan, B.R.; Amin, M.; Pedersen, K.S.; Park, H.; Suresh, R.; Lim, K.H.; et al. Pacritinib to inhibit jak/stat signaling in refractory metastatic colon and rectal cancer. J. Gastrointest. Oncol. 2017, 8, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Blondon, M.; Martinez de Tejada, B.; Glauser, F.; Righini, M.; Robert-Ebadi, H. Management of high-risk pulmonary embolism in pregnancy. Thromb. Res. 2021, 204, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Giri, J.; Sista, A.K.; Weinberg, I.; Kearon, C.; Kumbhani, D.J.; Desai, N.D.; Piazza, G.; Gladwin, M.T.; Chatterjee, S.; Kobayashi, T.; et al. Interventional therapies for acute pulmonary embolism: Current status and principles for the development of novel evidence: A scientific statement from the american heart association. Circulation 2019, 140, e774–e801. [Google Scholar] [CrossRef]
- Kawachi, M.; Nakayama, T.; Kayama, M.; Nomura, M.; Miyashita, H.; Bojo, O.; Rhodes, L.; Sym, S.; Pienaar, R.N.; Probert, I.; et al. Rappemonads are haptophyte phytoplankton. Curr. Biol. 2021, 31, 2395–2403.e4. [Google Scholar] [CrossRef]
- Ong, J.; Zhang, J.J.Y.; Lorusso, R.; MacLaren, G.; Ramanathan, K. Extracorporeal membrane oxygenation in pregnancy and the postpartum period: A systematic review of case reports. Int. J. Obstet. Anesth. 2020, 43, 106–113. [Google Scholar] [CrossRef]
- Moroi, S.I.; Weiss, E.; Stanciu, S.; Badila, E.; Iliesiu, A.M.; Balahura, A.M. Pregnancy-related thromboembolism-current challenges at the emergency department. J. Pers. Med. 2024, 14, 926. [Google Scholar] [CrossRef]
- Terragni, P.; Faggiano, C.; Ranieri, V.M. Extracorporeal membrane oxygenation in adult patients with acute respiratory distress syndrome. Curr. Opin. Crit. Care 2014, 20, 86–91. [Google Scholar] [CrossRef]
- Geli, J.; Capoccia, M.; Maybauer, D.M.; Maybauer, M.O. Direct thrombin inhibition in extracorporeal membrane oxygenation. Int. J. Artif. Organs 2022, 45, 652–655. [Google Scholar] [CrossRef]
- Dingman, J.S.; Smith, Z.R.; Coba, V.E.; Peters, M.A.; To, L. Argatroban dosing requirements in extracorporeal life support and other critically ill populations. Thromb. Res. 2020, 189, 69–76. [Google Scholar] [CrossRef]
- Coughlin, M.A.; Bartlett, R.H. Anticoagulation for extracorporeal life support: Direct thrombin inhibitors and heparin. ASAIO J. 2015, 61, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tang, Y.; Xiong, X.; Zhu, M.; Yu, H.; Cheng, D. Successful application of argatroban during vv-ecmo in a pregnant patient complicated with ards due to severe tuberculosis: A case report and literature review. Front. Pharmacol. 2022, 13, 866027. [Google Scholar] [CrossRef]
- Geli, J.; Capoccia, M.; Maybauer, D.M.; Maybauer, M.O. Argatroban anticoagulation for adult extracorporeal membrane oxygenation: A systematic review. J. Intensive Care Med. 2022, 37, 459–471. [Google Scholar] [CrossRef]
- Sanfilippo, F.; Asmussen, S.; Maybauer, D.M.; Santonocito, C.; Fraser, J.F.; Erdoes, G.; Maybauer, M.O. Bivalirudin for alternative anticoagulation in extracorporeal membrane oxygenation: A systematic review. J. Intensive Care Med. 2017, 32, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Dado, C.D.; Levinson, A.T.; Bourjeily, G. Pregnancy and pulmonary embolism. Clin. Chest Med. 2018, 39, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.S.; Tan, M.J.; Sng, M.K.; Teo, Z.; Phua, T.; Choo, C.C.; Li, L.; Zhu, P.; Tan, N.S. Cancer-associated fibroblasts enact field cancerization by promoting extratumoral oxidative stress. Cell Death Dis. 2017, 8, e2562. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Omura, H.; Tokuyasu, Y.; Nakamoto, S.; Tanaka, T. Successful management of primary mediastinal large b-cell lymphoma during pregnancy. Intern. Med. 2019, 58, 3455–3459. [Google Scholar] [CrossRef]
- Perez, C.A.; Amin, J.; Aguina, L.M.; Cioffi-Lavina, M.; Santos, E.S. Primary mediastinal large b-cell lymphoma during pregnancy. Case Rep. Hematol. 2012, 2012, 197347. [Google Scholar] [CrossRef]
- Brownhalls, L.; Gillett, A.; Whately, Y.; Tanaka, K. A pregnancy case of primary mediastinal large b cell lymphoma with superior vena cava syndrome. Case Rep. Obstet. Gynecol. 2021, 2021, 3438230. [Google Scholar] [CrossRef]
- Intravaia, R.; De Chiara, B.; Musca, F.; Casadei, F.; Santambrogio, G.; Spano, F.; Belli, O.; Quattrocchi, G.; Giannattasio, C.; Moreo, A. Primary mediastinal large b-cell lymphoma and pregnancy: A challenging clinical scenario. Monaldi Arch. Chest Dis. 2022, 92. [Google Scholar] [CrossRef]
- Bruni, A.; Garofalo, E.; Mazzitelli, M.; Voci, C.P.; Puglisi, A.; Quirino, A.; Marascio, N.; Trecarichi, E.M.; Matera, G.; Torti, C.; et al. Multidisciplinary approach to a septic COVID-19 patient undergoing veno-venous extracorporeal membrane oxygenation and receiving thoracic surgery. Clin. Case Rep. 2021, 9, e04828. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Normal Range | Day 0 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|---|
| WBC (×103/mL) | 5.2–12.4 | 20.47 | 13.84 | 5.28 | 7.01 | 2.45 |
| Neutrophils (%) | 40–74% | 89.9 | 85.5 | 91.1 | 76.1 | 71.4 |
| Lymphocytes (%) | 19–48 | 3.3 | 5.6 | 4.1 | 9.7 | 21 |
| RBC (×106/μL) | 4.2–5.9 | 4.15 | 2.93 | 2.94 | 2.73 | 3.41 |
| Platelets (n × 103/μL) | 130–400 | 236 | 205 | 315 | 338 | 454 |
| Procalcitonin (ng/mL) | <0.5 | 1.54 | 0.06 | 0.74 | 0.23 | 0.22 |
| Creatinine (mg/dL) | 0.81 | 0.35 | 0.18 | 0.24 | 0.15 | 0.21 |
| ALT (UI/L) | 4–36 | 368 | 26 | 39 | 16 | 16 |
| Total Bilirubin (mg/dL) | 0.1–1.2 | 0.36 | 0.50 | 0.30 | 0.19 | 0.26 |
| aPTT (seconds) | 22.0–36.6 | 28 | 44 | 31 | 31 | 31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neri, G.; Ielapi, J.; Bosco, V.; Mastrangelo, H.; Mellace, F.; Salerno, N.; Mazza, G.A.; Serraino, G.F.; Caracciolo, D.; Venturella, R.; et al. Severe Hemodynamic Instability in a Young Pregnant Woman with Massive Pericardial Effusion and Pulmonary Embolism Secondary to Primary Mediastinal Non-Hodgkin’s Lymphoma. J. Clin. Med. 2025, 14, 2670. https://doi.org/10.3390/jcm14082670
Neri G, Ielapi J, Bosco V, Mastrangelo H, Mellace F, Salerno N, Mazza GA, Serraino GF, Caracciolo D, Venturella R, et al. Severe Hemodynamic Instability in a Young Pregnant Woman with Massive Pericardial Effusion and Pulmonary Embolism Secondary to Primary Mediastinal Non-Hodgkin’s Lymphoma. Journal of Clinical Medicine. 2025; 14(8):2670. https://doi.org/10.3390/jcm14082670
Chicago/Turabian StyleNeri, Giuseppe, Jessica Ielapi, Vincenzo Bosco, Helenia Mastrangelo, Federica Mellace, Nadia Salerno, Giuseppe Antonio Mazza, Giuseppe Filiberto Serraino, Daniele Caracciolo, Roberta Venturella, and et al. 2025. "Severe Hemodynamic Instability in a Young Pregnant Woman with Massive Pericardial Effusion and Pulmonary Embolism Secondary to Primary Mediastinal Non-Hodgkin’s Lymphoma" Journal of Clinical Medicine 14, no. 8: 2670. https://doi.org/10.3390/jcm14082670
APA StyleNeri, G., Ielapi, J., Bosco, V., Mastrangelo, H., Mellace, F., Salerno, N., Mazza, G. A., Serraino, G. F., Caracciolo, D., Venturella, R., Torella, D., Mastroroberto, P., Chiappetta, M., Russo, A., Tagliaferri, P., Tassone, P., Zullo, F., Bruni, A., Longhini, F., & Garofalo, E., on behalf of the Collaborative-UMG Group. (2025). Severe Hemodynamic Instability in a Young Pregnant Woman with Massive Pericardial Effusion and Pulmonary Embolism Secondary to Primary Mediastinal Non-Hodgkin’s Lymphoma. Journal of Clinical Medicine, 14(8), 2670. https://doi.org/10.3390/jcm14082670







