Co-Existing Vestibular Hypofunction Impairs Postural Control, but Not Frailty and Well-Being, in Older Adults with Benign Paroxysmal Positional Vertigo
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.2.1. Well-Being
2.2.2. Frailty
2.2.3. Postural Control
- During the timed chair stand test [33], the participant was asked to stand up from a chair five times as fast as possible with the arms held against the chest. Total time (s), sit-to-stand time (s) and stand-to-sit time (s) were derived from the sensors on the sternum and lumbar vertebrae. An increased time implies poorer performance.
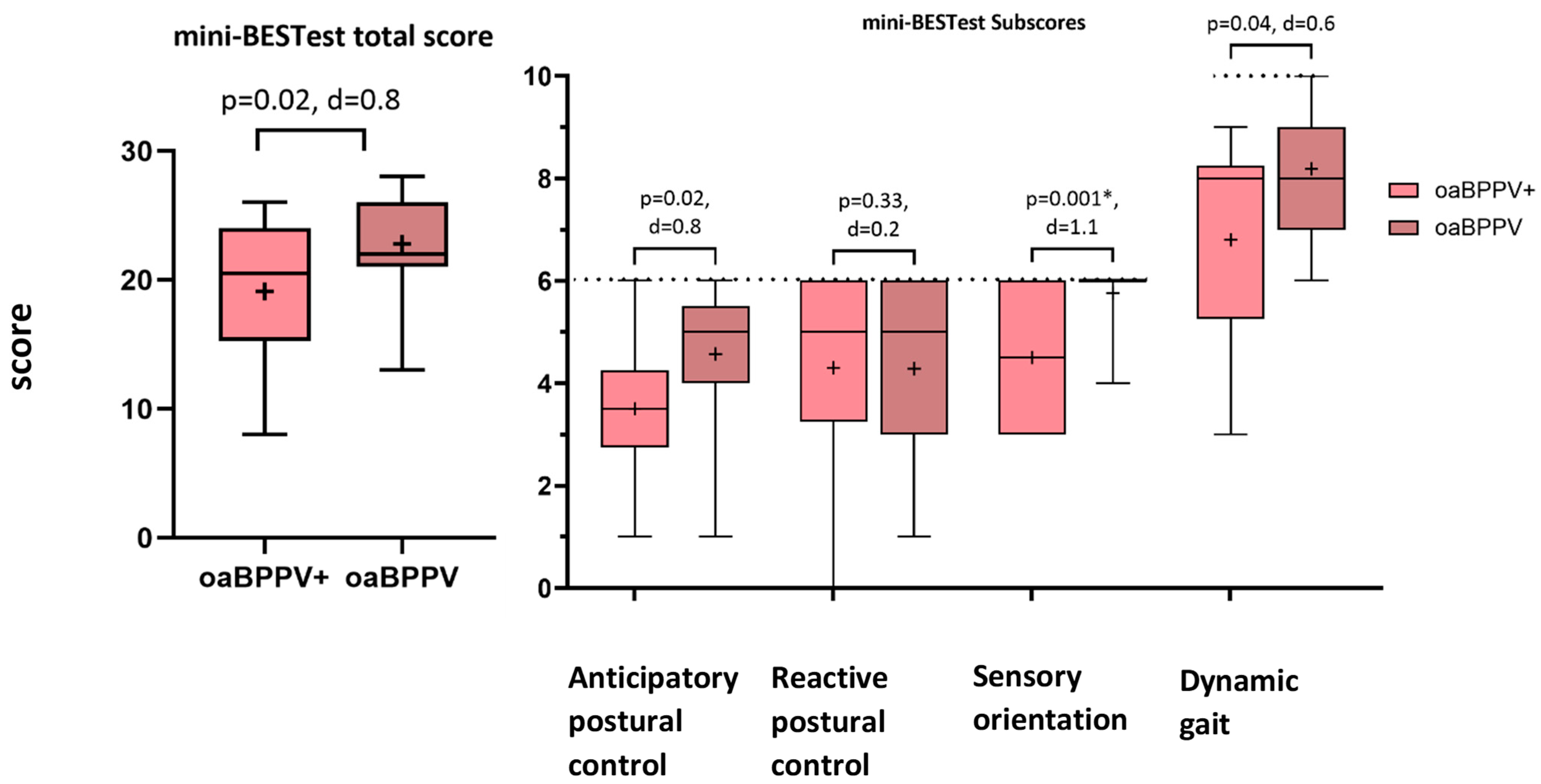
- The mini-balance evaluation systems test (Mini-BESTest) [34] was used to calculate the total score and subscores for anticipatory postural control, reactive postural control, sensory orientation and dynamic gait. A decreased score implies poorer performance.
- Some sub-items were evaluated in detail:
- ○
- For the timed up and go with and without a dual task (TUG and TUGdualtask), the total time (s), sit-to-stand time (s), stand-to-sit time (s) and turn duration (s) were derived from the sensors on the sternum and lumbar vertebrae. An increased time implies poorer performance. The dual task cost was calculated as [35]. A greater absolute value for the dual task cost (DTC) implicates poor performance deterioration under the dual task condition.
- ○
- For the 10-m walk test (10 MWT) at preferred gait speed and with head turns (10 MWHT), the gait speed (m/s), cadence (steps/min), stride length (m), stride length standard deviation (SD), double support (% gait cycle time), gait cycle duration (s) and gait cycle duration SD were derived from the mean of the bilateral sensors on the feet. A decreased gait speed, cadence, stride length with an increased stride length SD, double support, gait cycle duration and gait cycle duration SD implies poorer performance
- ○
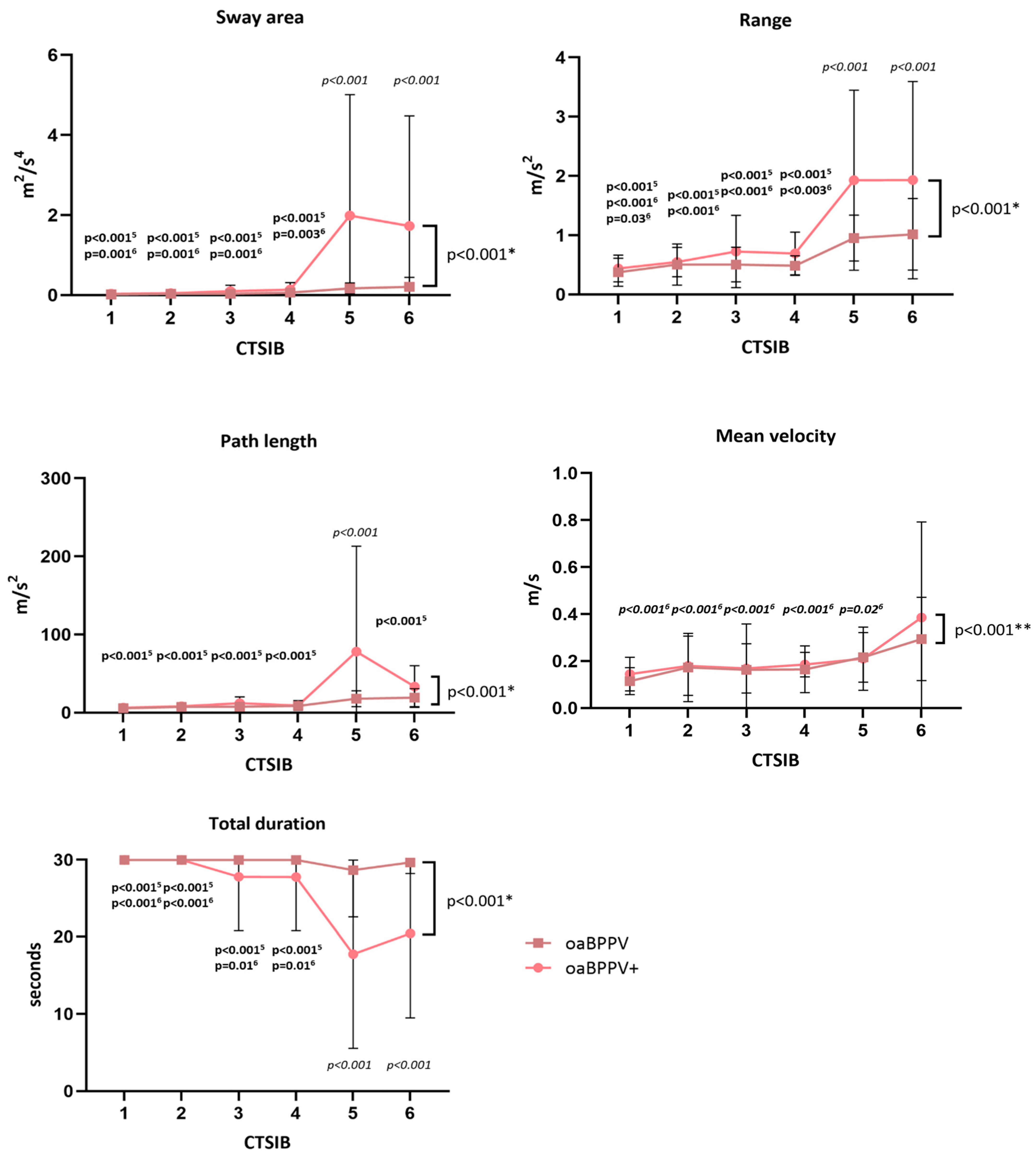
- For the longest trial of the worst side of the unilateral stance, the total time (s), sway area (m2/s4), mean velocity (m/s), path length (m/s2) and range (m/s2) of accelerations were derived from the lumbar sensor. A decreased time and increased sway area, mean velocity, path length and range of acceleration implies poorer performance.
2.2.4. Treatment with Repositioning Maneuvers
2.2.5. Caloric Irrigation Test
2.3. Statistics
3. Results
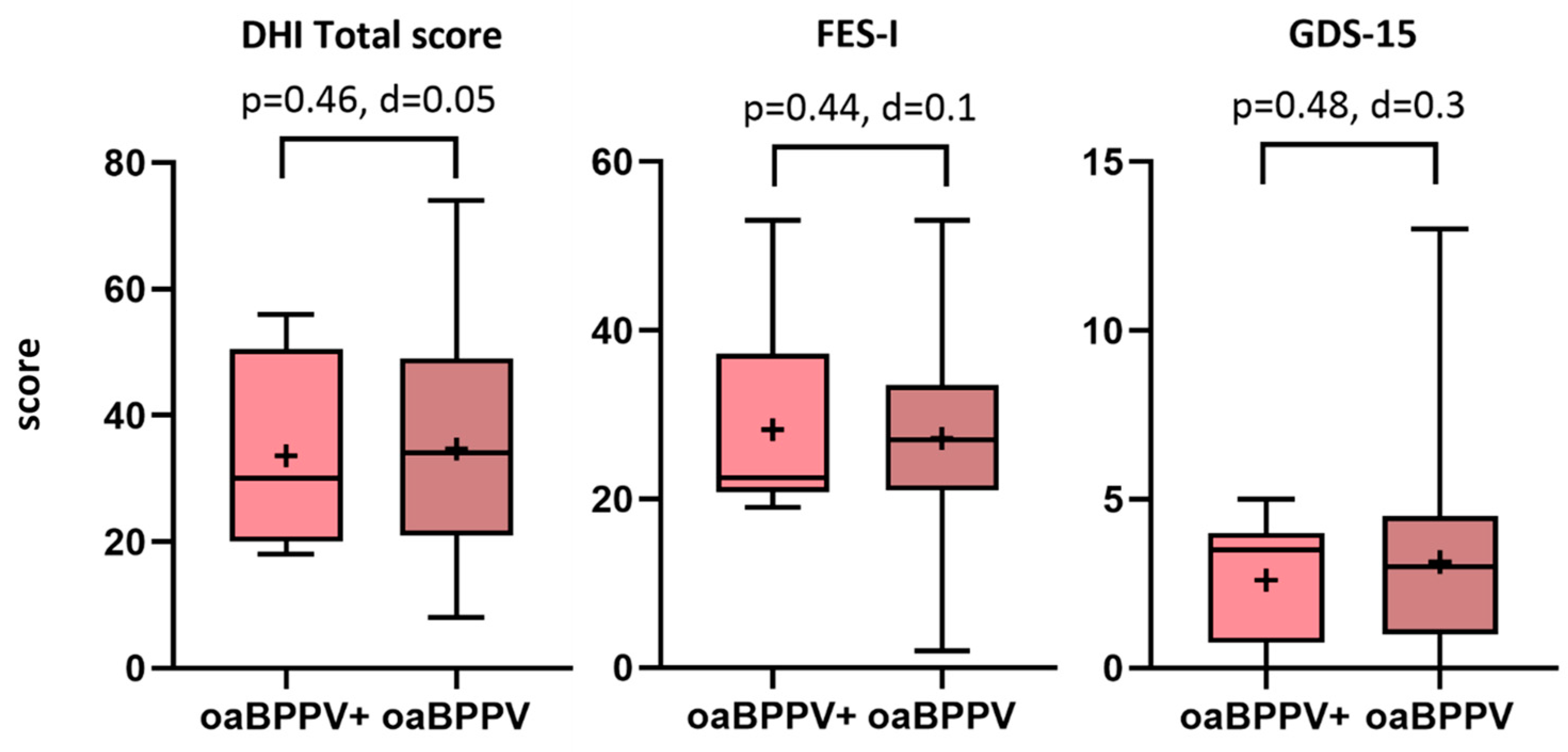
3.1. Well-Being
3.2. Frailty
3.3. Falls
3.4. Postural Control
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 10 MWT | 10-m walk test |
| 10 MWHT | 10-m walk test with head turns |
| BPPV | Benign Paroxysmal Positional Vertigo |
| CTSIB | Clinical test of sensory interaction on balance |
| DHI | Dizziness Handicap Inventory |
| FES-I | International Falls Efficacy Scale |
| GDS-15 | 15-item Geriatric Depression Scale |
| oaBPPV | Older adults with Benign Paroxysmal Positional Vertigo |
| oaBPPV+ | Older adults with Benign Paroxysmal Positional Vertigo and co-existing canal paresis |
| MOCA | Montreal Cognitive Assessment |
| RM | Repositioning maneuver |
| TUG | Timed up and go |
| TUGdualtask | Timed up and go with dual tasks |
| ZOL Genk | Hospital Oost-Limburg Genk |
References
- Katsarkas, A. Dizziness in aging: A retrospective study of 1194 cases. Otolaryngol. Head. Neck Surg. 1994, 110, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, J.H.; Kim, J.S. Update on benign paroxysmal positional vertigo. J. Neurol. 2021, 268, 1995–2000. [Google Scholar] [CrossRef] [PubMed]
- Parnes, L.S.; Agrawal, S.K.; Atlas, J. Diagnosis and management of benign paroxysmal positional vertigo (BPPV). Cmaj 2003, 169, 681–693. [Google Scholar] [PubMed]
- Bertholon, P.; Brandt, T.; Fife, T.; Imai, T.; Nuti, D.; Newman-toker, D. Benign paroxysmal positional vertigo: Diagnostic criteria. J. Vestib. Res. 2015, 25, 105–117. [Google Scholar] [CrossRef]
- Van Dam, V.S.; Maas, B.D.P.J.P.J.; Schermer, T.R.; van Benthem, P.-P.G.P.G.; Bruintjes, T.D. Two Symptoms Strongly Suggest Benign Paroxysmal Positional Vertigo in a Dizzy Patient. Front. Neurol. 2021, 11, 625776. [Google Scholar] [CrossRef]
- Piker, E.G.; Jacobson, G.P. Self-report symptoms differ between younger and older dizzy patients. Otol. Neurotol. 2014, 35, 873–879. [Google Scholar] [CrossRef]
- Pauwels, S.; Lemkens, N.; Lemmens, W.; Meijer, K.; Meyns, P.; Berg, R.V.D.; Spildooren, J. The importance of frailty in older adults with Benign Paroxysmal Positioning Vertigo. J. Neurol. Phys. Ther. 2025, 49, 99–107. [Google Scholar] [CrossRef]
- Oghalai, J.S.; Manolidis, S.; Barth, J.L.; Stewart, M.G.; Jenkins, H.A. Unrecognized benign paroxysmal positional vertigo in elderly patients. Otolaryngol. Neck Surg. 2000, 122, 630–634. [Google Scholar] [CrossRef]
- Lindell, E.; Kollen, L.; Johansson, M.; Karlsson, T.; Ryden, L.; Erhag, H.F.; Wetterberg, H.; Zettergren, A.; Skoog, I.; Finizia, C. Benign paroxysmal positional vertigo, dizziness, and health-related quality of life among older adults in a population-based setting. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 1637–1644. [Google Scholar] [CrossRef]
- Bhattacharyya, N.; Gubbels, S.P.; Schwartz, S.R.; Edlow, J.A.; El-Kashlan, H.; Fife, T.; Holmberg, J.M.; Mahoney, K.; Hollingsworth, D.B.; Roberts, R.; et al. Clinical Practice Guideline: Benign Paroxysmal Positional Vertigo (Update). Otolaryngol. Neck Surg. 2017, 156, S1–S47. [Google Scholar] [CrossRef]
- Laurent, G.; Vereeck, L.; Verbecque, E.; Herssens, N.; Casters, L.; Spildooren, J. Effect of age on treatment outcomes in benign paroxysmal positional vertigo: A systematic review. J. Am. Geriatr. Soc. 2022, 70, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Özgirgin, O.N.; Kingma, H.; Manzari, L.; Lacour, M. Residual dizziness after BPPV management: Exploring pathophysiology and treatment beyond canalith repositioning maneuvers. Front. Neurol. 2024, 15, 1382196. [Google Scholar] [CrossRef]
- Sfakianaki, I.; Binos, P.; Karkos, P.; Dimas, G.G.; Psillas, G. Risk Factors for Recurrence of Benign Paroxysmal Positional Vertigo. A Clinical Review. J. Clin. Med. 2021, 10, 4372. [Google Scholar] [CrossRef] [PubMed]
- Kabaya, K.; Katsumi, S.; Fukushima, A.; Esaki, S.; Minakata, T.; Iwasaki, S. Assessment of semicircular canal function in benign paroxysmal positional vertigo using the video head impulse test and caloric test. Laryngoscope Investig. Otolaryngol. 2023, 8, 525–531. [Google Scholar] [CrossRef]
- Song, N.; Wu, Y.; Li, X.; Wang, Q.; Ma, X.; Yang, X. Geriatric benign paroxysmal positional vertigo: A single-center study. Braz. J. Otorhinolaryngol. 2023, 89, 101277. [Google Scholar] [CrossRef]
- Starkov, D.; Strupp, M.; Pleshkov, M.; Kingma, H.; van de Berg, R. Diagnosing vestibular hypofunction: An update. J. Neurol. 2021, 268, 377–385. [Google Scholar] [CrossRef]
- Halmagyi, G.M.; Curthoys, I.S. A Clinical Sign of Canal Paresis. Arch. Neurol. 1988, 45, 737–739. [Google Scholar] [CrossRef]
- Paredis, S.; van Stiphout, L.; Remmen, E.; Strupp, M.; Gerards, M.C.; Kingma, H.; Van Rompaey, V.; Fornos, A.P.; Guinand, N.; van de Berg, R. DISCOHAT: An Acronym to Describe the Spectrum of Symptoms Related to Bilateral Vestibulopathy. Front. Neurol. 2021, 12, 771650. [Google Scholar] [CrossRef]
- Zhu, M.; van Stiphout, L.; Karabulut, M.; Pérez Fornos, A.; Guinand, N.; Meijer, K.; van de Berg, R.; McCrum, C. Assessing balance in people with bilateral vestibulopathy using the Mini-Balance Evaluation Systems Test (Mini-BESTest): Feasibility and comparison with healthy control data. J. Neurol. 2023, 270, 4423–4433. [Google Scholar] [CrossRef]
- Karabulut, M.; Viechtbauer, W.; Van Laer, L.; Mohamad, A.; Van Rompaey, V.; Guinand, N.; Perez Fornos, A.; Gerards, M.C.; van de Berg, R. Chronic Unilateral Vestibular Hypofunction: Insights into Etiologies, Clinical Subtypes, Diagnostics and Quality of Life. J. Clin. Med. 2024, 13, 5381. [Google Scholar] [CrossRef]
- Pollak, L.; Davies, R.A.; Luxon, L.L. Effectiveness of the particle repositioning maneuver in benign paroxysmal positional vertigo with and without additional vestibular pathology. Otol. Neurotol. 2002, 23, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Zhen, Z.; Zhang, J.; Zeng, Z.; Zhong, Z.; Wang, Q. The role of electrocochleography and the caloric test in predicting short-term recurrence of benign paroxysmal positional vertigo. Front. Neurol. 2023, 14, 1225857. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, S.; Lemkens, N.; Lemmens, W.; Meijer, K.; Bijnens, W.; Meyns, P. Physical Activity and Frailty Are Impaired in Older Adults with Benign Paroxysmal Positional Vertigo. J. Clin. Med. 2024, 13, 7542. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Jacobson, G.P.; Newman, C.W. Development of the Dizziness Handicap Inventory The. Arch. Otolaryngol. Neck Surg. 2013, 116, 424–427. [Google Scholar] [CrossRef]
- Yardley, L.; Beyer, N.; Hauer, K.; Kempen, G.; Piot-Ziegler, C.; Todd, C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005, 34, 614–619. [Google Scholar] [CrossRef]
- Brink, T.L.; Sciences, B.; Rose, L.; Alto, P. Development and validation of a geriatric depression report screening scale: A preliminary report. J. Psychiatr. Res. 1983, 17, 37–49. [Google Scholar]
- Vereeck, L.; Truijen, S.; Wuyts, F.; Heyning, P.H. Van De Test-retest reliability of the Dutch version of the Dizziness Handicap Inventory. B ENT 2020, 2, 75–80. [Google Scholar]
- Morgan, M.T.; Friscia, L.A.; Whitney, S.L.; Furman, J.M.; Sparto, P.J. Reliability and validity of the falls efficacy scale-international (FES-I) in individuals with dizziness and imbalance. Otol. Neurotol. 2013, 34, 1104–1108. [Google Scholar] [CrossRef]
- Krishnamoorthy, Y.; Rajaa, S.; Rehman, T. Diagnostic accuracy of various forms of geriatric depression scale for screening of depression among older adults: Systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2020, 87, 104002. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol.—Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Avila-Funes, J.A.; Helmer, C.; Amieva, H.; Barberger-Gateau, P.; Goff, M.L.; Ritchie, K.; Portet, F.; Carrière, I.; Tavernier, B.; Gutiérrez-Robledo, L.M.; et al. Frailty among community-dwelling elderly people in France: The three-city study. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Csuka, M.; McCarty, D.J. Simple method for measurement of lower extremity muscle strength. Am. J. Med. 1985, 78, 77–81. [Google Scholar] [CrossRef]
- Franchignoni, F.; Horak, F.; Godi, M.; Nardone, A.; Giordano, A. Using psychometric techniques to improve the balance evaluation systems test: The mini-bestest. J. Rehabil. Med. 2010, 42, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, E.; Warmerdam, E.; Romijnders, R.; Hansen, C.; Pontieri, F.E.; Maetzler, W. Cognitive dual-task cost depends on the complexity of the cognitive task, but not on age and disease. Front. Neurol. 2022, 13, 964207. [Google Scholar] [CrossRef]
- Cohen, H.; Blatchly, C.A.; Gombash, L.L.; Di Fabio, R.P. A study of the Clinical Test of Sensory Interaction and Balance. Phys. Ther. 1993, 73, 346–354. [Google Scholar] [CrossRef]
- Strupp, M.; Kim, J.S.; Murofushi, T.; Straumann, D.; Jen, J.C.; Rosengren, S.M.; Della Santina, C.C.; Kingma, H. Bilateral vestibulopathy: Diagnostic criteria consensus document of the classification committee of the barany society. J. Vestib. Res. Equilib. Orientat. 2017, 27, 177–189. [Google Scholar] [CrossRef]
- Shepard, N.T.; Jacobson, G.P. The caloric irrigation test. In Handbook of Clinical Neurology; Elsevier B.V.: Amsterdam, The Netherlands, 2016; Volume 137, pp. 119–131. [Google Scholar]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef]
- Seo, S. A Review and Comparison of Methods for Detecting Outliers in Univariate Data Sets. Doctoral Dissertation, University of Pittsburgh, Pittsburgh, PA, USA, 2006. [Google Scholar]
- Chowdhry, A.K.; Gondi, V.; Pugh, S.L. Missing Data in Clinical Studies. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1267–1271. [Google Scholar] [CrossRef]
- Holm, S. Board of the Foundation of the Scandinavian Journal of Statistics A Simple Sequentially Rejective Multiple Test Procedure A Simple Sequentially Rejective Multiple Test Procedure. Source Scand. J. Stat. Scand J Stat. 1979, 6, 65–70. [Google Scholar]
- Pauwels, S.; Casters, L.; Lemkens, N.; Lemmens, W.; Meijer, K.; Meyns, P.; Van De Berg, R.; Spildooren, J. Gait and Falls in Benign Paroxysmal Positional Vertigo: A Systematic Review and Meta-analysis. J. Neurol. Phys. Ther. 2023, 47, 127–138. [Google Scholar] [CrossRef] [PubMed]
- El Khiati, R.; Tighilet, B.; Chabbert, C. Vestibular Disorders and Hormonal Dysregulations: State of the Art and Clinical Perspectives. Cells 2023, 12, 656. [Google Scholar] [CrossRef] [PubMed]
- Neuhauser, H.K. The epidemiology of dizziness and vertigo. In Handbook of Clinical Neurology; Elsevier B.V.: Amsterdam, The Netherlands, 2016; Volume 137, pp. 67–82. [Google Scholar]
- Pollak, L.; Hreben, V.; Flechter, S. Benign Paroxysmal Positional Vertigo in Old Age: Our Experience and Review of the Literature. In Dizziness: Vertigo, Disequilibrium and Lightheadedness; Lindqvist, A., Nyman, G., Eds.; Nova Biomedical Books: Suite N Hauppauge, NY, USA, 2009; pp. 139–151. ISBN 978-1-60741-847-4. [Google Scholar]
- Li, S.; Wang, Z.; Liu, Y.; Cao, J.; Zheng, H.; Jing, Y.; Han, L.; Ma, X.; Xia, R.; Yu, L. Risk Factors for the Recurrence of Benign Paroxysmal Positional Vertigo: A Systematic Review and Meta-Analysis. Ear Nose Throat J. 2022, 101, NP112–NP134. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.S.; Mulavara, A.P.; Peters, B.T.; Sangi-Haghpeykar, H.; Bloomberg, J.J. Standing Balance Tests for Screening People With Vestibular Impairments. Laryngoscope 2014, 124, 545–550. [Google Scholar] [CrossRef][Green Version]
- Çelebisoy, N.; Bayam, E.; Güleç, F.; Köse, T.; Akyürekli, Ö. Balance in posterior and horizontal canal type benign paroxysmal positional vertigo before and after canalith repositioning maneuvers. Gait Posture 2009, 29, 520–523. [Google Scholar] [CrossRef]
- Assal, S.; Morsy, H.M.; Almagassbi, N.M.; Eldeeb, M. Assessment of sensory organization testing in benign paroxysmal positional vertigo patients before and after repositioning manoeuvre. Acta Otorrinolaringol. Esp. 2022, 73, 210–218. [Google Scholar] [CrossRef]
- Lim, Y.H.; Kang, K.; Lee, H.W.; Kim, J.S.; Kim, S.H. Gait in Benign Paroxysmal Positional Vertigo. Front. Neurol. 2021, 12, 633393. [Google Scholar] [CrossRef]
- Furman, J.M.; Raz, Y.; Whitney, S.L. Geriatric vestibulopathy assessment and management. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 386–391. [Google Scholar] [CrossRef]
- Pauwels, S.; Casters, L.; Meyns, P.; Lemkens, N.; Lemmens, W.; Meijer, K.; van de Berg, R.; Spildooren, J. Several components of postural control are affected by Benign Paroxysmal Positional Vertigo but improve after particle-repositioning maneuvers: A systematic review and meta-analysis. Clin. Rehabil. 2024, 39, 3–22. [Google Scholar] [CrossRef]
| Characteristics | oaBPPV+ n = 10 | oaBPPV n = 21 | p-Value |
|---|---|---|---|
| Female/Male | 9/1 | 10/11 | 0.04 |
| Age | 72.5 (4.5) | 72.62 (4.86) | 0.95 |
| Weight (kg) | 77.35 (11.45) | 75.99 (11.12) | 0.88 |
| Height (m) | 1.64 (0.06) | 1.66 (0.09) | 0.54 |
| BPPV | 0.25 | ||
| RPSCC (n, %) | 4, 40% | 9, 43% | |
| LPSCC (n, %) | 4, 40% | 9, 43% | |
| Bilateral PSCC (n, %) | 0, 0% | 0, 0% | |
| RLSCC geotropic (n, %)/apogeotropic (n, %) | 1, 10%/0, 0% | 0, 0%/3, 14% | |
| LLSCC geotropic (n, %)/apogeotropic (n, %) | 1, 10%/0, 0% | 0, 0% | |
| Number of repositioning maneuvers | 2 (3) | 2 (1.75) | 0.2 |
| Caloric irrigation test | |||
| UVH ipsilateral BPPV (n, %) | 8, 80% | 0, 0% | |
| UVH contralateral BPPV (n, %) | 2, 20% | 0, 0% | |
| No vestibular hypofunction (n, %) | 0, 0% | 21, 100% | |
| Duration of complaints | 0.1 | ||
| Some days (n) | 0, 0% | 3, 14% | |
| Several weeks (n) | 3, 30% | 2, 10% | |
| Several months (n) | 7, 70% | 16, 76% | |
| Walking aid | 0.12 | ||
| None (n) | 7, 70% | 20, 95% | |
| Crutch (n) | 2, 20% | 1, 5% | |
| Walker (n) | 1, 10% | 0, 0% | |
| Sleeping pattern | 0.56 | ||
| Good (n) | 7, 70% | 11, 52% | |
| Restless (n) | 1, 10% | 7, 33% | |
| Long time needed to fall asleep (n) | 1, 10% | 1, 5% | |
| Restless + long time needed (n) | 1, 10% | 2, 10% | |
| Number of medications | 5.7 (2.41) | 4.33 (2.72) | 0.09 |
| MOCA total score | 23.2 (4.85) | 24 (3.45) | 0.3 |
| Comorbidities | oaBPPV+ | oaBPPV | p-Value |
|---|---|---|---|
| Number of comorbidities | 4 (2) | 2 (3) | 0.03 |
| Cardiovascular (n, %) | 2, 20% | 6, 29% | 0.2 |
| Cerebrovascular (n, %) | 1, 10% | 1, 5% | 0.2 |
| Diabetes mellitus (n, %) | 1, 10% | 4, 19% | 0.2 |
| Hypertension (n, %) | 8, 80% | 11, 52% | 0.07 |
| Hypercholesterolemia (n, %) | 9, 90% | 11, 53% | 0.02 |
| Vitamin D deficiency (n, %) | 3, 30% | 5, 24% | 0.25 |
| Osteoporosis (n, %) | 3, 30% | 3, 15% | 0.14 |
| Other (n, %) | 6, 60% | 9, 43% | 0.3 |
| Frailty | oaBPPV+ | oaBPPV | p-Value | Cohen’s d |
|---|---|---|---|---|
| Robust (n, %) | 3, 30% | 5, 25% | 0.36 | 0.3 |
| Pre-frail (n, %) | 3, 30% | 9, 45% | ||
| Frail (n, %) | 4, 40% | 6, 30% | ||
| Unintentional weight loss | ||||
| Yes (n, %)/No (n) | 1, 10%/9 | 5, 25%/15 | 0.35 | 0.3 |
| Self-reported exhaustion | 0.2 | |||
| Yes (n, %)/No (n) | 6, 60%/4 | 10, 50%/10 | 0.45 | |
| Slowness | 0.5 | |||
| Yes (n, %)/No (n) | 5, 50%/5 | 6, 30%/14 | 0.22 | |
| Weakness | 0.1 | |||
| Yes (n, %)/No (n) | 3, 30%/7 | 7, 35%/13 | 0.56 | |
| Physical inactivity | 0 | |||
| Yes (n, %)/No (n) | 8, 80%/2 | 4, 20%/16 | 0.69 | |
| Timed chair stand test | ||||
| Total time (s) | 17.1 (11.6) | 16.2 (6.14) | 0.32 | 0.18 |
| Sit-to-stand time (s) | 1 (0.3) | 1 (0.4) | 0.48 | 0.02 |
| Stand-to-sit time (s) | 0.8 (0.3) | 0.7 (0.2) | 0.2 | 0.39 |
| Unilateral stance | ||||
| Area (m2/s4) | 1.4 (2.6) | 1.1 (2.1) | 0.29 | 0.19 |
| Velocity (m/s) | 0.3 (0.4) | 0.4 (0.4) | 0.21 | 0.31 |
| Path (m/s2) | 73.7 (47.14) | 59.1 (60.7) | 0.22 | 0.28 |
| Range (m/s2) | 2.2 (2.8) | 2.4 (3) | 0.47 | 0.03 |
| Time (s) | 4.8 (8.9) | 13.4 (14.3) | 0.01 | 0.84 |
| Falls | ||||
| Fall history Yes (n, %)/No (n) | 4, 40%/6 | 7, 33%/14 | 0.5 OR 1.3; 95% CI [0.28,6.3]; 0.08 | 0.1 |
| Number of falls | ||||
| 0 (n, %) | 6, 60% | 14, 67% | 0.4 | 0.3 |
| 1 (n, %) | 2, 20% | 3, 14% | ||
| 2 (n, %) | 2, 20% | 3, 14% | ||
| >2 (n, %) | 0, 0% | 1, 5% | ||
| Reason for falls | ||||
| Accidental (n, %) | 3, 30% | 4, 19% | 0.4 | 0.3 |
| Dizziness (n, %) | 1, 10% | 3, 14% | ||
| Syncope (n, %) | 0, 0% | 0, 0% | ||
| No falls (n, %) | 6, 60% | 14, 67% |
| Timed Up and Go | oaBPPV+ | oaBPPV |
|---|---|---|
| Total time (s) | 13.8 (3.8) | 12.2 (3.1) |
| Sit-to-stand time (s) | 1.1 (0.3) | 1.1 (0.5) |
| Stand-to-sit time (s) | 0.9 (0.2) | 0.9 (0.2) |
| Turn time (s) | 2.4 (0.4) | 2.4 (0.6) |
| Timed up and go with dual task | ||
| Total time (s) | 16.6 (8.1) | 13.4 (5.9) |
| Sit-to-stand time (s) | 1.1 (0.3) | 1 (0.3) |
| Stand-to-sit time (s) | 0.9 (0.2) | 0.9 (0.3) |
| Turn time (s) | 2.7 (0.6) | 2.7 (0.6) |
| Dual task cost (%) | 30.4 (21.5) | 26.4 (30.1) |
| 10-m walk test | ||
| Gait speed (m/s) | 0.9 (0.2) | 1 (0.2) |
| Cadance (step/min) | 99.9 (16) | 107.2 (10) |
| Stride length (m) | 1 (0.17) | 1.1 (0.2) |
| Stride length SD (m) | 0.04 (0.03) | 0.3 (0.02) |
| Double support time (%GCT) | 22.4 (4.8) | 21.8 (2.8) |
| Cycle duration (s) | 1.2 (1.3) | 1.1 (0.1) |
| Cycle duration SD (s) | 0.03 (0.01) | 0.03 (0.2) |
| 10-m walk test with head turns | ||
| Gait speed (m/s) | 0.7 (0.22) | 0.8 (0.2) |
| Gait speed (m/s) | 0.9 (0.2) | 1 (0.2) |
| Cadance (step/min) | 99.9 (16) | 107.2 (10) |
| Stride length (m) | 1 (0.17) | 1.1 (0.2) |
| Stride length SD (m) | 0.04 (0.07) | 0.3 (0.05) |
| Double support time (%GCT) | 22.4 (4.8) | 21.8 (2.8) |
| Cycle duration (s) | 1.2 (1.3) | 1.1 (0.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauwels, S.; Lemkens, N.; Lemmens, W.; Meijer, K.; Meyns, P.; van de Berg, R.; Spildooren, J. Co-Existing Vestibular Hypofunction Impairs Postural Control, but Not Frailty and Well-Being, in Older Adults with Benign Paroxysmal Positional Vertigo. J. Clin. Med. 2025, 14, 2666. https://doi.org/10.3390/jcm14082666
Pauwels S, Lemkens N, Lemmens W, Meijer K, Meyns P, van de Berg R, Spildooren J. Co-Existing Vestibular Hypofunction Impairs Postural Control, but Not Frailty and Well-Being, in Older Adults with Benign Paroxysmal Positional Vertigo. Journal of Clinical Medicine. 2025; 14(8):2666. https://doi.org/10.3390/jcm14082666
Chicago/Turabian StylePauwels, Sara, Nele Lemkens, Winde Lemmens, Kenneth Meijer, Pieter Meyns, Raymond van de Berg, and Joke Spildooren. 2025. "Co-Existing Vestibular Hypofunction Impairs Postural Control, but Not Frailty and Well-Being, in Older Adults with Benign Paroxysmal Positional Vertigo" Journal of Clinical Medicine 14, no. 8: 2666. https://doi.org/10.3390/jcm14082666
APA StylePauwels, S., Lemkens, N., Lemmens, W., Meijer, K., Meyns, P., van de Berg, R., & Spildooren, J. (2025). Co-Existing Vestibular Hypofunction Impairs Postural Control, but Not Frailty and Well-Being, in Older Adults with Benign Paroxysmal Positional Vertigo. Journal of Clinical Medicine, 14(8), 2666. https://doi.org/10.3390/jcm14082666






