Dynamic Neutrophil Subsets and Function in Lung Transplant Recipients: Insights from a One-Year Longitudinal Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Clinical Management
2.2.1. Surveillance Protocol
2.2.2. Immunosuppression Protocol
2.2.3. Antimicrobial Prophylaxis
2.3. Human Blood Samples and Neutrophil Isolation
2.3.1. ROS Production
2.3.2. Staining and Flow Cytometry
2.4. Statistical Analysis
3. Results
3.1. Study Cohort
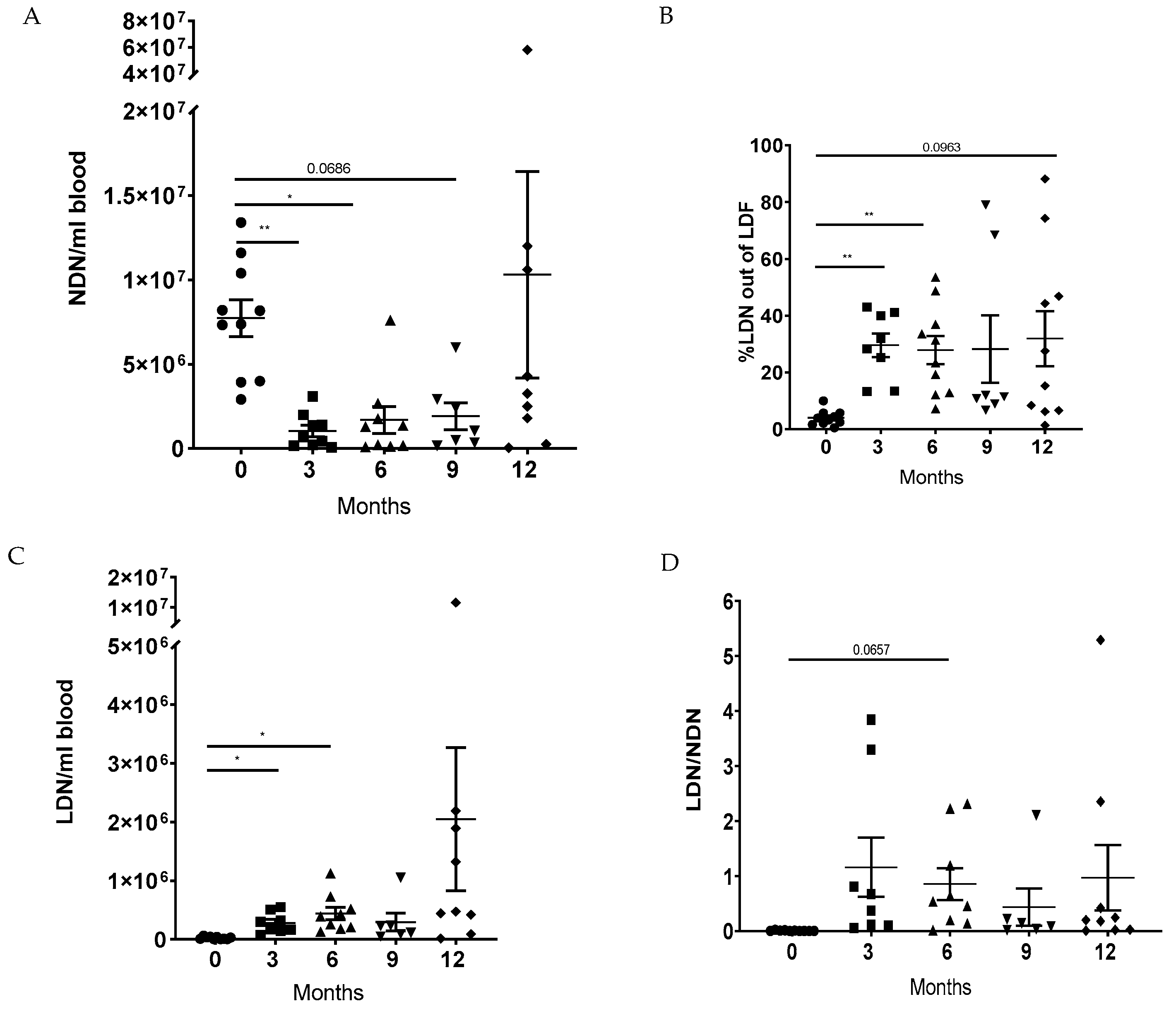
3.2. LTx Increased the Ratio of LDNs to NDNs in Peripheral Blood
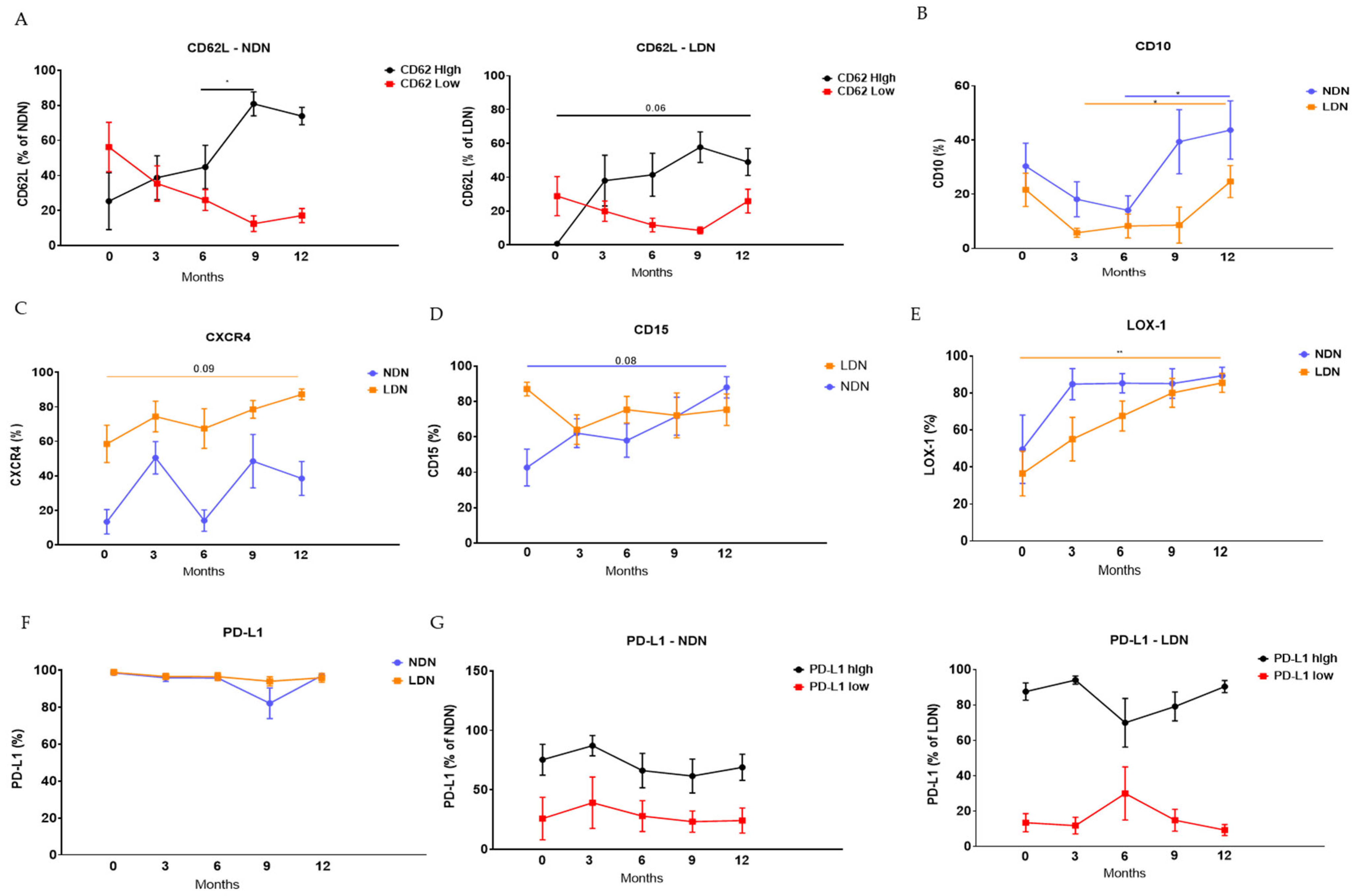
3.3. Modulation of Neutrophils’ Phenotype Following LTx
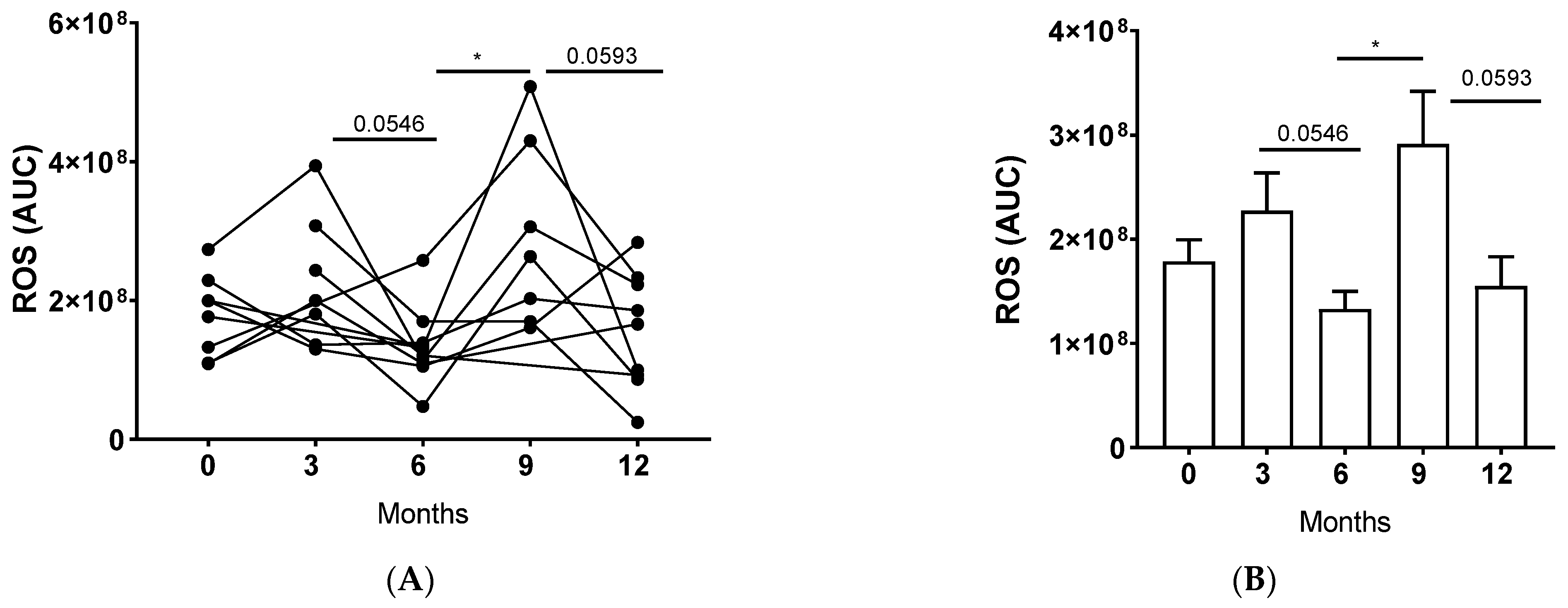
3.4. LTx Affected ROS Production by NDNs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACR | Acute Cellular Rejection |
| BOS | Bronchiolitis Obliterans Syndrome |
| CD | Cluster of Differentiation |
| CLAD | Chronic Lung Allograft Dysfunction |
| CMV | Cytomegalovirus |
| CXCR4 | C-X-C Chemokine Receptor 4 |
| FEV1 | Forced Expiratory Volume in 1 s |
| HRP | Horseradish Peroxidase |
| IQR | Interquartile Range |
| LDN | Low-Density Neutrophil |
| LTx | Lung Transplant |
| MMP8 | Matrix Metalloproteinase-8 |
| MMP9 | Matrix Metalloproteinase-9 |
| NDN | Normal-Density Neutrophil |
| NETs | Neutrophil Extracellular Traps |
| PMA | Phorbol Myristate Acetate |
| ROS | Reactive Oxygen Species |
References
- Koutsokera, A.; Levy, L.; Pal, P.; Orchanian-Cheff, A.; Martinu, T. Acute Cellular Rejection: Is It Still Relevant? Semin. Respir. Crit. Care Med. 2018, 39, 181–198. [Google Scholar] [CrossRef]
- Shilling, R.A.; Wilkes, D.S. Immunobiology of chronic lung allograft dysfunction: New insights from the bench and beyond. Am. J. Transpl. 2009, 9, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Schofield, Z.V.; Woodruff, T.M.; Halai, R.; Wu, M.C.-L.; Cooper, M.A. Neutrophils—A key component of ischemia-reperfusion injury. Shock 2013, 40, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Scozzi, D.; Ibrahim, M.; Menna, C.; Krupnick, A.S.; Kreisel, D.; Gelman, A.E. The Role of Neutrophils in Transplanted Organs. Am. J. Transpl. 2017, 17, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Beauvillain, C.; Cunin, P.; Doni, A.; Scotet, M.; Jaillon, S.; Loiry, M.-L.; Magistrelli, G.; Masternak, K.; Chevailler, A.; Delneste, Y.; et al. CCR7 is involved in the migration of neutrophils to lymph nodes. Blood 2011, 117, 1196–1204. [Google Scholar] [CrossRef]
- Leliefeld, P.H.C.; Koenderman, L.; Pillay, J. How Neutrophils Shape Adaptive Immune Responses. Front. Immunol. 2015, 6, 471. [Google Scholar] [CrossRef]
- Christoffersson, G.; Vågesjö, E.; Vandooren, J.; Lidén, M.; Massena, S.; Reinert, R.B.; Brissova, M.; Powers, A.C.; Opdenakker, G.; Phillipson, M. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood 2012, 120, 4653–4662. [Google Scholar] [CrossRef]
- Huynh, M.-L.N.; Fadok, V.A.; Henson, P.M. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J. Clin. Investig. 2002, 109, 41–50. [Google Scholar] [CrossRef]
- Pillay, J.; Kamp, V.M.; van Hoffen, E.; Visser, T.; Tak, T.; Lammers, J.-W.; Ulfman, L.H.; Leenen, L.P.; Pickkers, P.; Koenderman, L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J. Clin. Investig. 2012, 122, 327–336. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Fridlender, Z.G.; Glogauer, M.; Scapini, P. Neutrophil Diversity in Health and Disease. Trends Immunol. 2019, 40, 565–583. [Google Scholar] [CrossRef]
- Stewart, S.; Fishbein, M.C.; Snell, G.I.; Berry, G.J.; Boehler, A.; Burke, M.M.; Glanville, A.; Gould, F.K.; Magro, C.; Marboe, C.C.; et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J. Heart Lung Transpl. 2007, 26, 1229–1242. [Google Scholar] [CrossRef]
- Verleden, G.M.; Glanville, A.R.; Lease, E.D.; Fisher, A.J.; Calabrese, F.; Corris, P.A.; Ensor, C.R.; Gottlieb, J.; Hachem, R.R.; Lama, V.; et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J. Heart Lung Transpl. 2019, 38, 493–503. [Google Scholar] [CrossRef]
- Arpinati, L.; Kaisar-Iluz, N.; Shaul, M.E.; Groth, C.; Umansky, V.; Fridlender, Z.G. Tumor-Derived Factors Differentially Affect the Recruitment and Plasticity of Neutrophils. Cancers 2021, 13, 5082. [Google Scholar] [CrossRef]
- Kaisar-Iluz, N.; Arpinati, L.; Shaul, M.E.; Mahroum, S.; Qaisi, M.; Tidhar, E.; Fridlender, Z.G. The Bilateral Interplay between Cancer Immunotherapies and Neutrophils’ Phenotypes and Sub-Populations. Cells 2022, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V.; Fridlender, Z.G.; Granot, Z. The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment. Cancer Microenviron. Off. J. Int. Cancer Microenviron. Soc. 2014, 8, 125–158. [Google Scholar] [CrossRef] [PubMed]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Mishalian, I.; Singhal, S.; Cheng, G.; Kapoor, V.; Horng, W.; Fridlender, G.; Bayuh, R.; Worthen, G.S.; et al. Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PLoS ONE 2012, 7, e31524. [Google Scholar] [CrossRef]
- Sagiv, J.Y.; Michaeli, J.; Assi, S.; Mishalian, I.; Kisos, H.; Levy, L.; Damti, P.; Lumbroso, D.; Polyansky, L.; Sionov, R.V.; et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015, 10, 562–573. [Google Scholar] [CrossRef]
- Ferrari, R.S.; Andrade, C.F. Oxidative Stress and Lung Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2015, 2015, 590987. [Google Scholar] [CrossRef]
- Hardison, M.T.; Galin, F.S.; Calderon, C.E.; Djekic, U.V.; Parker, S.B.; Wille, K.M.; Jackson, P.L.; Oster, R.A.; Young, K.R.; Blalock, J.E.; et al. The presence of a matrix-derived neutrophil chemoattractant in bronchiolitis obliterans syndrome after lung transplantation. J. Immunol. 2009, 182, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.C.; Chuang, Y.H.; Tsai, Y.F.; Yu, H.P. Role of neutrophil extracellular traps following injury. Shock 2014, 41, 491–498. [Google Scholar] [CrossRef]
- Sayah, D.M.; Mallavia, B.; Liu, F.; Ortiz-Muñoz, G.; Caudrillier, A.; DerHovanessian, A.; Ross, D.J.; Lynch, J.P., 3rd; Saggar, R.; Ardehali, A.; et al. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am. J. Respir. Crit. Care Med. 2015, 191, 455–463. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.; Villca-Gonzales, R.; Gómez-Martín, D.; Zentella-Dehesa, A.; Tapia-Rodríguez, M.; Uribe-Uribe, N.O.; Morales-Buenrostro, L.E.; Alberú, J. A potential role of neutrophil extracellular traps (NETs) in kidney acute antibody mediated rejection. Transpl. Immunol. 2020, 60, 101286. [Google Scholar] [CrossRef]
- Schoen, J.; Euler, M.; Schauer, C.; Schett, G.; Herrmann, M.; Knopf, J.; Yaykasli, K.O. Neutrophils’ Extracellular Trap Mechanisms: From Physiology to Pathology. Int. J. Mol. Sci. 2022, 23, 12855. [Google Scholar] [CrossRef]
- Jones, N.D.; Brook, M.O.; Carvalho-Gaspar, M.; Luo, S.; Wood, K.J. Regulatory T cells can prevent memory CD8+ T-cell-mediated rejection following polymorphonuclear cell depletion. Eur. J. Immunol. 2010, 40, 3107–3116. [Google Scholar] [CrossRef] [PubMed]
- Kish, D.D.; Gorbachev, A.V.; Parameswaran, N.; Gupta, N.; Fairchild, R.L. Neutrophil expression of Fas ligand and perforin directs effector CD8 T cell infiltration into antigen-challenged skin. J. Immunol. 2012, 189, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Wasowska, B.A. Mechanisms involved in antibody- and complement-mediated allograft rejection. Immunol. Res. 2010, 47, 25–44. [Google Scholar] [CrossRef]
- Saini, D.; Angaswamy, N.; Tiriveedhi, V.; Fukami, N.; Ramachandran, S.; Hachem, R.; Trulock, E.; Meyers, B.; Patterson, A.; Mohanakumar, T. Synergistic effect of antibodies to human leukocyte antigens and defensins in pathogenesis of bronchiolitis obliterans syndrome after human lung transplantation. J. Heart Lung Transpl. 2010, 29, 1330–1336. [Google Scholar] [CrossRef]
- Ruttens, D.; Wauters, E.; Kiciński, M.; Verleden, S.E.; Vandermeulen, E.; Vos, R.; Van Raemdonck, D.E.; Nawrot, T.S.; Lambrechts, D.; Verleden, G.M.; et al. Genetic variation in interleukin-17 receptor A is functionally associated with chronic rejection after lung transplantation. J. Heart Lung Transpl. 2013, 32, 1233–1240. [Google Scholar] [CrossRef]
| N = 11 | |
|---|---|
| Baseline Characteristics | |
| Recipient age at transplant, year, median (IQR) | 54 (47, 60) |
| Sex, male, n (%) | 2 (18.2) |
| Native lung disease, n (%) | |
| Pulmonary fibrosis | 6 (54.5) |
| Chronic obstructive pulmonary disease | 1 (9.1) |
| COVID-19 ARDS | 3 (27.3) |
| Bronchiectasis | 1 (9.1) |
| CMV serology, n (%) | |
| D+R− | 1(9.1) |
| D+R+ | 9 (81.8) |
| D−R+ | 1 (9.1) |
| D−R− | 0 |
| PGD at 72 h post-transplant, n (%) | |
| Grade 0 | 10 (90.9) |
| Grade 1 | 0 |
| Grade 2 | 0 |
| Grade 3 | 1 (9.1) |
| Acute rejection episodes during follow-up, n (%) | |
| AX | 1 (1.5) |
| A0 | 48 (72.7) |
| A1 | 8 (12.1) |
| ≥A2 | 3 (4.5) |
| Not done | 6 (9.1) |
| Follow-up time, months, median (IQR) | 27.6 (24.5, 30.9) |
| CLAD development during follow-up, n (%) | 1 (9.1) |
| Death or retransplant during follow-up, n (%) | 2 (18.2) |
| 2-Week Post-Tx | 6-Week Post-Tx | 3-Month Post-Tx | 6-Month Post-Tx | 9-Month Post-Tx | 12-Month Post-Tx | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AR Score | Infection | AR Score | Infection | AR Score | Infection | AR Score | Infection | AR Score | Infection | AR Score | Infection | |
| 1 | A0BX | Y | A1BX | Y | A0BX | Y | A0BX | N | N | N | A0B0 | Y |
| 2 | A2B0 | Y | A0BX | Y | A0B0 | Y | A0B0 | N | A0BX | Y | A0BX | Y |
| 3 | A0BX | Y | A1BX | Y | A0BX | Y | A0BX | Y | A0B0 | Y | A2B0 | Y |
| 4 | A0BX | N | A0BX | N | A0BX | Y | A0B0 | Y | A0B0 | Y | A1B0 | NA |
| 5 | A0BX | Y | A0BX | Y | A0BX | Y | NA | Y | NA | NA | NA | NA |
| 6 | NA | Y | A2BX | Y | A0B0 | N | A1BX | Y | A0B0 | Y | A2BX | Y |
| 7 | NA | Y | A0BX | Y | AXBX | Y | A0BX | Y | A0BX | Y | A0BX | Y |
| 8 | A0BX | Y | A0BX | Y | A0B0 | Y | A0BX | Y | A0BX | Y | A1BX | Y |
| 9 | A1BX | N | A0BX | Y | A0BX | Y | A0BX | Y | A0BX | Y | A0BX | Y |
| 10 | A1BX | N | A0BX | N | A0B0 | N | A0B0 | N | A1BX | Y | A0BX | Y |
| 11 | A0BX | Y | A1BX | Y | A0BX | N | A0BX | N | A0B0 | Y | A0BX | N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaisar-Iluz, N.; Shaul, M.E.; Deri, O.; Huszti, E.; Peled, M.; Bezalel, Y.; Fridlender, Z.G.; Levy, L. Dynamic Neutrophil Subsets and Function in Lung Transplant Recipients: Insights from a One-Year Longitudinal Pilot Study. J. Clin. Med. 2025, 14, 2660. https://doi.org/10.3390/jcm14082660
Kaisar-Iluz N, Shaul ME, Deri O, Huszti E, Peled M, Bezalel Y, Fridlender ZG, Levy L. Dynamic Neutrophil Subsets and Function in Lung Transplant Recipients: Insights from a One-Year Longitudinal Pilot Study. Journal of Clinical Medicine. 2025; 14(8):2660. https://doi.org/10.3390/jcm14082660
Chicago/Turabian StyleKaisar-Iluz, Naomi, Merav E. Shaul, Ofir Deri, Ella Huszti, Michael Peled, Yael Bezalel, Zvi G. Fridlender, and Liran Levy. 2025. "Dynamic Neutrophil Subsets and Function in Lung Transplant Recipients: Insights from a One-Year Longitudinal Pilot Study" Journal of Clinical Medicine 14, no. 8: 2660. https://doi.org/10.3390/jcm14082660
APA StyleKaisar-Iluz, N., Shaul, M. E., Deri, O., Huszti, E., Peled, M., Bezalel, Y., Fridlender, Z. G., & Levy, L. (2025). Dynamic Neutrophil Subsets and Function in Lung Transplant Recipients: Insights from a One-Year Longitudinal Pilot Study. Journal of Clinical Medicine, 14(8), 2660. https://doi.org/10.3390/jcm14082660







