Leptin and Insulin in COPD: Unveiling the Metabolic-Inflammatory Axis—A Narrative Review
Abstract
1. Introduction
2. Leptin: A Metabolic and Immunological Hormone
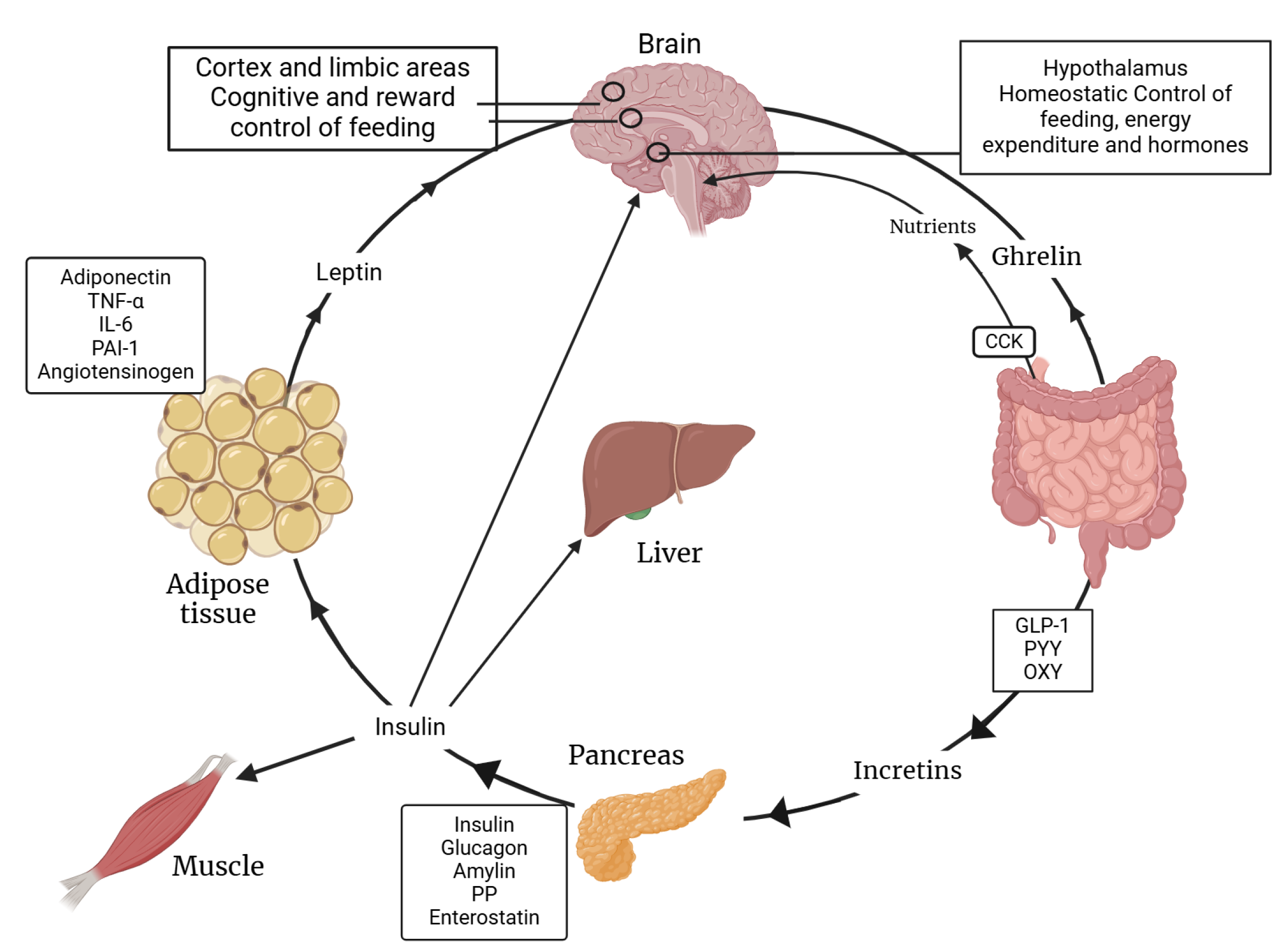
3. Insulin: Role Beyond Glucose Homeostasis
4. Insulin–Leptin Interplay in the Central Nervous System
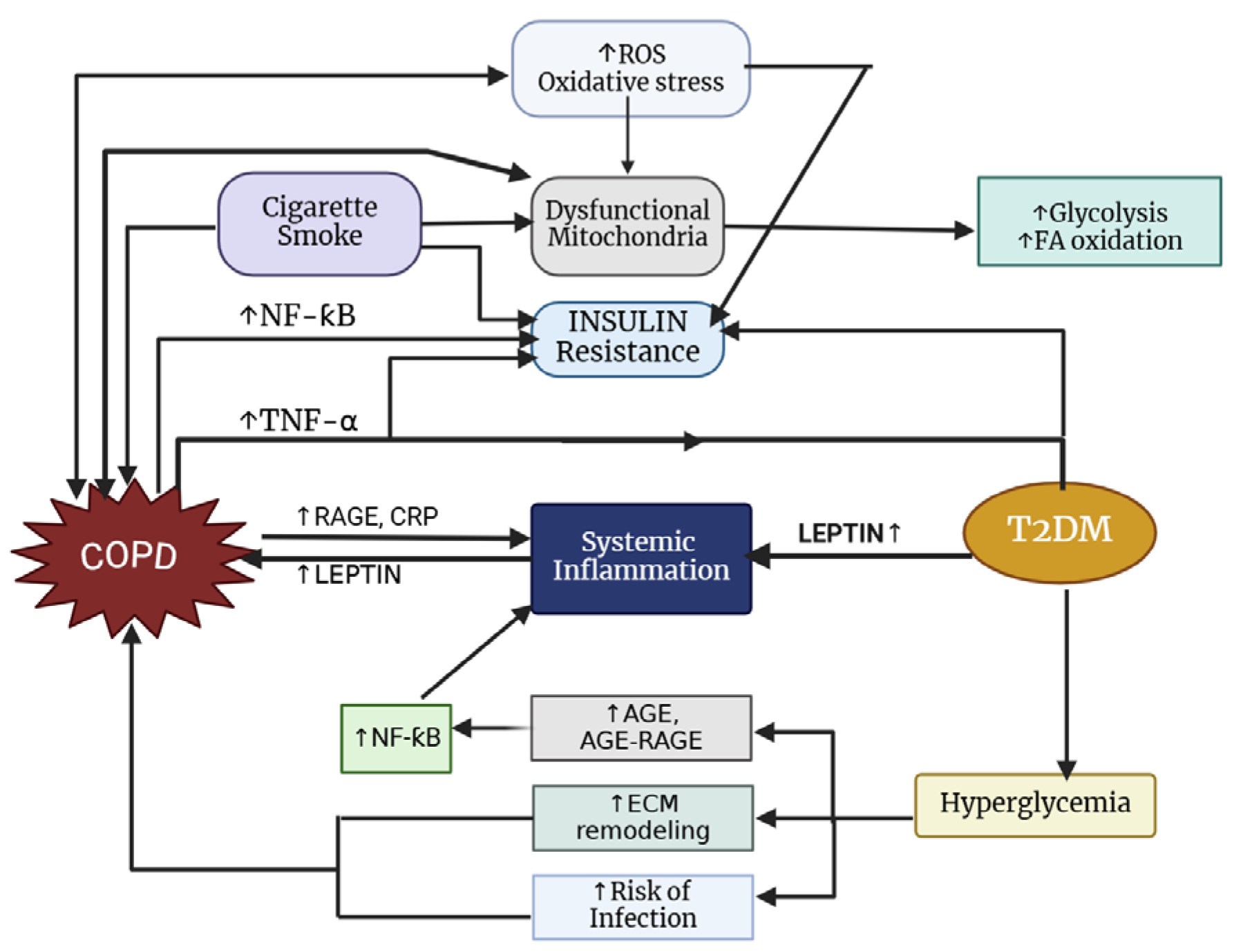
5. Pathophysiological Links Between COPD, Insulin, and Leptin
5.1. Leptin Dysregulation in COPD
5.1.1. Systemic and Local Inflammation
5.1.2. Leptin Resistance and COPD
5.2. Insulin Resistance and COPD
6. Crosstalk Between Insulin and Leptin in COPD Pathophysiology
6.1. Inflammation
6.2. Phenotype-Specific Pathways
6.3. Hypoxia
6.4. Oxidative Stress
6.5. Temporal Causality, Aging, and Epigenetic Impacts
6.6. Metabolomics and Lung Inflammation in Metabolic Dysregulation
7. Clinical Implications of the Metabolic–Inflammatory Axis
7.1. COPD and Metabolic Syndrome
7.2. COPD and Type 2 Diabetes Mellitus
7.3. COPD and Cardiovascular Disease
7.4. COPD, Muscle Wasting, and Dysfunction
7.5. COPD and Obesity
8. COPD in Low- and Middle-Income Countries
9. Therapeutic Management and Future Directions
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- 2025 GOLD Report—Global Initiative for Chronic Obstructive Lung Disease—GOLD. Available online: https://goldcopd.org/2025-gold-report/ (accessed on 4 February 2025).
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, B.; McBurnie, M.A.; Vollmer, W.M.; Gudmundsson, G.; Welte, T.; Nizankowska-Mogilnicka, E.; Studnicka, M.; Bateman, E.; Anto, J.M.; Burney, P.; et al. COPD in Never Smokers: Results from the Population-Based Burden of Obstructive Lung Disease Study. Chest 2011, 139, 752. [Google Scholar] [CrossRef]
- FastStats—Chronic Lower Respiratory Disease. Available online: https://www.cdc.gov/nchs/fastats/copd.htm (accessed on 28 January 2025).
- Lange, P.; Ahmed, E.; Lahmar, Z.M.; Martinez, F.J.; Bourdin, A. Natural History and Mechanisms of COPD. Respirology 2021, 26, 298–321. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, D.; Chua, S.; Lee, C.; Basquill, C.; Papana, A.; Theodoratou, E.; Nair, H.; Gasevic, D.; Sridhar, D.; Campbell, H.; et al. Global and Regional Estimates of COPD Prevalence: Systematic Review and Meta-Analysis. J. Glob. Health 2015, 5, 020415. [Google Scholar] [CrossRef]
- Boers, E.; Barrett, M.; Su, J.G.; Benjafield, A.V.; Sinha, S.; Kaye, L.; Zar, H.J.; Vuong, V.; Tellez, D.; Gondalia, R.; et al. Global Burden of Chronic Obstructive Pulmonary Disease Through 2050. JAMA Netw. Open 2023, 6, e2346598. [Google Scholar] [CrossRef]
- Fallahzadeh, A.; Sharifnejad Tehrani, Y.; Sheikhy, A.; Ghamari, S.H.; Mohammadi, E.; Saeedi Moghaddam, S.; Esfahani, Z.; Nasserinejad, M.; Shobeiri, P.; Rashidi, M.M.; et al. The Burden of Chronic Respiratory Disease and Attributable Risk Factors in North Africa and Middle East: Findings from Global Burden of Disease Study (GBD) 2019. Respir. Res. 2022, 23, 268. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.R.; Hao, K.; Yang, T. Air Pollution and Chronic Obstructive Pulmonary Disease. Chronic Dis. Transl. Med. 2020, 6, 260–269. [Google Scholar] [CrossRef]
- Nugmanova, D.; Feshchenko, Y.; Iashyna, L.; Gyrina, O.; Malynovska, K.; Mammadbayov, E.; Akhundova, I.; Nurkina, N.; Tariq, L.; Makarova, J.; et al. The Prevalence, Burden and Risk Factors Associated with Chronic Obstructive Pulmonary Disease in Commonwealth of Independent States (Ukraine, Kazakhstan and Azerbaijan): Results of the CORE Study. BMC Pulm. Med. 2018, 18, 26. [Google Scholar] [CrossRef]
- Chapmann, K.R.; Mannino, D.M.; Soriano, J.B.; Vermeire, P.A.; Buist, A.S.; Thun, M.J.; Connell, C.; Lee, T.A.; Miravitlles, M.; Aldington, S.; et al. Epidemiology and Costs of Chronic Obstructive Pulmonary Disease. Eur. Respir. J. 2006, 27, 188–207. [Google Scholar] [CrossRef]
- Ho, T.; Cusack, R.P.; Chaudhary, N.; Satia, I.; Kurmi, O.P. Under- and over-Diagnosis of COPD: A Global Perspective. Breathe 2019, 15, 24. [Google Scholar] [CrossRef]
- Decramer, M.; Janssens, W.; Miravitlles, M. Chronic Obstructive Pulmonary Disease. Lancet 2012, 379, 1341. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Celli, B.R. Systemic Manifestations and Comorbidities of COPD. Eur. Respir. J. 2009, 33, 1165–1185. [Google Scholar] [CrossRef] [PubMed]
- Zatloukal, J.; Brat, K.; Neumannova, K.; Volakova, E.; Hejduk, K.; Kocova, E.; Kudela, O.; Kopecky, M.; Plutinsky, M.; Koblizek, V. Chronic Obstructive Pulmonary Disease—Diagnosis and Management of Stable Disease; a Personalized Approach to Care, Using the Treatable Traits Concept Based on Clinical Phenotypes. Position Paper of the Czech Pneumological and Phthisiological Society. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2020, 164, 325–356. [Google Scholar] [CrossRef]
- Mannino, D.M.; Watt, G.; Hole, D.; Gillis, C.; Hart, C.; McConnachie, A.; Smith, G.D.; Upton, M.; Hawthorne, V.; Sin, D.D.; et al. The Natural History of Chronic Obstructive Pulmonary Disease. Eur. Respir. J. 2006, 27, 627–643. [Google Scholar] [CrossRef]
- Miravitlles, M.; Ribera, A. Understanding the Impact of Symptoms on the Burden of COPD. Respir. Res. 2017, 18, 67. [Google Scholar] [CrossRef]
- Vestbo, J.; Hurd, S.S.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Wedzicha, J.A. Controversies in Treatment of Chronic Obstructive Pulmonary Disease. Lancet 2011, 378, 1038–1047. [Google Scholar] [CrossRef]
- Sin, D.D.; Man, S.F.P. Impaired Lung Function and Serum Leptin in Men and Women with Normal Body Weight: A Population Based Study. Thorax 2003, 58, 695–698. [Google Scholar] [CrossRef]
- Agusti, A. Thomas a. Neff Lecture. Chronic Obstructive Pulmonary Disease: A Systemic Disease. Proc. Am. Thorac. Soc. 2006, 3, 478–481. [Google Scholar] [CrossRef]
- Yanbaeva, D.G.; Dentener, M.A.; Creutzberg, E.C.; Wesseling, G.; Wouters, E.F.M. Systemic Effects of Smoking. Chest 2007, 131, 1557–1566. [Google Scholar] [CrossRef]
- Kahnert, K.; Lucke, T.; Biertz, F.; Lechner, A.; Watz, H.; Alter, P.; Bals, R.; Behr, J.; Holle, R.; Huber, R.M.; et al. Transfer Factor for Carbon Monoxide in Patients with COPD and Diabetes: Results from the German COSYCONET Cohort. Respir. Res. 2017, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R. Insulin Signaling in Health and Disease. J. Clin. Investig. 2021, 131, e142241. [Google Scholar] [CrossRef] [PubMed]
- Schols, A.M.W.J.; Creutzberg, E.C.; Buurman, W.A.; Campfield, L.A.; Saris, W.H.M.; Wouters, E.F.M. Plasma Leptin Is Related to Proinflammatory Status and Dietary Intake in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1999, 160, 1220–1226. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The Fat-Derived Hormone Adiponectin Reverses Insulin Resistance Associated with Both Lipoatrophy and Obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional Cloning of the Mouse Obese Gene and Its Human Homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Sierra-Honigmann, M.R.; Nath, A.K.; Murakami, C.; García-Cardeña, G.; Papapetropoulos, A.; Sessa, W.C.; Madge, L.A.; Schechner, J.S.; Schwabb, M.B.; Polverini, P.J.; et al. Biological Action of Leptin as an Angiogenic Factor. Science 1998, 281, 1683–1686. [Google Scholar] [CrossRef]
- Emilsson, V.; Liu, Y.L.; Cawthorne, M.A.; Morton, N.M.; Davenport, M. Expression of the Functional Leptin Receptor MRNA in Pancreatic Islets and Direct Inhibitory Action of Leptin on Insulin Secretion. Diabetes 1997, 46, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Saladin, R.; De Vos, P.; Guerre-Millot, M.; Leturque, A.; Girard, J.; Staels, B.; Auwerx, J. Transient Increase in Obese Gene Expression after Food Intake or Insulin Administration. Nature 1995, 377, 527–528. [Google Scholar] [CrossRef]
- Widjaja, A.; Schürmeyer, T.H.; Von Zur Mühlen, A.; Brabant, G. Determinants of Serum Leptin Levels in Cushing’s Syndrome. J. Clin. Endocrinol. Metab. 1998, 83, 600–603. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Toro, A.; Vilariño-García, T.; Maymó, J.; Guadix, P.; Dueñas, J.L.; Fernández-Sánchez, M.; Varone, C.; Sánchez-Margalet, V. Leptin Action in Normal and Pathological Pregnancies. J. Cell Mol. Med. 2018, 22, 716–727. [Google Scholar] [CrossRef]
- White, D.W.; Tartaglia, L.A. Leptin and OB-R: Body Weight Regulation by a Cytokine Receptor. Cytokine Growth Factor Rev. 1996, 7, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.B.S. Leptin–Much More than a Satiety Signal. Annu. Rev. Nutr. 2000, 20, 45–75. [Google Scholar] [CrossRef] [PubMed]
- Stephens, T.W.; Basinski, M.; Bristow, P.K.; Bue-Valleskey, J.M.; Burgett, S.G.; Craft, L.; Hale, J.; Hoffmann, J.; Hsiung, H.M.; Kriauciunas, A.; et al. The Role of Neuropeptide Y in the Antiobesity Action of the Obese Gene Product. Nature 1995, 377, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Okano, H.J.; Li, C.; Lee, G.H.; Zhao, C.; Darnell, R.; Friedman, J.M. Anatomic Localization of Alternatively Spliced Leptin Receptors (Ob-R) in Mouse Brain and Other Tissues. Proc. Natl. Acad. Sci. USA 1997, 94, 7001. [Google Scholar] [CrossRef]
- Li, M.D. Leptin and Beyond: An Odyssey to the Central Control of Body Weight. Yale J. Biol. Med. 2011, 84, 1. [Google Scholar]
- Cowley, M.A.; Smart, J.L.; Rubinstein, M.; Cerdán, M.G.; Diano, S.; Horvath, T.L.; Cone, R.D.; Low, M.J. Leptin Activates Anorexigenic POMC Neurons through a Neural Network in the Arcuate Nucleus. Nature 2001, 411, 480–484. [Google Scholar] [CrossRef]
- Coppari, R.; Ichinose, M.; Lee, C.E.; Pullen, A.E.; Kenny, C.D.; McGovern, R.A.; Tang, V.; Liu, S.M.; Ludwig, T.; Chua, S.C.; et al. The Hypothalamic Arcuate Nucleus: A Key Site for Mediating Leptin’s Effects on Glucose Homeostasis and Locomotor Activity. Cell Metab. 2005, 1, 63–72. [Google Scholar] [CrossRef]
- Rossi, J.; Balthasar, N.; Olson, D.; Scott, M.; Berglund, E.; Lee, C.E.; Choi, M.J.; Lauzon, D.; Lowell, B.B.; Elmquist, J.K. Melanocortin-4-Receptors Expressed by Cholinergic Neurons Regulate Energy Balance and Glucose Homeostasis. Cell Metab. 2011, 13, 195. [Google Scholar] [CrossRef]
- Zhang, R.; Dhillon, H.; Yin, H.; Yoshimura, A.; Lowell, B.B.; Maratos-Flier, E.; Flier, J.S. Selective Inactivation of Socs3 in SF1 Neurons Improves Glucose Homeostasis Without Affecting Body Weight. Endocrinology 2008, 149, 5654. [Google Scholar] [CrossRef]
- La Cava, A.; Matarese, G. The Weight of Leptin in Immunity. Nat. Rev. Immunol. 2004, 4, 371–379. [Google Scholar] [CrossRef]
- Lord, G.M.; Matarese, G.; Howard, J.K.; Baker, R.J.; Bloom, S.R.; Lechler, R.I. Leptin Modulates the T-Cell Immune Response and Reverses Starvation-Induced Immunosuppression. Nature 1998, 394, 897–901. [Google Scholar] [CrossRef]
- Faggioni, R.; Feingold, K.R.; Grunfeld, C. Leptin Regulation of the Immune Response and the Immunodeficiency of Malnutrition. FASEB J. 2001, 15, 2565–2571. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.; Netea, M.G.; Radstake, T.R.D.S.; Van Riel, P.L.; Barrera, P.; Van Der Meer, J.W.M. Markers of Inflammation Are Negatively Correlated with Serum Leptin in Rheumatoid Arthritis. Ann. Rheum. Dis. 2005, 64, 1195. [Google Scholar] [CrossRef] [PubMed]
- Baumann, H.; Morella, K.K.; White, D.W.; Dembski, M.; Bailon, P.S.; Kim, H.; Lai, C.F.; Tartaglia, L.A. The Full-Length Leptin Receptor Has Signaling Capabilities of Interleukin 6-Type Cytokine Receptors. Proc. Natl. Acad. Sci. USA 1996, 93, 8374. [Google Scholar] [CrossRef] [PubMed]
- Bjørbæk, C.; Elmquist, J.K.; Frantz, J.D.; Shoelson, S.E.; Flier, J.S. Identification of SOCS-3 as a Potential Mediator of Central Leptin Resistance. Mol. Cell 1998, 1, 619–625. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of Interleukin (IL)-6-Type Cytokine Signalling and Its Regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef]
- Csajbók, É.A.; Tamás, G. Cerebral Cortex: A Target and Source of Insulin? Diabetologia 2016, 59, 1609–1615. [Google Scholar] [CrossRef]
- Vasiljević, J.; Torkko, J.M.; Knoch, K.P.; Solimena, M. The Making of Insulin in Health and Disease. Diabetologia 2020, 63, 1981–1989. [Google Scholar] [CrossRef]
- Suckale, J.; Solimena, M. The Insulin Secretory Granule as a Signaling Hub. Trends Endocrinol. Metab. 2010, 21, 599–609. [Google Scholar] [CrossRef]
- Yang, B.Y.; Zhai, G.; Gong, Y.L.; Su, J.Z.; Peng, X.Y.; Shang, G.H.; Han, D.; Jin, J.Y.; Liu, H.K.; Du, Z.Y.; et al. Different Physiological Roles of Insulin Receptors in Mediating Nutrient Metabolism in Zebrafish. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E38–E51. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical Nodes in Signalling Pathways: Insights into Insulin Action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Baron, A.D.; Brechtel, G.; Wallace, P.; Edelman, S.V. Rates and Tissue Sites of Non-Insulin- and Insulin-Mediated Glucose Uptake in Humans. Am. J. Physiol. 1988, 255, E769–E774. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, M.A.; Hannan, M.A.; Uddin, M.J.; Pang, M.G. Role of Insulin in Health and Disease: An Update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar] [CrossRef] [PubMed]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of Endogenous Fat and Carbohydrate Metabolism in Relation to Exercise Intensity and Duration. Am. J. Physiol. 1993, 265, E380–E391. [Google Scholar] [CrossRef] [PubMed]
- Defronzo, R.A. Banting Lecture. From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef]
- Smith, U. Impaired (‘diabetic’) Insulin Signaling and Action Occur in Fat Cells Long before Glucose Intolerance—Is Insulin Resistance Initiated in the Adipose Tissue? Int. J. Obes. 2002, 26, 897–904. [Google Scholar] [CrossRef]
- Cignarelli, A.; Genchi, V.A.; Perrini, S.; Natalicchio, A.; Laviola, L.; Giorgino, F. Insulin and Insulin Receptors in Adipose Tissue Development. Int. J. Mol. Sci. 2019, 20, 759. [Google Scholar] [CrossRef]
- Barac, A.; Campia, U.; Panza, J.A. Methods for Evaluating Endothelial Function in Humans. Hypertension 2007, 49, 748–760. [Google Scholar] [CrossRef]
- Vicent, D.; Ilany, J.; Kondo, T.; Naruse, K.; Fisher, S.J.; Kisanuki, Y.Y.; Bursell, S.; Yanagisawa, M.; King, G.L.; Kahn, C.R. The Role of Endothelial Insulin Signaling in the Regulation of Vascular Tone and Insulin Resistance. J. Clin. Investig. 2003, 111, 1373–1380. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, K.H.; Cheong, J.H. Hepatitis B Virus X Protein Impairs Hepatic Insulin Signaling Through Degradation of IRS1 and Induction of SOCS3. PLoS ONE 2010, 5, e8649. [Google Scholar] [CrossRef][Green Version]
- Kim, J.B.; Siddiqui, S.; Kim, K.W.; Hosoi, T.; Thon, M.; Ozawa, K. Possible Integrative Actions of Leptin and Insulin Signaling in the Hypothalamus Targeting Energy Homeostasis. Front. Endocrinol. 2016, 7, 138. [Google Scholar] [CrossRef]
- Bruning, J.C.; Gautam, D.; Burks, D.J.; Gillette, J.; Schubert, M.; Orban, P.C.; Klein, R.; Krone, W.; Muller-Wieland, D.; Kahn, C.R. Role of Brain Insulin Receptor in Control of Body Weight and Reproduction. Science 2000, 289, 2122–2125. [Google Scholar] [CrossRef]
- Baskin, D.G.; Breininger, J.F.; Schwartz, M.W. Leptin Receptor MRNA Identifies a Subpopulation of Neuropeptide Y Neurons Activated by Fasting in Rat Hypothalamus. Diabetes 1999, 48, 828–833. [Google Scholar] [CrossRef]
- Cheung, C.C.; Clifton, D.K.; Steiner, R.A. Proopiomelanocortin Neurons Are Direct Targets for Leptin in the Hypothalamus. Endocrinology 1997, 138, 4489–4492. [Google Scholar] [CrossRef] [PubMed]
- Baskin, D.G.; Wilcox, B.J.; Figlewicz, D.P.; Dorsa, D.M. Insulin and Insulin-like Growth Factors in the CNS. Trends Neurosci. 1988, 11, 107–111. [Google Scholar] [CrossRef]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central Nervous System Control of Food Intake and Body Weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Thon, M.; Hosoi, T.; Ozawa, K. Insulin Enhanced Leptin-Induced STAT3 Signaling by Inducing GRP78. Sci. Rep. 2016, 6, 34312. [Google Scholar] [CrossRef]
- Niswender, K.D.; Schwartz, M.W. Insulin and Leptin Revisited: Adiposity Signals with Overlapping Physiological and Intracellular Signaling Capabilities. Front. Neuroendocrinol. 2003, 24, 1–10. [Google Scholar] [CrossRef]
- Benomar, Y.; Roy, A.F.; Aubourg, A.; Djiane, J.; Taouis, M. Cross Down-Regulation of Leptin and Insulin Receptor Expression and Signalling in a Human Neuronal Cell Line. Biochem. J. 2005, 388, 929. [Google Scholar] [CrossRef]
- Kim, M.S.; Pak, Y.K.; Jang, P.G.; Namkoong, C.; Choi, Y.S.; Won, J.C.; Kim, K.S.; Kim, S.W.; Kim, H.S.; Park, J.Y.; et al. Role of Hypothalamic Foxo1 in the Regulation of Food Intake and Energy Homeostasis. Nat. Neurosci. 2006, 9, 901–906. [Google Scholar] [CrossRef]
- Xu, A.W.; Kaelin, C.B.; Takeda, K.; Akira, S.; Schwartz, M.W.; Barsh, G.S. PI3K Integrates the Action of Insulin and Leptin on Hypothalamic Neurons. J. Clin. Investig. 2005, 115, 951. [Google Scholar] [CrossRef] [PubMed]
- Niswender, K.D.; Morrison, C.D.; Clegg, D.J.; Olson, R.; Baskin, D.G.; Myers, M.G.; Seeley, R.J.; Schwartz, M.W. Insulin Activation of Phosphatidylinositol 3-Kinase in the Hypothalamic Arcuate Nucleus: A Key Mediator of Insulin-Induced Anorexia. Diabetes 2003, 52, 227–231. [Google Scholar] [CrossRef]
- Plum, L.; Lin, H.V.; Dutia, R.; Tanaka, J.; Aizawa, K.S.; Matsumoto, M.; Kim, A.J.; Cawley, N.X.; Paik, J.H.; Loh, Y.P.; et al. The Obesity Susceptibility Gene Carboxypeptidase E Links FoxO1 Signaling in Hypothalamic Pro–Opiomelanocortin Neurons with Regulation of Food Intake. Nat. Med. 2009, 15, 1195. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Uetani, N.; Simoncic, P.D.; Chaubey, V.P.; Lee-Loy, A.; McGlade, C.J.; Kennedy, B.P.; Tremblay, M.L. Attenuation of Leptin Action and Regulation of Obesity by Protein Tyrosine Phosphatase 1B. Dev. Cell 2002, 2, 497–503. [Google Scholar] [CrossRef]
- Kenner, K.A.; Anyanwu, E.; Olefsky, J.M.; Kusari, J. Protein-Tyrosine Phosphatase 1B Is a Negative Regulator of Insulin- and Insulin-like Growth Factor-I-Stimulated Signaling. J. Biol. Chem. 1996, 271, 19810–19816. [Google Scholar] [CrossRef] [PubMed]
- Elchebly, M.; Payette, P.; Michaliszyn, E.; Cromlish, W.; Collins, S.; Loy, A.L.; Normandin, D.; Cheng, A.; Himms-Hagen, J.; Chan, C.C.; et al. Increased Insulin Sensitivity and Obesity Resistance in Mice Lacking the Protein Tyrosine Phosphatase-1B Gene. Science 1999, 283, 1544–1548. [Google Scholar] [CrossRef]
- Emanuelli, B.; Peraldi, P.; Filloux, C.; Sawka-Verhelle, D.; Hilton, D.; Van Obberghen, E. SOCS-3 Is an Insulin-Induced Negative Regulator of Insulin Signaling. J. Biol. Chem. 2000, 275, 15985–15991. [Google Scholar] [CrossRef]
- Reed, A.S.; Unger, E.K.; Olofsson, L.E.; Piper, M.L.; Myers, M.G.; Xu, A.W. Functional Role of Suppressor of Cytokine Signaling 3 Upregulation in Hypothalamic Leptin Resistance and Long-Term Energy Homeostasis. Diabetes 2010, 59, 894–906. [Google Scholar] [CrossRef]
- Ernst, M.B.; Wunderlich, C.M.; Hess, S.; Paehler, M.; Mesaros, A.; Koralov, S.B.; Kleinridders, A.; Husch, A.; Münzberg, H.; Hampel, B.; et al. Enhanced Stat3 Activation in POMC Neurons Provokes Negative Feedback Inhibition of Leptin and InsulinSignaling in Obesity. J. Neurosci. 2009, 29, 11582–11593. [Google Scholar] [CrossRef]
- Kievit, P.; Howard, J.K.; Badman, M.K.; Balthasar, N.; Coppari, R.; Mori, H.; Lee, C.E.; Elmquist, J.K.; Yoshimura, A.; Flier, J.S. Enhanced Leptin Sensitivity and Improved Glucose Homeostasis in Mice Lacking Suppressor of Cytokine Signaling-3 in POMC-Expressing Cells. Cell Metab. 2006, 4, 123–132. [Google Scholar] [CrossRef]
- Hogg, J.C. Pathophysiology of Airflow Limitation in Chronic Obstructive Pulmonary Disease. Lancet 2004, 364, 709–721. [Google Scholar] [CrossRef]
- Broekhuizen, R.; Vernooy, J.H.J.; Schols, A.M.W.J.; Dentener, M.A.; Wouters, E.F.M. Leptin as Local Inflammatory Marker in COPD. Respir. Med. 2005, 99, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Vernooy, J.H.J.; Drummen, N.E.A.; Van Suylen, R.J.; Cloots, R.H.E.; Möller, G.M.; Bracke, K.R.; Zuyderduyn, S.; Dentener, M.A.; Brusselle, G.G.; Hiemstra, P.S.; et al. Enhanced Pulmonary Leptin Expression in Patients with Severe COPD and Asymptomatic Smokers. Thorax 2009, 64, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.J.; Yoo, W.J.; Bae, C.H.; Song, S.Y.; Kim, Y.W.; Park, S.Y.; Kim, Y.D. Leptin Up-Regulates MUC5B Expression in Human Airway Epithelial Cells via Mitogen-Activated Protein Kinase Pathway. Exp. Lung Res. 2010, 36, 262–269. [Google Scholar] [CrossRef]
- Breyer, M.K.; Rutten, E.P.A.; Vernooy, J.H.J.; Spruit, M.A.; Dentener, M.A.; Van Der Kallen, C.; Vangreevenbroek, M.M.J.; Wouters, E.F.M. Gender Differences in the Adipose Secretome System in Chronic Obstructive Pulmonary Disease (COPD): A Pivotal Role of Leptin. Respir. Med. 2011, 105, 1046–1053. [Google Scholar] [CrossRef]
- Breyer, M.K.; Rutten, E.P.A.; Locantore, N.W.; Watkins, M.L.; Miller, B.E.; Wouters, E.F.M. Dysregulated Adipokine Metabolism in Chronic Obstructive Pulmonary Disease. Eur. J. Clin. Investig. 2012, 42, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Vernooy, J.H.J.; Ubags, N.D.J.; Brusselle, G.G.; Tavernier, J.; Suratt, B.T.; Joos, G.F.; Wouters, E.F.M.; Bracke, K.R. Leptin as Regulator of Pulmonary Immune Responses: Involvement in Respiratory Diseases. Pulm. Pharmacol. Ther. 2013, 26, 464. [Google Scholar] [CrossRef]
- Gaki, E.; Kontogianni, K.; Papaioannou, A.I.; Bakakos, P.; Gourgoulianis, K.I.; Kostikas, K.; Alchanatis, M.; Papiris, S.; Loukides, S. Associations between BODE Index and Systemic Inflammatory Biomarkers in COPD. COPD J. Chronic Obstr. Pulm. Dis. 2011, 8, 408–413. [Google Scholar] [CrossRef]
- Lambert, A.A.; Putcha, N.; Drummond, M.B.; Boriek, A.M.; Hanania, N.A.; Kim, V.; Kinney, G.L.; McDonald, M.L.N.; Brigham, E.P.; Wise, R.A.; et al. Obesity Is Associated With Increased Morbidity in Moderate to Severe COPD. Chest 2016, 151, 68. [Google Scholar] [CrossRef]
- Matarese, G.; Procaccini, C.; De Rosa, V.; Horvath, T.L.; La Cava, A. Regulatory T Cells in Obesity: The Leptin Connection. Trends Mol. Med. 2010, 16, 247–256. [Google Scholar] [CrossRef]
- Bolton, C.E.; Evans, M.; Ionescu, A.A.; Edwards, S.M.; Morris, R.H.K.; Dunseath, G.; Luzio, S.D.; Owens, D.R.; Shale, D.J. Insulin Resistance and Inflammation—A Further Systemic Complication of COPD. COPD J. Chronic Obstr. Pulm. Dis. 2007, 4, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Poulain, M.; Doucet, M.; Major, G.C.; Drapeau, V.; Sériès, F.; Boulet, L.P.; Tremblay, A.; Maltais, F. The Effect of Obesity on Chronic Respiratory Diseases: Pathophysiology and Therapeutic Strategies. CMAJ Can. Med. Assoc. J. 2006, 174, 1293. [Google Scholar] [CrossRef]
- Franssen, F.M.E.; O’Donnell, D.E.; Goossens, G.H.; Blaak, E.E.; Schols, A.M.W.J. Obesity and the Lung: 5. Obesity and COPD. Thorax 2008, 63, 1110–1117. [Google Scholar] [CrossRef]
- Landbo, C.; Prescott, E.; Lange, P.; Vestbo, J.; Almdal, T.P. Prognostic Value of Nutritional Status in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1999, 160, 1856–1861. [Google Scholar] [CrossRef]
- Vestbo, J.; Prescott, E.; Almdal, T.; Dahl, M.; Nordestgaard, B.G.; Andersen, T.; Sørensen, T.I.A.; Lange, P. Body Mass, Fat-Free Body Mass, and Prognosis in Patients with Chronic Obstructive Pulmonary Disease from a Random Population Sample: Findings from the Copenhagen City Heart Study. Am. J. Respir. Crit. Care Med. 2006, 173, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; Sánchez-Margalet, V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Qasim, A.; Reilly, M.P. Leptin Resistance: A Possible Interface of Inflammation and Metabolism in Obesity-Related Cardiovascular Disease. J. Am. Coll. Cardiol. 2008, 52, 1201. [Google Scholar] [CrossRef]
- Vilariño-García, T.; Polonio-González, M.L.; Pérez-Pérez, A.; Ribalta, J.; Arrieta, F.; Aguilar, M.; Obaya, J.C.; Gimeno-Orna, J.A.; Iglesias, P.; Navarro, J.; et al. Role of Leptin in Obesity, Cardiovascular Disease, and Type 2 Diabetes. Int. J. Mol. Sci. 2024, 25, 2338. [Google Scholar] [CrossRef]
- Frederich, R.C.; Hamann, A.; Anderson, S.; Löllmann, B.; Lowell, B.B.; Flier, J.S. Leptin Levels Reflect Body Lipid Content in Mice: Evidence for Diet-Induced Resistance to Leptin Action. Nat. Med. 1995, 1, 1311–1314. [Google Scholar] [CrossRef]
- McGuinness, A.J.A.; Sapey, L. Oxidative Stress in COPD: Sources, Markers, and Potential Mechanisms. J. Clin. Med. 2017, 6, 21. [Google Scholar] [CrossRef]
- McMurray, F.; Patten, D.A.; Harper, M.E. Reactive Oxygen Species and Oxidative Stress in Obesity-Recent Findings and Empirical Approaches. Obesity 2016, 24, 2301–2310. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.M.M.; Shaw, J. Global and Societal Implications of the Diabetes Epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef]
- Abstracts 2007. Diabetologia 2007, 50, 1–538. [CrossRef] [PubMed]
- Zhao, X.; An, X.; Yang, C.; Sun, W.; Ji, H.; Lian, F. The Crucial Role and Mechanism of Insulin Resistance in Metabolic Disease. Front. Endocrinol. 2023, 14, 1149239. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Kahn, C.R. Insulin Signalling and the Regulation of Glucose and Lipid Metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Freidenberg, G.R.; Reichart, D.; Olefsky, J.M.; Henry, R.R. Reversibility of Defective Adipocyte Insulin Receptor Kinase Activity in Non-Insulin-Dependent Diabetes Mellitus. Effect of Weight Loss. J. Clin. Investig. 1988, 82, 1398. [Google Scholar] [CrossRef]
- Machado, F.V.C.; Pitta, F.; Hernandes, N.A.; Bertolini, G.L. Physiopathological Relationship between Chronic Obstructive Pulmonary Disease and Insulin Resistance. Endocrine 2018, 61, 17–22. [Google Scholar] [CrossRef]
- Donath, M.Y.; Dalmas, É.; Sauter, N.S.; Böni-Schnetzler, M. Inflammation in Obesity and Diabetes: Islet Dysfunction and Therapeutic Opportunity. Cell Metab. 2013, 17, 860–872. [Google Scholar] [CrossRef]
- Nieto-Vazquez, I.; Fernández-Veledo, S.; Krämer, D.K.; Vila-Bedmar, R.; Garcia-Guerra, L.; Lorenzo, M. Insulin Resistance Associated to Obesity: The Link TNF-Alpha. Arch. Physiol. Biochem. 2008, 114, 183–194. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Integrating Mechanisms for Insulin Resistance: Common Threads and Missing Links. Cell 2012, 148, 852. [Google Scholar] [CrossRef]
- Chen, K.; Li, F.; Li, J.; Cai, H.; Strom, S.; Bisello, A.; Kelley, D.E.; Friedman-Einat, M.; Skibinski, G.A.; McCrory, M.A.; et al. Induction of Leptin Resistance through Direct Interaction of C-Reactive Protein with Leptin. Nat. Med. 2006, 12, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Eker, S.; Ayaz, L.; Tamer, L.; Ulubas, B. Leptin, Visfatin, Insulin Resistance, and Body Composition Change in Chronic Obstructive Pulmonary Disease. Scand. J. Clin. Lab. Investig. 2010, 70, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Streba, L.; Popovici, V.; Mihai, A.; Mititelu, M.; Lupu, C.E.; Matei, M.; Vladu, I.M.; Iovănescu, M.L.; Cioboată, R.; Călărașu, C.; et al. Integrative Approach to Risk Factors in Simple Chronic Obstructive Airway Diseases of the Lung or Associated with Metabolic Syndrome-Analysis and Prediction. Nutrients 2024, 16, 1851. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R. Overview and New Insights into the Metabolic Syndrome: Risk Factors and Emerging Variables in the Development of Type 2 Diabetes and Cerebrocardiovascular Disease. Medicina 2023, 59, 561. [Google Scholar] [CrossRef]
- Minas, M.; Kostikas, K.; Papaioannou, A.I.; Mystridou, P.; Karetsi, E.; Georgoulias, P.; Liakos, N.; Pournaras, S.; Gourgoulianis, K.I. The Association of Metabolic Syndrome with Adipose Tissue Hormones and Insulin Resistance in Patients with COPD without Co-Morbidities. COPD J. Chronic Obstr. Pulm. Dis. 2011, 8, 414–420. [Google Scholar] [CrossRef]
- Takabatake, N.; Nakamura, H.; Abe, S.; Hino, T.; Saito, H.; Yuki, H.; Kato, S.; Tomoike, H. Circulating Leptin in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1999, 159, 1215–1219. [Google Scholar] [CrossRef]
- Schols, A.M.; Broekhuizen, R.; Weling-Scheepers, C.A.; Wouters, E.F. Body Composition and Mortality in Chronic Obstructive Pulmonary Disease. Am. J. Clin. Nutr. 2005, 82, 53–59. [Google Scholar] [CrossRef]
- Wells, C.E.; Polkey, M.I.; Baker, E.H. Insulin Resistance Is Associated with Skeletal Muscle Weakness in COPD. Respirology 2016, 21, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.M.H.; Selemidis, S.; Bozinovski, S.; Vlahos, R. Pathobiological Mechanisms Underlying Metabolic Syndrome (MetS) in Chronic Obstructive Pulmonary Disease (COPD): Clinical Significance and Therapeutic Strategies. Pharmacol. Ther. 2019, 198, 160–188. [Google Scholar] [CrossRef]
- Clini, E.; Crisafulli, E.; Radaeli, A.; Malerba, M. COPD and the Metabolic Syndrome: An Intriguing Association. Intern. Emerg. Med. 2013, 8, 283–289. [Google Scholar] [CrossRef]
- Díez-Manglano, J.; Barquero-Romero, J.; Almagro, P.; Cabrera, F.J.; López García, F.; Montero, L.; Soriano, J.B. COPD Patients with and Without Metabolic Syndrome: Clinical and Functional Differences. Intern. Emerg. Med. 2014, 9, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory Mechanisms in Obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Bruun, J.M.; Pedersen, S.B.; Kristensen, K.; Richelsen, B. Opposite Regulation of Interleukin-8 and Tumor Necrosis Factor-Alpha by Weight Loss. Obes. Res. 2002, 10, 499–506. [Google Scholar] [CrossRef]
- Zumbach, M.S.; Boehme, M.W.J.; Wahl, P.; Stremmel, W.; Ziegler, R.; Nawroth, P.P. Tumor Necrosis Factor Increases Serum Leptin Levels in Humans. J. Clin. Endocrinol. Metab. 1997, 82, 4080–4082. [Google Scholar] [CrossRef]
- Grunfeld, C.; Zhao, C.; Fuller, J.; Pollock, A.; Moser, A.; Friedman, J.; Feingold, K.R. Endotoxin and Cytokines Induce Expression of Leptin, the Ob Gene Product, in Hamsters. J. Clin. Investig. 1996, 97, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, P.; Frederich, R.C.; Turner, E.M.; Ma, G.; Jaskowiak, N.T.; Rivet, D.J.; Flier, J.S.; Lowell, B.B.; Fraker, D.L.; Alexander, H.R. Multiple Cytokines and Acute Inflammation Raise Mouse Leptin Levels: Potential Role in Inflammatory Anorexia. J. Exp. Med. 1997, 185, 171–175. [Google Scholar] [CrossRef]
- Saetta, M.; Di Stefano, A.; Maestrelli, P.; Turato, G.; Ruggieri, M.P.; Roggeri, A.; Calcagni, P.; Mapp, C.E.; Ciaccia, A.; Fabbri, L.M. Airway Eosinophilia in Chronic Bronchitis During Exacerbations. Am. J. Respir. Crit. Care Med. 1994, 150, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Radford, K.; Fanat, A.; Janssen, L.J.; Peters-Golden, M.; Cox, P.G. The Effects of Leptin on Airway Smooth Muscle Responses. Am. J. Respir. Cell Mol. Biol. 2008, 39, 475–481. [Google Scholar] [CrossRef]
- Clerk, L.H.; Vincent, M.A.; Jahn, L.A.; Liu, Z.; Lindner, J.R.; Barrett, E.J. Obesity Blunts Insulin-Mediated Microvascular Recruitment in Human Forearm Muscle. Diabetes 2006, 55, 1436–1442. [Google Scholar] [CrossRef]
- Barnes, P.J. Chronic Obstructive Pulmonary Disease: Effects Beyond the Lungs. PLoS Med. 2010, 7, e1000220. [Google Scholar] [CrossRef]
- Price, D.B.; Russell, R.; Mares, R.; Burden, A.; Skinner, D.; Mikkelsen, H.; Ding, C.; Brice, R.; Chavannes, N.H.; Kocks, J.W.H.; et al. Metabolic Effects Associated with ICS in Patients with COPD and Comorbid Type 2 Diabetes: A Historical Matched Cohort Study. PLoS ONE 2016, 11, e0162903. [Google Scholar] [CrossRef]
- Cruickshank-Quinn, C.I.; Jacobson, S.; Hughes, G.; Powell, R.L.; Petrache, I.; Kechris, K.; Bowler, R.; Reisdorph, N. Metabolomics and Transcriptomics Pathway Approach Reveals Outcome-Specific Perturbations in COPD. Sci. Rep. 2018, 8, 17132. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.D.; Chase, R.P.; Parker, M.M.; Glass, K.; Seo, M.; Divo, M.; Owen, C.A.; Castaldi, P.; Demeo, D.L.; Silverman, E.K.; et al. RNA-Sequencing across Three Matched Tissues Reveals Shared and Tissue-Specific Gene Expression and Pathway Signatures of COPD. Respir. Res. 2019, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Kohler, M.; Heyder, T.; Forsslund, H.; Garberg, H.K.; Karimi, R.; Grunewald, J.; Berven, F.S.; Nyrén, S.; Magnus Sköld, C.; et al. Proteomic Profiling of Lung Immune Cells Reveals Dysregulation of Phagocytotic Pathways in Female-Dominated Molecular COPD Phenotype. Respir. Res. 2018, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Ye, R.; Wang, C.; Sun, P.; Wang, D.; Yue, Y.; Wang, H.; Wu, S.; Yu, M.; Xi, S.; et al. Identification of Proteomic Signatures in Chronic Obstructive Pulmonary Disease Emphysematous Phenotype. Front. Mol. Biosci. 2021, 8, 650604. [Google Scholar] [CrossRef]
- Garudadri, S.; Woodruff, P.G. Targeting Chronic Obstructive Pulmonary Disease Phenotypes, Endotypes, and Biomarkers. Ann. Am. Thorac. Soc. 2018, 15, S234–S238. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef]
- Kent, B.D.; Mitchell, P.D.; Mcnicholas, W.T. Hypoxemia in Patients with COPD: Cause, Effects, and Disease Progression. Int. J. Chronic Obstr. Pulm. Dis. 2011, 6, 199–208. [Google Scholar] [CrossRef]
- Gileles-Hillel, A.; Kheirandish-Gozal, L.; Gozal, D. Biological Plausibility Linking Sleep Apnoea and Metabolic Dysfunction. Nat. Rev. Endocrinol. 2016, 12, 290–298. [Google Scholar] [CrossRef]
- Kim, J.W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-Mediated Expression of Pyruvate Dehydrogenase Kinase: A Metabolic Switch Required for Cellular Adaptation to Hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef]
- Regazzetti, C.; Peraldi, P.; Grémeaux, T.; Najem-Lendom, R.; Ben-Sahra, I.; Cormont, M.; Bost, F.; Marchand-Brustel, Y.L.; Tanti, J.F.; Giorgetti-Peraldi, S. Hypoxia Decreases Insulin Signaling Pathways in Adipocytes. Diabetes 2009, 58, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, I.; Bozinovski, S.; Vlahos, R. Targeting Oxidant-Dependent Mechanisms for the Treatment of COPD and Its Comorbidities. Pharmacol. Ther. 2015, 155, 60–79. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Grigoryev, D.N.; Ye, S.Q.; Thorne, L.; Schwartz, A.R.; Smith, P.L.; O’Donnell, C.P.; Polotsky, V.Y. Chronic Intermittent Hypoxia Upregulates Genes of Lipid Biosynthesis in Obese Mice. J. Appl. Physiol. 2005, 99, 1643–1648. [Google Scholar] [CrossRef]
- Murphy, A.M.; Thomas, A.; Crinion, S.J.; Kent, B.D.; Tambuwala, M.M.; Fabre, A.; Pepin, J.L.; Roche, H.M.; Arnaud, C.; Ryan, S. Intermittent Hypoxia in Obstructive Sleep Apnoea Mediates Insulin Resistance through Adipose Tissue Inflammation. Eur. Respir. J. 2017, 49, 1601731. [Google Scholar] [CrossRef]
- Yokoe, T.; Alonso, L.C.; Romano, L.C.; Rosa, T.C.; O’Doherty, R.M.; Garcia-Ocana, A.; Minoguchi, K.; O’Donnell, C.P. Intermittent Hypoxia Reverses the Diurnal Glucose Rhythm and Causes Pancreatic Beta-Cell Replication in Mice. J. Physiol. 2008, 586, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Qu, A.; Taylor, M.; Xue, X.; Matsubara, T.; Metzger, D.; Chambon, P.; Gonzalez, F.J.; Shah, Y.M. Hypoxia-Inducible Transcription Factor 2α Promotes Steatohepatitis through Augmenting Lipid Accumulation, Inflammation, and Fibrosis. Hepatology 2011, 54, 472–483. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Finger, E.C.; Krieg, A.J.; Wu, C.; Diep, A.N.; Lagory, E.L.; Wei, K.; McGinnis, L.M.; Yuan, J.; Kuo, C.J.; et al. Cross-Talk between Hypoxia and Insulin Signaling through Phd3 Regulates Hepatic Glucose and Lipid Metabolism and Ameliorates Diabetes. Nat. Med. 2013, 19, 1325–1330. [Google Scholar] [CrossRef]
- Wei, K.; Piecewicz, S.M.; McGinnis, L.M.; Taniguchi, C.M.; Wiegand, S.J.; Anderson, K.; Chan, C.W.M.; Mulligan, K.X.; Kuo, D.; Yuan, J.; et al. A Liver Hif-2α-Irs2 Pathway Sensitizes Hepatic Insulin Signaling and Is Modulated by Vegf Inhibition. Nat. Med. 2013, 19, 1331–1337. [Google Scholar] [CrossRef]
- Thomas, A.; Belaidi, E.; Moulin, S.; Horman, S.; Van Der Zon, G.C.; Viollet, B.; Levy, P.; Bertrand, L.; Pepin, J.L.; Godin-Ribuot, D.; et al. Chronic Intermittent Hypoxia Impairs Insulin Sensitivity but Improves Whole-Body Glucose Tolerance by Activating Skeletal Muscle AMPK. Diabetes 2017, 66, 2942–2951. [Google Scholar] [CrossRef]
- Gamboa, J.L.; Garcia-Cazarin, M.L.; Andrade, F.H. Chronic Hypoxia Increases Insulin-Stimulated Glucose Uptake in Mouse Soleus Muscle. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 300, R85–R91. [Google Scholar] [CrossRef]
- Kirkham, P.A.; Barnes, P.J. Oxidative Stress in COPD. Chest 2013, 144, 266–273. [Google Scholar] [CrossRef]
- Taniguchi, A.; Tsuge, M.; Miyahara, N.; Tsukahara, H. Reactive Oxygen Species and Antioxidative Defense in Chronic Obstructive Pulmonary Disease. Antioxidants 2021, 10, 1537. [Google Scholar] [CrossRef]
- Van Der Toorn, M.; Rezayat, D.; Kauffman, H.F.; Bakker, S.J.L.; Gans, R.O.B.; Koëter, G.H.; Choi, A.M.K.; Van Oosterhout, A.J.M.; Slebos, D.J. Lipid-Soluble Components in Cigarette Smoke Induce Mitochondrial Production of Reactive Oxygen Species in Lung Epithelial Cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 297, L109–L114. [Google Scholar] [CrossRef]
- Osoata, G.O.; Hanazawa, T.; Brindicci, C.; Ito, M.; Barnes, P.J.; Kharitonov, S.; Ito, K. Peroxynitrite Elevation in Exhaled Breath Condensate of COPD and Its Inhibition by Fudosteine. Chest 2009, 135, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, C.; Newbold, P.; White, P.; Thong, B.; Stone, H.; Stockley, R.A. 3-Chlorotyrosine in Sputum of COPD Patients: Relationship with Airway Inflammation. COPD J. Chronic Obstr. Pulm. Dis. 2010, 7, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Miklós, Z.; Horváth, I. The Role of Oxidative Stress and Antioxidants in Cardiovascular Comorbidities in COPD. Antioxidants 2023, 12, 1196. [Google Scholar] [CrossRef] [PubMed]
- Negre-Salvayre, A.; Coatrieux, C.; Ingueneau, C.; Salvayre, R. Advanced Lipid Peroxidation End Products in Oxidative Damage to Proteins. Potential Role in Diseases and Therapeutic Prospects for the Inhibitors. Br. J. Pharmacol. 2008, 153, 6–20. [Google Scholar] [CrossRef]
- Kirkham, P.A.; Caramori, G.; Casolari, P.; Papi, A.A.; Edwards, M.; Shamji, B.; Triantaphyllopoulos, K.; Hussain, F.; Pinart, M.; Khan, Y.; et al. Oxidative Stress–Induced Antibodies to Carbonyl-Modified Protein Correlate with Severity of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2011, 184, 796. [Google Scholar] [CrossRef]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.L.; Montezano, A.C. Oxidative Stress: A Unifying Paradigm in Hypertension. Can. J. Cardiol. 2020, 36, 659–670. [Google Scholar] [CrossRef]
- Devallance, E.; Li, Y.; Jurczak, M.J.; Cifuentes-Pagano, E.; Pagano, P.J. The Role of NADPH Oxidases in the Etiology of Obesity and Metabolic Syndrome: Contribution of Individual Isoforms and Cell Biology. Antioxid. Redox Signal. 2019, 31, 687–709. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Kolka, C.M.; Bergman, R.N. The Endothelium in Diabetes: Its Role in Insulin Access and Diabetic Complications. Rev. Endocr. Metab. Disord. 2013, 14, 13–19. [Google Scholar] [CrossRef]
- Tarantino, G.; Caputi, A. JNKs, Insulin Resistance and Inflammation: A Possible Link between NAFLD and Coronary Artery Disease. World J. Gastroenterol. WJG 2011, 17, 3785. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef]
- Savini, I.; Catani, M.V.; Evangelista, D.; Gasperi, V.; Avigliano, L. Obesity-Associated Oxidative Stress: Strategies Finalized to Improve Redox State. Int. J. Mol. Sci. 2013, 14, 10497–10538. [Google Scholar] [CrossRef]
- Colak, E.; Pap, D. The Role of Oxidative Stress in the Development of Obesity and Obesity-Related Metabolic Disorders. J. Med. Biochem. 2021, 40, 1–9. [Google Scholar] [CrossRef]
- Glass, C.K.; Olefsky, J.M. Inflammation and Lipid Signaling in the Etiology of Insulin Resistance. Cell Metab. 2012, 15, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Aras, M.; Tchang, B.G.; Pape, J. Obesity and Diabetes. Nurs. Clin. N. Am. 2021, 56, 527–541. [Google Scholar] [CrossRef]
- Recinella, L.; Orlando, G.; Ferrante, C.; Chiavaroli, A.; Brunetti, L.; Leone, S. Adipokines: New Potential Therapeutic Target for Obesity and Metabolic, Rheumatic, and Cardiovascular Diseases. Front. Physiol. 2020, 11, 578966. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, K. NF-ΚB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative Stress, Insulin Resistance, Dyslipidemia and Type 2 Diabetes Mellitus. World J. Diabetes 2015, 6, 456. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.; Caramori, G.; Oates, T.; Capelli, A.; Lusuardi, M.; Gnemmi, I.; Ioli, F.; Chung, K.F.; Donner, C.F.; Barnes, P.J.; et al. Increased Expression of Nuclear Factor-KappaB in Bronchial Biopsies from Smokers and Patients with COPD. Eur. Respir. J. 2002, 20, 556–563. [Google Scholar] [CrossRef]
- Barnes, P.J. Cellular and Molecular Mechanisms of Asthma and COPD. Clin. Sci. 2017, 131, 1541–1558. [Google Scholar] [CrossRef]
- Fabbri, L.M.; Rabe, K.F. From COPD to Chronic Systemic Inflammatory Syndrome? Lancet 2007, 370, 797–799. [Google Scholar] [CrossRef]
- Decramer, M.; Rennard, S.; Troosters, T.; Mapel, D.W.; Giardino, N.; Mannino, D.; Wouters, E.; Sethi, S.; Cooper, C.B. COPD as a Lung Disease with Systemic Consequences—Clinical Impact, Mechanisms, and Potential for Early Intervention. COPD J. Chronic Obstr. Pulm. Dis. 2008, 5, 235–256. [Google Scholar] [CrossRef]
- Easter, M.; Bollenbecker, S.; Barnes, J.W.; Krick, S. Targeting Aging Pathways in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2020, 21, 6294. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Cordero, A.I.; Yang, C.X.; Milne, S.; Li, X.; Hollander, Z.; Chen, V.; Ng, R.; Tebbutt, S.J.; Leung, J.M.; Sin, D.D. Epigenetic Blood Biomarkers of Ageing and Mortality in COPD. Eur. Respir. J. 2021, 58, 2101890. [Google Scholar] [CrossRef]
- Breen, M.; Nwanaji-Enwerem, J.C.; Karrasch, S.; Flexeder, C.; Schulz, H.; Waldenberger, M.; Kunze, S.; Ollert, M.; Weidinger, S.; Colicino, E.; et al. Accelerated Epigenetic Aging as a Risk Factor for Chronic Obstructive Pulmonary Disease and Decreased Lung Function in Two Prospective Cohort Studies. Aging 2020, 12, 16539–16554. [Google Scholar] [CrossRef]
- Górski, P.; Białas, A.J.; Piotrowski, W.J. Aging Lung: Molecular Drivers and Impact on Respiratory Diseases—A Narrative Clinical Review. Antioxidants 2024, 13, 1480. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, D.; Casas-Recasens, S.; Faner, R.; Palange, P.; Agusti, A. When GETomics Meets Aging and Exercise in COPD. Respir. Med. 2023, 216, 107294. [Google Scholar] [CrossRef]
- Martino, D.J.; Bui, D.S.; Li, S.; Idrose, S.; Perret, J.; Lowe, A.J.; Lodge, C.J.; Bowatte, G.; Moodley, Y.; Thomas, P.S.; et al. Genetic and Epigenetic Associations with Pre-COPD Lung Function Trajectories. Am. J. Respir. Crit. Care Med. 2023, 208, 1135–1137. [Google Scholar] [CrossRef]
- Feng, Y.; Xie, M.; Liu, Q.; Weng, J.; Wei, L.; Chung, K.F.; Adcock, I.M.; Chang, Q.; Li, M.; Huang, Y.; et al. Changes in Targeted Metabolomics in Lung Tissue of Chronic Obstructive Pulmonary Disease. J. Thorac. Dis. 2023, 15, 2544–2558. [Google Scholar] [CrossRef]
- Diao, W.; Labaki, W.W.; Han, M.K.; Yeomans, L.; Sun, Y.; Smiley, Z.; Kim, J.H.; McHugh, C.; Xiang, P.; Shen, N.; et al. Disruption of Histidine and Energy Homeostasis in Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Zeng, Y.; Lin, R.; Qu, H.Q.; Zhang, T.; Zhang, X.D.; Liang, Y.; Zhen, Y.; Chen, H.; Huang, Z.; et al. Metabolomic Profiling of Anaerobic and Aerobic Energy Metabolic Pathways in Chronic Obstructive Pulmonary Disease. Exp. Biol. Med. 2021, 246, 1586–1596. [Google Scholar] [CrossRef]
- Kim, J.; Suresh, B.; Lim, M.N.; Hong, S.H.; Kim, K.S.; Song, H.E.; Lee, H.Y.; Yoo, H.J.; Kim, W.J. Metabolomics Reveals Dysregulated Sphingolipid and Amino Acid Metabolism Associated with Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Kolmert, J.; Yang, M.; Reinke, S.N.; Kamleh, M.A.; Snowden, S.; Heyder, T.; Levänen, B.; Erle, D.J.; Sköld, C.M.; et al. Metabolomics Analysis Identifies Sex-Associated Metabotypes of Oxidative Stress and the Autotaxin–LysoPA Axis in COPD. Eur. Respir. J. 2017, 49, 1602322. [Google Scholar] [CrossRef]
- Zinellu, A.; Mangoni, A.A. Arginine, Transsulfuration, and Folic Acid Pathway Metabolomics in Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Cells 2023, 12, 2180. [Google Scholar] [CrossRef]
- Adamko, D.J.; Nair, P.; Mayers, I.; Tsuyuki, R.T.; Regush, S.; Rowe, B.H. Metabolomic Profiling of Asthma and Chronic Obstructive Pulmonary Disease: A Pilot Study Differentiating Diseases. J. Allergy Clin. Immunol. 2015, 136, 571–580.e3. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory Mechanisms Linking Obesity and Metabolic Disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mihai, A.; Mititelu, M.; Matei, M.; Lupu, E.C.; Streba, L.; Vladu, I.M.; Iovănescu, M.L.; Cioboată, R.; Călărașu, C.; Busnatu, Ș.S.; et al. Assessment of Behavioral Risk Factors in Chronic Obstructive Airway Diseases of the Lung Associated with Metabolic Syndrome. J. Clin. Med. 2024, 13, 1037. [Google Scholar] [CrossRef] [PubMed]
- Cebron Lipovec, N.; Beijers, R.J.H.C.G.; van den Borst, B.; Doehner, W.; Lainscak, M.; Schols, A.M.W.J. The Prevalence of Metabolic Syndrome in Chronic Obstructive Pulmonary Disease: A Systematic Review. COPD J. Chronic Obstr. Pulm. Dis. 2016, 13, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Vujic, T.; Nagorni Obradovic, L.; Maric, G.; Popovic, L.; Jankovic, J. Metabolic Syndrome in Patients with Chronic Obstructive Pulmonary Disease: Frequency and Relationship with Systemic Inflammation. Hippokratia 2016, 20, 110. [Google Scholar]
- Watz, H.; Waschki, B.; Kirsten, A.; Müller, K.C.; Kretschmar, G.; Meyer, T.; Holz, O.; Magnussen, H. The Metabolic Syndrome in Patients with Chronic Bronchitis and COPD: Frequency and Associated Consequences for Systemic Inflammation and Physical Inactivity. Chest 2009, 136, 1039–1046. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity and Its Impact on Metabolic Syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Hutcheson, R.; Rocic, P. The Metabolic Syndrome, Oxidative Stress, Environment, and Cardiovascular Disease: The Great Exploration. Exp. Diabetes Res. 2012, 2012, 271028. [Google Scholar] [CrossRef] [PubMed]
- Cyphert, T.J.; Morris, R.T.; House, L.M.; Barnes, T.M.; Otero, Y.F.; Barham, W.J.; Hunt, R.P.; Zaynagetdinov, R.; Yull, F.E.; Blackwell, T.S.; et al. NF-ΚB-Dependent Airway Inflammation Triggers Systemic Insulin Resistance. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 309, R1144–R1152. [Google Scholar] [CrossRef]
- Monteiro, F.; Camillo, C.A.; Vitorasso, R.; Sant’Anna, T.; Hernandes, N.A.; Probst, V.S.; Pitta, F. Obesity and Physical Activity in the Daily Life of Patients with COPD. Lung 2012, 190, 403–410. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol. Cell Physiol. 2020, 320, C375–C391. [Google Scholar] [CrossRef]
- Sánchez-Margalet, V.; Fernández-Riejos, P.; Najib, S.; Santos-Alvarez, J.; Martín-Romero, C.; Pérez-Pérez, A.; González-Yanes, C. Role of Leptin in the Activation of Immune Cells. Mediat. Inflamm. 2010, 2010, 568343. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Vilariño-García, T.; Fernández-Riejos, P.; Martín-González, J.; Segura-Egea, J.J.; Sánchez-Margalet, V. Role of Leptin as a Link Between Metabolism and the Immune System. Cytokine Growth Factor Rev. 2017, 35, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.M.; Westerman, R.A.; De Courten, M.P.; Collier, G.R.; Zimmet, P.Z.; Alberti, K.G.M.M. Is Leptin Sensitivity the Link between Smoking Cessation and Weight Gain? Int. J. Obes. 1997, 21, 50–53. [Google Scholar] [CrossRef]
- Eliasson, B.; Smith, U. Leptin Levels in Smokers and Long-Term Users of Nicotine Gum. Eur. J. Clin. Investig. 1999, 29, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Bettoncelli, G.; Sessa, E.; Cricelli, C.; Biscione, G. Prevalence of Comorbidities in Patients with Chronic Obstructive Pulmonary Disease. Respiration 2010, 80, 112–119. [Google Scholar] [CrossRef]
- Yin, H.L.; Yin, S.Q.; Lin, Q.Y.; Xu, Y.; Xu, H.W.; Liu, T. Prevalence of Comorbidities in Chronic Obstructive Pulmonary Disease Patients: A Meta-Analysis. Medicine 2017, 96, e6863. [Google Scholar] [CrossRef]
- Cazzola, M.; Rogliani, P.; Calzetta, L.; Lauro, D.; Page, C.; Matera, M.G. Targeting Mechanisms Linking COPD to Type 2 Diabetes Mellitus. Trends Pharmacol. Sci. 2017, 38, 940–951. [Google Scholar] [CrossRef]
- Oh, J.Y.; Sin, D.D. Lung Inflammation in COPD: Why Does It Matter? F1000 Med. Rep. 2012, 4, 23. [Google Scholar] [CrossRef]
- Cravo, J.; Esquinas, A.M. Obesity and COPD Exacerbations—It’s Not That Simple. Respir. Med. 2017, 125, 103. [Google Scholar] [CrossRef][Green Version]
- Kinney, G.L.; Black-Shinn, J.L.; Wan, E.S.; Make, B.; Regan, E.; Lutz, S.; Soler, X.; Silverman, E.K.; Crapo, J.; Hokanson, J.E. Pulmonary Function Reduction in Diabetes with and Without Chronic Obstructive Pulmonary Disease. Diabetes Care 2014, 37, 389–395. [Google Scholar] [CrossRef]
- Sedgwick, J.B.; Menon, I.; Gern, J.E.; Busse, W.W. Effects of Inflammatory Cytokines on the Permeability of Human Lung Microvascular Endothelial Cell Monolayers and Differential Eosinophil Transmigration. J. Allergy Clin. Immunol. 2002, 110, 752–756. [Google Scholar] [CrossRef] [PubMed]
- McKeever, T.M.; Weston, P.J.; Hubbard, R.; Fogarty, A. Lung Function and Glucose Metabolism: An Analysis of Data from the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 2005, 161, 546–556. [Google Scholar] [CrossRef]
- Sparvero, L.J.; Asafu-Adjei, D.; Kang, R.; Tang, D.; Amin, N.; Im, J.; Rutledge, R.; Lin, B.; Amoscato, A.A.; Zeh, H.J.; et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE Ligands, and Their Role in Cancer and Inflammation. J. Transl. Med. 2009, 7, 17. [Google Scholar] [CrossRef]
- Singh, S.; Bodas, M.; Bhatraju, N.K.; Pattnaik, B.; Gheware, A.; Parameswaran, P.K.; Thompson, M.; Freeman, M.; Mabalirajan, U.; Gosens, R.; et al. Hyperinsulinemia Adversely Affects Lung Structure and Function. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L837–L845. [Google Scholar] [CrossRef]
- Baker, E.H.; Janaway, C.H.; Philips, B.J.; Brennan, A.L.; Baines, D.L.; Wood, D.M.; Jones, P.W. Hyperglycaemia Is Associated with Poor Outcomes in Patients Admitted to Hospital with Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Thorax 2006, 61, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Parappil, A.; Depczynski, B.; Collett, P.; Marks, G.B. Effect of Comorbid Diabetes on Length of Stay and Risk of Death in Patients Admitted with Acute Exacerbations of COPD. Respirology 2010, 15, 918–922. [Google Scholar] [CrossRef]
- Ceriello, A. Oxidative Stress and Diabetes-Associated Complications. Endocr. Pract. 2006, 12 (Suppl. S1), 60–62. [Google Scholar] [CrossRef]
- Tiengo, A.; Fadini, G.P.; Avogaro, A. The Metabolic Syndrome, Diabetes and Lung Dysfunction. Diabetes Metab. 2008, 34, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Calzetta, L.; Rogliani, P.; Lauro, D.; Novelli, L.; Page, C.P.; Kanabar, V.; Matera, M.G. High Glucose Enhances Responsiveness of Human Airways Smooth Muscle via the Rho/ROCK Pathway. Am. J. Respir. Cell Mol. Biol. 2012, 47, 509–516. [Google Scholar] [CrossRef]
- Ottenheijm, C.A.C.; Heunks, L.M.A.; Dekhuijzen, R.P.N. Diaphragm Adaptations in Patients with COPD. Respir. Res. 2008, 9, 12. [Google Scholar] [CrossRef]
- Friedman, J. 20 Years of Leptin: Leptin at 20: An Overview. J. Endocrinol. 2014, 223, T1–T8. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Seeley, R.J.; Campfield, L.A.; Burn, P.; Baskin, D.G. Identification of Targets of Leptin Action in Rat Hypothalamus. J. Clin. Investig. 1996, 98, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- German, J.P.; Thaler, J.P.; Wisse, B.E.; Oh-I, S.; Sarruf, D.A.; Matsen, M.E.; Fischer, J.D.; Taborsky, G.J.; Schwartz, M.W.; Morton, G.J. Leptin Activates a Novel CNS Mechanism for Insulin-Independent Normalization of Severe Diabetic Hyperglycemia. Endocrinology 2011, 152, 394–404. [Google Scholar] [CrossRef]
- Tang, X.; Li, J.; Xiang, W.; Cui, Y.; Xie, B.; Wang, X.; Xu, Z.; Gan, L. Metformin Increases Hepatic Leptin Receptor and Decreases Steatosis in Mice. J. Endocrinol. 2016, 230, 227–237. [Google Scholar] [CrossRef]
- Hocking, S.; Samocha-Bonet, D.; Milner, K.L.; Greenfield, J.R.; Chisholm, D.J. Adiposity and Insulin Resistance in Humans: The Role of the Different Tissue and Cellular Lipid Depots. Endocr. Rev. 2013, 34, 463–500. [Google Scholar] [CrossRef] [PubMed]
- Sin, D.D.; Man, S.F.P. Chronic Obstructive Pulmonary Disease as a Risk Factor for Cardiovascular Morbidity and Mortality. Proc. Am. Thorac. Soc. 2005, 2, 8–11. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Conde, B.; Fragoso, E.; Boléo-Tomé, J.P.; Areias, V.; Cardoso, J. COPD and Cardiovascular Disease. Pulmonology 2019, 25, 168–176. [Google Scholar] [CrossRef]
- Howe, M.; Leidal, A.; Montgomery, D.; Jackson, E. Role of Cigarette Smoking and Gender in Acute Coronary Syndrome Events. Am. J. Cardiol. 2011, 108, 1382–1386. [Google Scholar] [CrossRef]
- Mariniello, D.F.; D’Agnano, V.; Cennamo, D.; Conte, S.; Quarcio, G.; Notizia, L.; Pagliaro, R.; Schiattarella, A.; Salvi, R.; Bianco, A.; et al. Comorbidities in COPD: Current and Future Treatment Challenges. J. Clin. Med. 2024, 13, 743. [Google Scholar] [CrossRef]
- Cheyne, W.S.; Gelinas, J.C.; Eves, N.D. Hemodynamic Effects of Incremental Lung Hyperinflation. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H474–H481. [Google Scholar] [CrossRef]
- Rabe, K.F.; Hurst, J.R.; Suissa, S. Cardiovascular Disease and COPD: Dangerous Liaisons? Eur. Respir. Rev. 2018, 27, 180057. [Google Scholar] [CrossRef] [PubMed]
- Cosio, M.G.; Saetta, M.; Agusti, A. Immunologic Aspects of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2009, 360, 2445–2454. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.C.; Hurst, J.R.; Smith, C.J.; Hubbard, R.B.; Wedzicha, J.A. Increased Risk of Myocardial Infarction and Stroke Following Exacerbation of COPD. Chest 2010, 137, 1091–1097. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, Atherosclerosis, and Coronary Artery Disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Athyros, V.G.; Katsiki, N.; Doumas, M.; Karagiannis, A.; Mikhailidis, D.P. Effect of Tobacco Smoking and Smoking Cessation on Plasma Lipoproteins and Associated Major Cardiovascular Risk Factors: A Narrative Review. Curr. Med. Res. Opin. 2013, 29, 1263–1274. [Google Scholar] [CrossRef]
- Man, S.F.P.; Van Eeden, S.; Sin, D.D. Vascular Risk in Chronic Obstructive Pulmonary Disease: Role of Inflammation and Other Mediators. Can. J. Cardiol. 2012, 28, 653–661. [Google Scholar] [CrossRef]
- Mirrakhimov, A.E.; Mirrakhimov, E.M. Chronic Obstructive Pulmonary Disease and Vascular Risk: Should We Account for Diabetes Mellitus and Renal Disease? Can. J. Cardiol. 2013, 29, 639.e9. [Google Scholar] [CrossRef]
- Dong, M.; Ren, J. What Fans the Fire: Insights into Mechanisms of Leptin in Metabolic Syndrome-Associated Heart Diseases. Curr. Pharm. Des. 2014, 20, 652–658. [Google Scholar] [CrossRef]
- Katsiki, N.; Mikhailidis, D.P.; Banach, M. Leptin, Cardiovascular Diseases and Type 2 Diabetes Mellitus. Acta Pharmacol. Sin. 2018, 39, 1176. [Google Scholar] [CrossRef]
- Ren, J. Leptin and Hyperleptinemia—From Friend to Foe for Cardiovascular Function. J. Endocrinol. 2004, 181, 1–10. [Google Scholar] [CrossRef]
- Puurunen, V.P.; Kiviniemi, A.; Lepojärvi, S.; Piira, O.P.; Hedberg, P.; Junttila, J.; Ukkola, O.; Huikuri, H. Leptin Predicts Short-Term Major Adverse Cardiac Events in Patients with Coronary Artery Disease. Ann. Med. 2017, 49, 448–454. [Google Scholar] [CrossRef]
- Puurunen, V.P.; Lepojärvi, E.S.; Piira, O.P.; Hedberg, P.; Junttila, M.J.; Ukkola, O.; Huikuri, H.V. High Plasma Leptin Levels Are Associated with Impaired Diastolic Function in Patients with Coronary Artery Disease. Peptides 2016, 84, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Farcaş, A.D.; Rusu, A.; Stoia, M.A.; Vida-Simiti, L.A. Plasma Leptin, but Not Resistin, TNF-α and Adiponectin, Is Associated with Echocardiographic Parameters of Cardiac Remodeling in Patients with Coronary Artery Disease. Cytokine 2018, 103, 46–49. [Google Scholar] [CrossRef]
- Chen, M.C.; Wang, J.H.; Lee, C.J.; Hsu, B.G. Association between Hyperleptinemia and Cardiovascular Outcomes in Patients with Coronary Artery Disease. Ther. Clin. Risk Manag. 2018, 14, 1855. [Google Scholar] [CrossRef] [PubMed]
- Decramer, M.; Janssens, W. Chronic Obstructive Pulmonary Disease and Comorbidities. Lancet Respir. Med. 2013, 1, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Lahousse, L.; Verhamme, K.M.; Stricker, B.H.; Brusselle, G.G. Cardiac Effects of Current Treatments of Chronic Obstructive Pulmonary Disease. Lancet Respir. Med. 2016, 4, 149–164. [Google Scholar] [CrossRef]
- Heindl, S.; Lehnert, M.; Criée, C.P.; Hasenfuss, G.; Andreas, S. Marked Sympathetic Activation in Patients with Chronic Respiratory Failure. Am. J. Respir. Crit. Care Med. 2001, 164, 597–601. [Google Scholar] [CrossRef]
- Gershon, A.; Croxford, R.; Calzavara, A.; To, T.; Stanbrook, M.B.; Upshur, R.; Stukel, T.A. Cardiovascular Safety of Inhaled Long-Acting Bronchodilators in Individuals with Chronic Obstructive Pulmonary Disease. JAMA Intern. Med. 2013, 173, 1175–1184. [Google Scholar] [CrossRef]
- Passey, S.L.; Hansen, M.J.; Bozinovski, S.; McDonald, C.F.; Holland, A.E.; Vlahos, R. Emerging Therapies for the Treatment of Skeletal Muscle Wasting in Chronic Obstructive Pulmonary Disease. Pharmacol. Ther. 2016, 166, 56–70. [Google Scholar] [CrossRef]
- Sergi, G.; Coin, A.; Marin, S.; Vianello, A.; Manzan, A.; Peruzza, S.; Inelmen, E.M.; Busetto, L.; Mulone, S.; Enzi, G. Body Composition and Resting Energy Expenditure in Elderly Male Patients with Chronic Obstructive Pulmonary Disease. Respir. Med. 2006, 100, 1918–1924. [Google Scholar] [CrossRef]
- Schols, A.M.W.J. Body Composition in COPD.; Stepping Back or Moving Forward? Respir. Med. 2010, 104, 157–158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Swallow, E.B.; Reyes, D.; Hopkinson, N.S.; Man, W.D.C.; Porcher, R.; Cetti, E.J.; Moore, A.J.; Moxham, J.; Polkey, M.I. Quadriceps Strength Predicts Mortality in Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease. Thorax 2007, 62, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Debigaré, R.; Côté, C.H.; Hould, F.S.; LeBlanc, P.; Maltais, F. In Vitro and In Vivo Contractile Properties of the Vastus Lateralis Muscle in Males with COPD. Eur. Respir. J. 2003, 21, 273–278. [Google Scholar] [CrossRef]
- Jobin, J.; Maltais, F.; Doyon, J.F.; LeBlanc, P.; Simard, P.M.; Simard, A.A.; Simard, C. Chronic Obstructive Pulmonary Disease: Capillarity and Fiber-Type Characteristics of Skeletal Muscle. J. Cardiopulm. Rehabil. Prev. 1998, 18, 432–437. [Google Scholar] [CrossRef]
- Vogiatzis, I.; Terzis, G.; Stratakos, G.; Cherouveim, E.; Athanasopoulos, D.; Spetsioti, S.; Nasis, I.; Manta, P.; Roussos, C.; Zakynthinos, S. Effect of Pulmonary Rehabilitation on Peripheral Muscle Fiber Remodeling in Patients with COPD in GOLD Stages II to IV. Chest 2011, 140, 744–752. [Google Scholar] [CrossRef]
- Whittom, F.; Jobin, J.; Simard, P.M.; Leblanc, P.; Simard, C.; Bernard, S.; Belleau, R.; Maltais, F. Histochemical and Morphological Characteristics of the Vastus Lateralis Muscle in Patients with Chronic Obstructive Pulmonary Disease. Med. Sci. Sports Exerc. 1998, 30, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Gysel, T.; Cambier, D.; Roman De Mettelinge, T. Association Between Insulin Resistance, Lean Mass and Muscle Torque/Force in Proximal Versus Distal Body Parts in Healthy Young Men. J. Musculoskelet. Neuronal Interact. 2014, 14, 41–49. [Google Scholar]
- Barzilay, J.I.; Cotsonis, G.A.; Walston, J.; Schwartz, A.V.; Satterfield, S.; Miljkovic, I.; Harris, T.B. Insulin Resistance Is Associated With Decreased Quadriceps Muscle Strength in Nondiabetic Adults Aged ≥ 70 Years. Diabetes Care 2009, 32, 736. [Google Scholar] [CrossRef]
- Seok, W.P.; Goodpaster, B.H.; Strotmeyer, E.S.; Kuller, L.H.; Broudeau, R.; Kammerer, C.; De Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; et al. Accelerated Loss of Skeletal Muscle Strength in Older Adults with Type 2 Diabetes: The Health, Aging, and Body Composition Study. Diabetes Care 2007, 30, 1507–1512. [Google Scholar] [CrossRef]
- Guillet, C.; Boirie, Y. Insulin Resistance: A Contributing Factor to Age-Related Muscle Mass Loss? Diabetes Metab. 2005, 31, 5S20–5S26. [Google Scholar] [CrossRef]
- Du, J.; Wang, X.; Miereles, C.; Bailey, J.L.; Debigare, R.; Zheng, B.; Price, S.R.; Mitch, W.E. Activation of Caspase-3 Is an Initial Step Triggering Accelerated Muscle Proteolysis in Catabolic Conditions. J. Clin. Investig. 2004, 113, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.T.; Rajani, S.; Dhadda, R.S.; Tisdale, M.J. Mechanism of Induction of Muscle Protein Loss by Hyperglycaemia. Exp. Cell Res. 2009, 315, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Attvall, S.; Fowelin, J.; Lager, I.; Von Schenck, H.; Smith, U. Smoking Induces Insulin Resistance—A Potential Link with the Insulin Resistance Syndrome. J. Intern. Med. 1993, 233, 327–332. [Google Scholar] [CrossRef]
- Boettcher, M.; Machann, J.; Stefan, N.; Thamer, C.; Häring, H.U.; Claussen, C.D.; Fritsche, A.; Schick, F. Intermuscular Adipose Tissue (IMAT): Association with Other Adipose Tissue Compartments and Insulin Sensitivity. J. Magn. Reson. Imaging 2009, 29, 1340–1345. [Google Scholar] [CrossRef]
- Shields, G.S.; Coissi, G.S.; Jimenez-Royo, P.; Gambarota, G.; Dimber, R.; Hopkinson, N.S.; Matthews, P.M.; Brown, A.P.; Polkey, M.I. Bioenergetics and Intermuscular Fat in Chronic Obstructive Pulmonary Disease-Associated Quadriceps Weakness. Muscle Nerve 2015, 51, 214–221. [Google Scholar] [CrossRef]
- Andreas, S.; Anker, S.D.; Scanlon, P.D.; Somers, V.K. Neurohumoral Activation as a Link to Systemic Manifestations of Chronic Lung Disease. Chest 2005, 128, 3618–3624. [Google Scholar] [CrossRef] [PubMed]
- Malli, F.; Papaioannou, A.I.; Gourgoulianis, K.I.; Daniil, Z. The Role of Leptin in the Respiratory System: An Overview. Respir. Res. 2010, 11, 152. [Google Scholar] [CrossRef]
- Takabatake, N.; Nakamura, H.; Minamihaba, O.; Inage, M.; Inoue, S.; Kagaya, S.; Yamaki, M.; Tomoike, H. A Novel Pathophysiologic Phenomenon in Cachexic Patients with Chronic Obstructive Pulmonary Disease: The Relationship Between the Circadian Rhythm of Circulating Leptin and the Very Low-Frequency Component of Heart Rate Variability. Am. J. Respir. Crit. Care Med. 2001, 163, 1314–1319. [Google Scholar] [CrossRef]
- Yao, S.; Zeng, L.; Wang, F.; Chen, K. Obesity Paradox in Lung Diseases: What Explains It? Obes. Facts 2023, 16, 411–426. [Google Scholar] [CrossRef]
- Tenda, E.D.; Henrina, J.; Setiadharma, A.; Felix, I.; Yulianti, M.; Pitoyo, C.W.; Kho, S.S.; Tay, M.C.K.; Purnamasari, D.S.; Soejono, C.H.; et al. The Impact of Body Mass Index on Mortality in COPD: An Updated Dose–Response Meta-Analysis. Eur. Respir. Rev. 2024, 33, 230261. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, L.; Mykytiuk, M.; Cinato, M.; Tronchere, H.; Kunduzova, O.; Kaidashev, I.P. IL-26 in the Induced Sputum Is Associated with the Level of Systemic Inflammation, Lung Functions and Body Weight in COPD Patients. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Çalikoglu, M.; Şahin, G.; Unlu, A.; Ozturk, C.; Tamer, L.; Ercan, B.; Kanik, A.; Atik, U. Leptin and TNF-Alpha Levels in Patients with Chronic Obstructive Pulmonary Disease and Their Relationship to Nutritional Parameters. Respiration 2004, 71, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yuan, C.; Zhang, J.; Yu, R.; Huang, M.; Adcock, I.M.; Yao, X. Circulating Leptin Concentrations in Patients with Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Respiration 2013, 86, 512–522. [Google Scholar] [CrossRef]
- Halpin, D.M.G.; Celli, B.R.; Criner, G.J.; Frith, P.; López Varela, M.V.; Salvi, S.; Vogelmeier, C.F.; Chen, R.; Mortimer, K.; Montes de Oca, M.; et al. The GOLD Summit on Chronic Obstructive Pulmonary Disease in Low- and Middle-Income Countries. Int. J. Tuberc. Lung Dis. 2019, 23, 1131–1141. [Google Scholar] [CrossRef]
- Meghji, J.; Mortimer, K.; Agusti, A.; Allwood, B.W.; Asher, I.; Bateman, E.D.; Bissell, K.; Bolton, C.E.; Bush, A.; Celli, B.; et al. Improving Lung Health in Low-Income and Middle-Income Countries: From Challenges to Solutions. Lancet 2021, 397, 928–940. [Google Scholar] [CrossRef]
- Perret, J.L.; Abramson, M.J. Biomass Smoke COPD: A Phenotype or a Different Disease? Respirology 2018, 23, 124–125. [Google Scholar] [CrossRef]
- Ramírez-Venegas, A.; Montiel-Lopez, F.; Lara-Albisua, J.L.P.; Aranda-Chávez, A.; Perea-Gutiérrez, H.; Falfán-Valencia, R.; Pérez-Rubio, G.; Pérez-Padilla, R.; Ramírez-Díaz, M.; Martínez-Gómez, M.L.; et al. Association between Chronic Obstructive Pulmonary Disease and Biomass Smoke in Rural Areas. Int. J. Tuberc. Lung Dis. 2022, 26, 1191–1193. [Google Scholar] [CrossRef]
- Ozoh, O.B.; Ayo-Olagunju, T.; Mortimer, K. Meeting Unmet Needs in Chronic Obstructive Pulmonary Disease Diagnosis and Treatment in Low- and Middle-Income Countries. Am. J. Respir. Crit. Care Med. 2023, 208, 352–354. [Google Scholar] [CrossRef]
- Florman, K.E.H.; Siddharthan, T.; Pollard, S.L.; Alupo, P.; Barber, J.A.; Chandyo, R.K.; Flores-Flores, O.; Kirenga, B.; Mendes, R.G.; Miranda, J.J.; et al. Unmet Diagnostic and Therapeutic Opportunities for Chronic Obstructive Pulmonary Disease in Low- and Middle-Income Countries. Am. J. Respir. Crit. Care Med. 2023, 208, 442–450. [Google Scholar] [CrossRef]
- Lim, H.; Kim, D.-Y.; Park, J.H.; Lim, H. The Global Non-Communicable Diseases Epidemic and Association Between Dietary Factors and Non-Communicable Diseases by Income Levels. Proc. Nutr. Soc. 2020, 79, E606. [Google Scholar] [CrossRef]
- Van Iersel, L.E.J.; Beijers, R.J.H.C.G.; Gosker, H.R.; Schols, A.M.W.J. Nutrition as a Modifiable Factor in the Onset and Progression of Pulmonary Function Impairment in COPD: A Systematic Review. Nutr. Rev. 2022, 80, 1434–1444. [Google Scholar] [CrossRef]
- Leme, A.C.B.; Hou, S.; Fisberg, R.M.; Fisberg, M.; Haines, J. Adherence to Food-Based Dietary Guidelines: A Systemic Review of High-Income and Low- and Middle-Income Countries. Nutrients 2021, 13, 1038. [Google Scholar] [CrossRef]
- Alupo, P.; Baluku, J.; Bongomin, F.; Siddharthan, T.; Katagira, W.; Ddungu, A.; Hurst, J.R.; van Boven, J.F.M.; Worodria, W.; Kirenga, B.J. Overcoming Challenges of Managing Chronic Obstructive Pulmonary Disease in Low- and Middle-Income Countries. Expert Rev. Respir. Med. 2024, 18, 873–882. [Google Scholar] [CrossRef]
- Beran, D.; Zar, H.J.; Perrin, C.; Menezes, A.M.; Burney, P. Burden of Asthma and Chronic Obstructive Pulmonary Disease and Access to Essential Medicines in Low-Income and Middle-Income Countries. Lancet Respir. Med. 2015, 3, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Gross, N.J. Novel Antiinflammatory Therapies for COPD. Chest 2012, 142, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Hanania, N.A.; Page, C.P.; Matera, M.G. Novel Anti-Inflammatory Approaches to COPD. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 1333–1352. [Google Scholar] [CrossRef] [PubMed]
- Matera, M.G.; Cazzola, M.; Page, C. Prospects for COPD Treatment. Curr. Opin. Pharmacol. 2020, 56, 74–84. [Google Scholar] [CrossRef]
- Bolger, G.B. Therapeutic Targets and Precision Medicine in COPD: Inflammation, Ion Channels, Both, or Neither? Int. J. Mol. Sci. 2023, 24, 17363. [Google Scholar] [CrossRef]
- Lo Bello, F.; Hansbro, P.M.; Donovan, C.; Coppolino, I.; Mumby, S.; Adcock, I.M.; Caramori, G. New Drugs Under Development for COPD. Expert Opin. Emerg. Drugs 2020, 25, 419–431. [Google Scholar] [CrossRef]
- Ray, A.; Paik, J.M.; Wexler, D.J.; Sreedhara, S.K.; Bykov, K.; Feldman, W.B.; Patorno, E. Glucose-Lowering Medications and Risk of Chronic Obstructive Pulmonary Disease Exacerbations in Patients with Type 2 Diabetes. JAMA Intern. Med. 2025, 185, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yi, H.; Zhao, C.; Zhang, Y.; Zhu, L.; Liu, B.; He, P.; Zhou, M. Glucagon-like Peptide-1 Receptor (GLP-1R) Signaling Ameliorates Dysfunctional Immunity in COPD Patients. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3191–3202. [Google Scholar] [CrossRef]
- Wang, W.; Mei, A.; Qian, H.; Li, D.; Xu, H.; Chen, J.; Yang, H.; Min, X.; Li, C.; Cheng, L.; et al. The Role of Glucagon-Like Peptide-1 Receptor Agonists in Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 129–137. [Google Scholar] [CrossRef] [PubMed]
- See, X.Y.; Xanthavanij, N.; Lee, Y.-C.; Ong, T.E.; Wang, T.-H.; Ahmed, O.; Chang, Y.-C.; Peng, C.-Y.; Chi, K.-Y.; Chang, Y.-C.; et al. Pulmonary Outcomes of Incretin-Based Therapies in COPD Patients Receiving Single-Inhaler Triple Therapy. ERJ Open Res. 2024, 00803–02024. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Sánchez-García, A.; Linden-Torres, E.; Simental-Mendía, M. Effect of Glucagon-like Peptide-1 Receptor Agonists on Circulating Levels of Leptin and Resistin: A Meta-Analysis of Randomized Controlled Trials. Diabetes Res. Clin. Pract. 2021, 177, 108899. [Google Scholar] [CrossRef]
- Borg, M.; Ibsen, R.; Hilberg, O.; Løkke, A. Real-Life Nationwide Outcomes of Bronchoscopic Lung Volume Reduction with Endobronchial Valves in Severe COPD. Respiration 2024, 103, 322–331. [Google Scholar] [CrossRef]
- Zhong, C.; You, Z.; Li, S. Application and Innovation of Bronchoscopic Lung Volume Reduction with Endobronchial Valve. Zhonghua Jie He He Hu Xi Za Zhi 2024, 47, 1015–1018. [Google Scholar] [CrossRef]
- Buttery, S.C.; Banya, W.; Bilancia, R.; Boyd, E.; Buckley, J.; Greening, N.J.; Housley, K.; Jordan, S.; Kemp, S.V.; Kirk, A.J.B.; et al. Lung Volume Reduction Surgery versus Endobronchial Valves: A Randomised Controlled Trial. Eur. Respir. J. 2023, 61, 2202063. [Google Scholar] [CrossRef]
- Simoff, M.J.; Diaz-Mendoza, J.I.; Khan, A.Y.; Bechara, R.I. Bronchoscopic Lung Volume Reduction. Clin. Chest Med. 2013, 34, 445–457. [Google Scholar] [CrossRef]
- Calvache-Mateo, A.; López-López, L.; Heredia-Ciuró, A.; Martín-Núñez, J.; Rodríguez-Torres, J.; Ortiz-Rubio, A.; Valenza, M.C. Efficacy of Web-Based Supportive Interventions in Quality of Life in COPD Patients, a Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 12692. [Google Scholar] [CrossRef]
- Jonkman, N.H.; Westland, H.; Trappenburg, J.C.A.; Groenwold, R.H.H.; Bischoff, E.W.M.A.; Bourbeau, J.; Bucknall, C.E.; Coultas, D.; Effing, T.W.; Epton, M.J.; et al. Do Self-Management Interventions in COPD Patients Work and Which Patients Benefit Most? An Individual Patient Data Meta-Analysis. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2063–2074. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gregersen, T.L.; Green, A.; Frausing, E.; Ringbæk, T.; Brøndum, E.; Ulrik, C.S. Do Telemedical Interventions Improve Quality of Life in Patients with COPD? A Systematic Review. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 809–822. [Google Scholar] [CrossRef]
- Newham, J.J.; Presseau, J.; Heslop-Marshall, K.; Russell, S.; Ogunbayo, O.J.; Netts, P.; Hanratty, B.; Kaner, E. Features of Self-Management Interventions for People with COPD Associated with Improved Health-Related Quality of Life and Reduced Emergency Department Visits: A Systematic Review and Meta-Analysis. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1705–1720. [Google Scholar] [CrossRef] [PubMed]


| Author (Year) | Main Outcomes |
|---|---|
| Bolton et al. (2007) [93] | COPD patients had greater insulin resistance than the healthy subjects |
| Breyer et al. (2011) [87] | Leptin is increased in COPD women when compared to healthy women and compared to COPD men and to a greater extent in overweight women with COPD |
| Breyer et al. (2012) [88] | Female COPD subjects had higher leptin and higher ratios of leptin/FM and leptin/adiponectin compared with male COPD subjects |
| Eker et al. (2010) [114] | Leptin levels were lower in COPD patients compared to controls. COPD patients had higher HOMA-IR scores |
| Minas et al. (2011) [117] | Patients with COPD and MetS show higher leptin levels and greater insulin resistance compared to those without MetS. |
| Takabatake et al. (1999) [118] | Serum leptin was lower in COPD patients compared to healthy controls, yet it remains physiologically regulated even amidst cachexia. |
| Schols et al. (1999) [25] | Emphysematous patients had lower leptin concentrations compared with bronchitic patients. |
| Broekhuizen et al. (2005) [119] | Leptin is detectable in COPD sputum, inversely correlates with plasma leptin, and is significantly linked to CRP and TNF-α. |
| Vernooy et al. (2009) [85] | Ex-smokers, with or without severe COPD, show higher leptin expression in bronchial epithelial cells and alveolar macrophages than never-smokers. |
| Gaki et al. (2011) [90] | In patients with COPD, both BODE index and FFMI presented significant positive and negative associations, respectively, with leptin levels. |
| Wells et al. (2016) [120] | Insulin resistance inversely correlates with quadricep strength, and patients with quadricep weakness exhibit significantly higher insulin resistance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catana, O.M.; Nemes, A.F.; Cioboata, R.; Toma, C.L.; Mitroi, D.M.; Calarasu, C.; Streba, C.T. Leptin and Insulin in COPD: Unveiling the Metabolic-Inflammatory Axis—A Narrative Review. J. Clin. Med. 2025, 14, 2611. https://doi.org/10.3390/jcm14082611
Catana OM, Nemes AF, Cioboata R, Toma CL, Mitroi DM, Calarasu C, Streba CT. Leptin and Insulin in COPD: Unveiling the Metabolic-Inflammatory Axis—A Narrative Review. Journal of Clinical Medicine. 2025; 14(8):2611. https://doi.org/10.3390/jcm14082611
Chicago/Turabian StyleCatana, Oana Maria, Alexandra Floriana Nemes, Ramona Cioboata, Claudia Lucia Toma, Denisa Maria Mitroi, Cristina Calarasu, and Costin Teodor Streba. 2025. "Leptin and Insulin in COPD: Unveiling the Metabolic-Inflammatory Axis—A Narrative Review" Journal of Clinical Medicine 14, no. 8: 2611. https://doi.org/10.3390/jcm14082611
APA StyleCatana, O. M., Nemes, A. F., Cioboata, R., Toma, C. L., Mitroi, D. M., Calarasu, C., & Streba, C. T. (2025). Leptin and Insulin in COPD: Unveiling the Metabolic-Inflammatory Axis—A Narrative Review. Journal of Clinical Medicine, 14(8), 2611. https://doi.org/10.3390/jcm14082611








