Carotid Web as a Cause of Ischemic Stroke: Effective Treatment with Endovascular Techniques
Abstract
1. Introduction
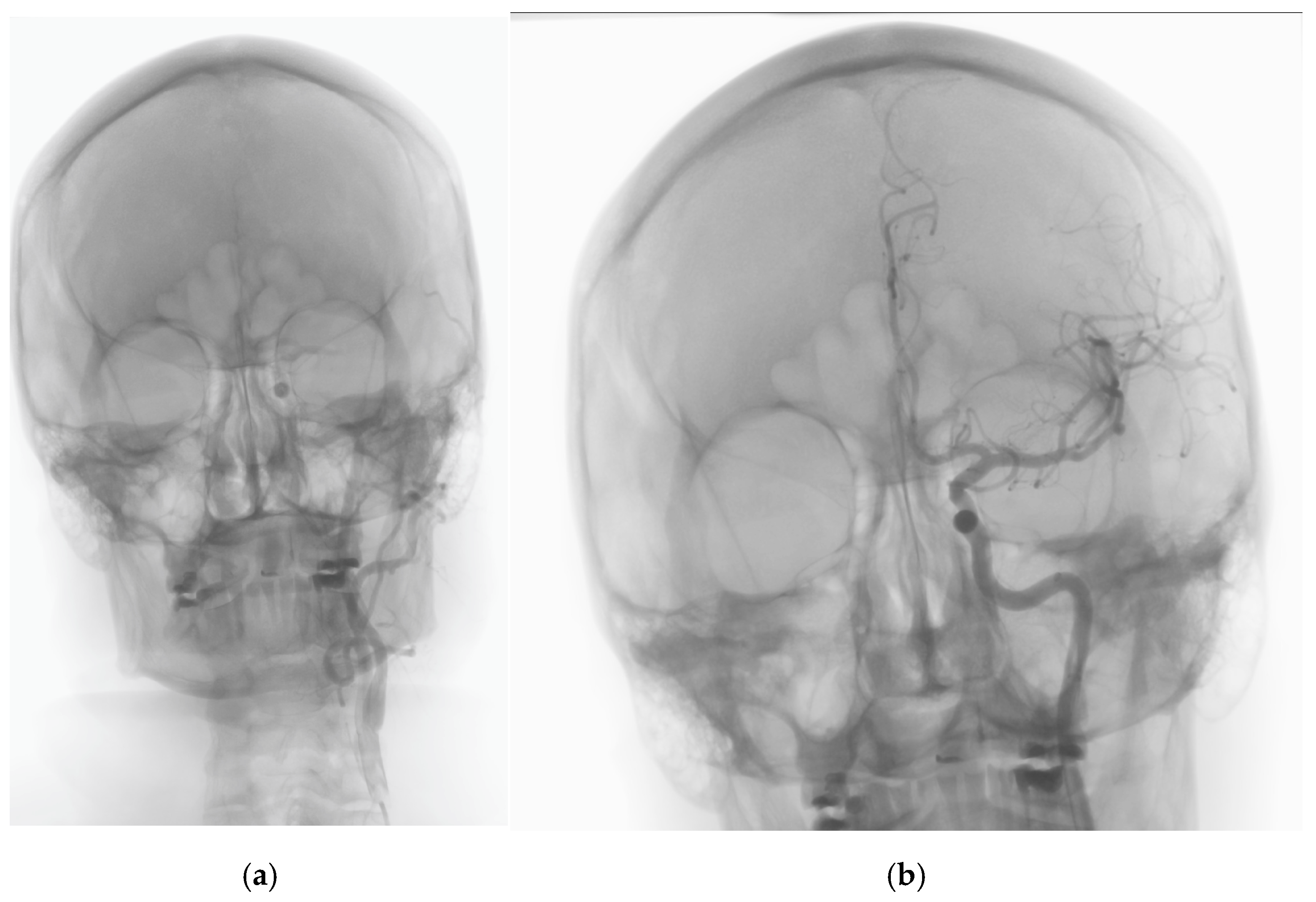
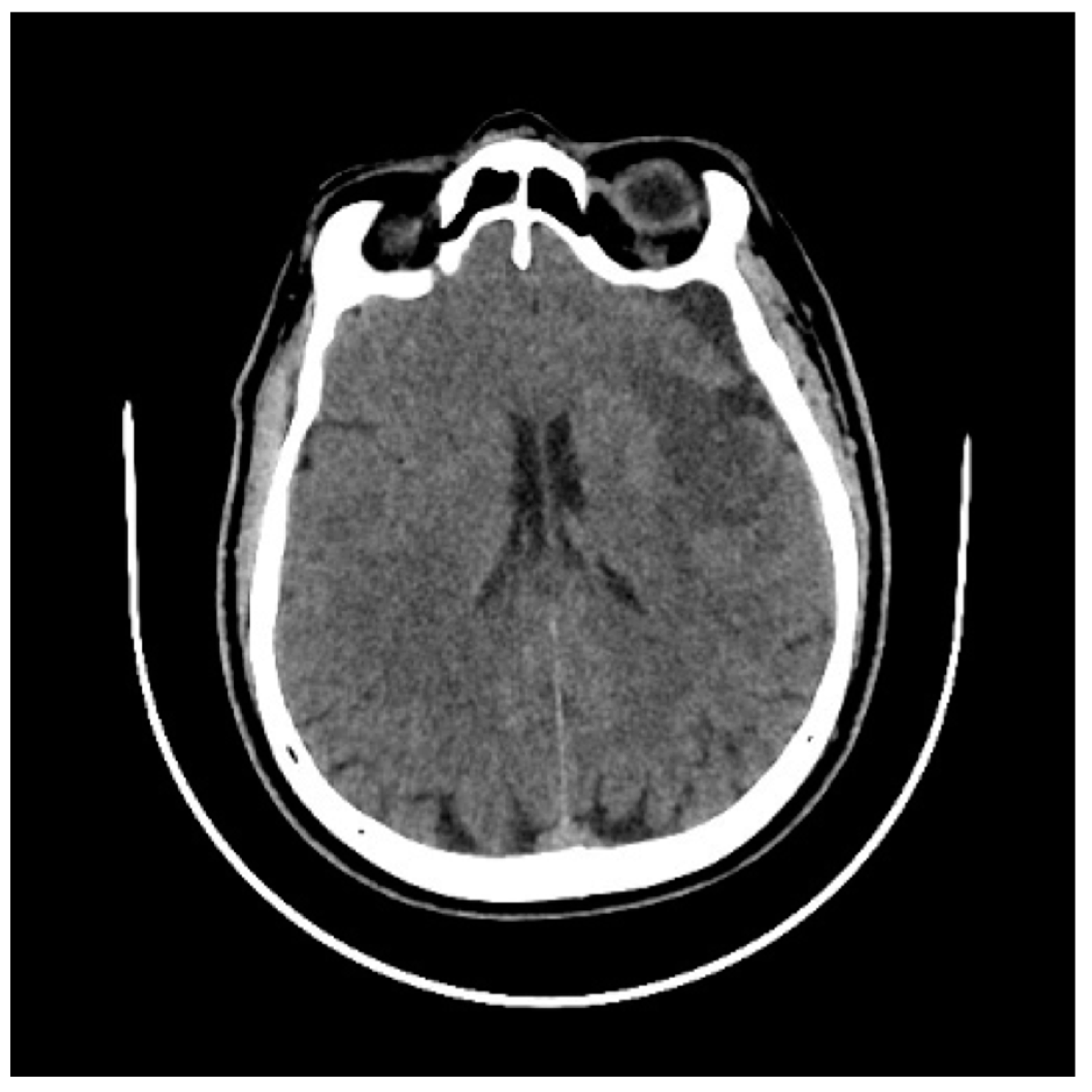
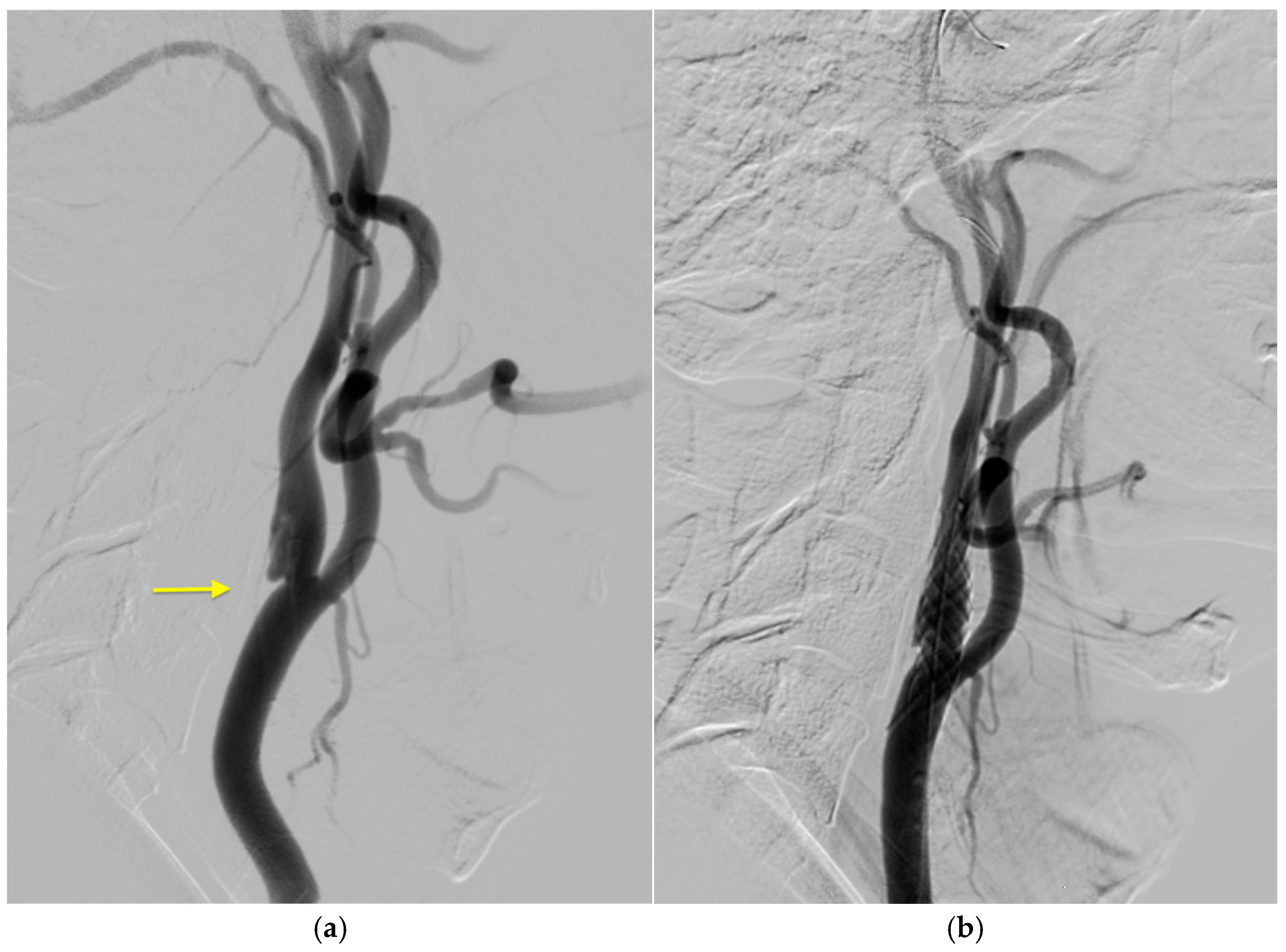
2. Case History
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mac Grory, B.; Emmer, B.J.; Roosendaal, S.D.; Zagzag, D.; Yaghi, S.; Nossek, E. Carotid web: An occult mechanism of embolic stroke. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.M.; Singh, D.; Trivedi, A.; Qazi, E.; George, D.; Wong, J.; Demchuk, A.M.; Goyal, M.; Hill, M.D.; Menon, B.K. Carotid Webs and Recurrent Ischemic Strokes in the Era of CT Angiography. Am. J. Neuroradiol. 2015, 36, 2134–2139. [Google Scholar] [CrossRef]
- Compagne, K.C.J.; van Es, A.C.G.M.; Berkhemer, O.A.; Borst, J.; Roos, Y.B.W.E.M.; van Oostenbrugge, R.J.; van Zwam, W.H.; Majoie, C.B.L.M.; Marquering, H.A.; Dippel, D.W.J.; et al. Prevalence of Carotid Web in Patients with Acute Intracranial Stroke Due to Intracranial Large Vessel Occlusion. Radiology 2018, 286, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Turpinat, C.; Collemiche, F.L.; Arquizan, C.; Molinari, N.; Cagnazzo, F.; Mourand, I.; Lefèvre, P.H.; Henneton, P.; Corti, L.; Gascou, G.; et al. Prevalence of carotid web in a French cohort of cryptogenic stroke. J. Neurol. Sci. 2021, 427, 117513. [Google Scholar] [CrossRef]
- Olindo, S.; Chausson, N.; Signate, A.; Mecharles, S.; Hennequin, J.-L.; Saint-Vil, M.; Edimonana-Kaptue, M.; Jeannin, S.; Landais, A.; Cabre, P.; et al. Stroke Recurrence in First-Ever Symptomatic Carotid Web: A Cohort Study. J. Stroke 2021, 23, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Joux, J.; Boulanger, M.; Jeannin, S.; Chausson, N.; Hennequin, J.-L.; Molinié, V.; Smadja, D.; Touzé, E.; Olindo, S. Association Between Carotid Bulb Diaphragm and Ischemic Stroke in Young Afro-Caribbean Patients. Stroke 2016, 47, 2641–2644. [Google Scholar] [CrossRef]
- Chen, H.; Colasurdo, M.; Costa, M.; Nossek, E.; Kan, P. Carotid webs: A review of pathophysiology, diagnostic findings, and treatment options. J. Neurointerv. Surg. 2024, 16, 1294–1299. [Google Scholar] [CrossRef]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef]
- Zaidat, O.O.; Yoo, A.J.; Khatri, P.; Tomsick, T.A.; von Kummer, R.; Saver, J.L.; Marks, M.P.; Prabhakaran, S.; Kallmes, D.F.; Fitzsimmons, B.-F.M.; et al. Recommendations on Angiographic Revascularization Grading Standards for Acute Ischemic Stroke. Stroke 2013, 44, 2650–2663. [Google Scholar] [CrossRef]
- Sajedi, P.I.; Gonzalez, J.N.; Cronin, C.A.; Kouo, T.; Steven, A.; Zhuo, J.; Thompson, O.; Castellani, R.; Kittner, S.J.; Gandhi, D.; et al. Carotid Bulb Webs as a Cause of “Cryptogenic” Ischemic Stroke. Am. J. Neuroradiol. 2017, 38, 1399–1404. [Google Scholar] [CrossRef]
- Haussen, D.C.; Grossberg, J.A.; Koch, S.; Malik, A.; Yavagal, D.; Gory, B.; Leesch, W.; Hassan, A.E.; Derelle, A.-L.; Richard, S.; et al. Multicenter Experience with Stenting for Symptomatic Carotid Web. Interv. Neurol. 2018, 7, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Multon, S.; Denier, C.; Charbonneau, P.; Sarov, M.; Boulate, D.; Mitilian, D.; Mougin, J.; Chassin, O.; Legris, N.; Fadel, E.; et al. Carotid webs management in symptomatic patients. J. Vasc. Surg. 2020, 73, 1290–1297. [Google Scholar] [CrossRef]
- Kyaw, K.; Latt, H.; Aung, S.S.M.; Babu, J.; Rangaswamy, R. A rare case of carotid web presenting with ischemic stroke in a young woman and a brief review of the literature. Case Rep. Med. 2018, 2018, 3195679. [Google Scholar] [CrossRef] [PubMed]
- El-Masri, S.; Wilson, M.M.; Kleinig, T. Systematic review and meta-analysis of ipsilateral and contralateral carotid web prevalence in embolic supratentorial strokes of undetermined source. J. Clin. Neurosci. 2022, 107, 118–123. [Google Scholar] [CrossRef]
- Zhang, A.J.; Dhruv, P.; Choi, P.; Bakker, C.; Koffel, J.; Anderson, D.; Kim, J.; Jagadeesan, B.; Menon, B.K.; Streib, C. A Systematic Literature Review of Patients With Carotid Web and Acute Ischemic Stroke. Stroke 2018, 49, 2872–2876. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, L.; Guidolin, B.; Viguier, A.; Gollion, C.; Barbieux, M.; Larrue, V. Ultrasound characteristics of carotid web. J. Neuroimaging 2022, 32, 894–901. [Google Scholar] [CrossRef]
- Olin, J.W.; Gornik, H.L.; Bacharach, J.M.; Biller, J.; Fine, L.J.; Gray, B.H.; Gray, W.A.; Gupta, R.; Hamburg, N.M.; Katzen, B.T.; et al. Fibromuscular Dysplasia: State of the Science and Critical Unanswered Questions. Circulation 2014, 129, 1048–1078. [Google Scholar] [CrossRef]
- Rzepka, M.; Chmiela, T.; Bosowska, J.; Cebula, M.; Krzystanek, E. Fibromuscular Dysplasia/Carotid Web in Angio-CT Imaging: A Rare Cause of Ischemic Stroke. Medicina 2021, 57, 1112. [Google Scholar] [CrossRef]
- Kim, S.J.; Nogueira, R.G.; Haussen, D.C. Current Understanding and Gaps in Research of Carotid Webs in Ischemic Strokes: A Review. JAMA Neurol. 2019, 76, 355–361. [Google Scholar] [CrossRef]
- Liang, S.; Qin, P.; Xie, L.; Niu, S.; Luo, J.; Chen, F.; Chen, X.; Zhang, J.; Wang, G. The carotid web: Current research status and imaging features. Front. Neurosci. 2023, 17, 1104212. [Google Scholar] [CrossRef]
- Labeyrie, M.-A.; Serrano, F.; Civelli, V.; Jourdaine, C.; Reiner, P.; Saint-Maurice, J.-P.; Chabriat, H.; Houdart, E. Carotid artery webs in embolic stroke of undetermined source with large intracranial vessel occlusion. Int. J. Stroke 2020, 16, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Tabibian, B.E.; Parr, M.; Salehani, A.; Mahavadi, A.; Rahm, S.; Kaur, M.; Howell, S.; Jones, J.G.; Liptrap, E.; Harrigan, M.R. Morphological characteristics of symptomatic and asymptomatic carotid webs. J. Neurosurg. 2022, 137, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Allen, J.W.; Bouslama, M.; Nahab, F.; Frankel, M.R.; Nogueira, R.G.; Haussen, D.C. Carotid Webs in Cryptogenic Ischemic Strokes: A Matched Case-Control Study. J. Stroke Cerebrovasc. Dis. 2019, 28, 104402. [Google Scholar] [CrossRef] [PubMed]
- Madaelil, T.P.; Grossberg, J.A.; Nogueira, R.G.; Anderson, A.; Barreira, C.; Frankel, M.; Haussen, D.C. Multimodality Imaging in Carotid Web. Front. Neurol. 2019, 10, 220. [Google Scholar] [CrossRef]
- Zelada-Ríos, L.; Barrientos-Imán, D.; Simbrón-Ribbeck, L.; Argomedo, C.A.; Ramírez-Quiñones, J.; La Rosa, P.C.; Chávez, A.V.; Otiniano-Sifuentes, R. Importance of multiplanar reformation angiographic images for the detection of carotid web. Brain Circ. 2023, 9, 44–47. [Google Scholar] [CrossRef]
- Wintermark, M.; Jawadi, S.S.; Rapp, J.H.; Tihan, T.; Tong, E.; Glidden, D.V.; Abedin, S.; Schaeffer, S.; Acevedo-Bolton, G.; Boudignon, B.; et al. High-Resolution CT Imaging of Carotid Artery Atherosclerotic Plaques. Am. J. Neuroradiol. 2008, 29, 875–882. [Google Scholar] [CrossRef]
- Sharashidze, V.; Nogueira, R.G.; Al-Bayati, A.R.; Bhatt, N.; Nahab, F.B.; Yun, J.; Allen, J.W.; Frankel, M.; Haussen, D.C. Carotid Web Phenotype Is Uncommonly Associated With Classic Fibromuscular Dysplasia: A Retrospective Observational Study. Stroke 2022, 53, E33–E36. [Google Scholar] [CrossRef]
- Zeng, F.; Pan, Q.; Wang, X.; Wang, Z.; Ni, J. The Efficacy and Safety of Endovascular Treatment on Large Vessel Occlusion of Intracranial Atherosclerosis Versus Embolism: A Meta-Analysis. Cardiol. Rev. 2024. [Google Scholar] [CrossRef]
- Guglielmi, V.; Compagne, K.C.J.; Sarrami, A.H.; Sluis, W.M.; van den Berg, L.A.; van der Sluijs, P.M.; Mandell, D.M.; van der Lugt, A.; Roos, Y.B.W.E.M.; Majoie, C.B.L.M.; et al. Assessment of Recurrent Stroke Risk in Patients With a Carotid Web. JAMA Neurol. 2021, 78, 826–833. [Google Scholar] [CrossRef]
- Haussen, D.C.; Grossberg, J.A.; Bouslama, M.; Pradilla, G.; Belagaje, S.; Bianchi, N.; Allen, J.W.; Frankel, M.; Nogueira, R.G. Carotid Web (Intimal Fibromuscular Dysplasia) Has High Stroke Recurrence Risk and Is Amenable to Stenting. Stroke 2017, 48, 3134–3137. [Google Scholar] [CrossRef]
- Marnat, G.; Holay, Q.; Darcourt, J.; Desilles, J.-P.; Obadia, M.; Viguier, A.; Caroff, J.; Denier, C.; Papillon, L.; Barreau, X.; et al. Dual-layer carotid stenting for symptomatic carotid web: Results from the Caroweb study. J. Neuroradiol. 2022, 50, 444–448. [Google Scholar] [CrossRef]
- Musiałek, P.; Tekieli, L.; Umemoto, T. Carotid Antiembolic (“Mesh”) Stents: Not Created Equal. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 102429. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K. Clinical Insights on My Acute Ischemic Stroke Caused by a Carotid Web. Cureus 2024, 16, e76093. [Google Scholar] [CrossRef]
- Takahashi, T.; Yanaka, K.; Aiyama, H.; Saura, M.; Kajita, M.; Takahashi, N.; Marushima, A.; Matsumaru, Y.; Ishikawa, E. Spontaneous asymptomatic common carotid artery dissection resembling a carotid web: Illustrative case. J. Neurosurg. Case Lessons 2024, 8, CASE24344. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, K.; Milburn, J.; Vidal, G.; Steven, A. Carotid Webs: Radiographic Appearance and Significance. Ochsner J. 2018, 18, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Perez, R.; Lownie, S.P.; Pandey, S.K.; Boulton, M.R. Stent Placement for Carotid Web. World Neurosurg. 2017, 98, 879.e9–879.e11. [Google Scholar] [CrossRef]
- Elmokadem, A.H.; Ansari, S.A.; Sangha, R.; Prabhakaran, S.; Shaibani, A.; Hurley, M.C. Neurointerventional management of carotid webs associated with recurrent and acute cerebral ischemic syndromes. Interv. Neuroradiol. 2016, 22, 432–437. [Google Scholar] [CrossRef]

| Case Reports | Age | Gender | Race | Stroke | Cardiovasc. Disease | Diabetes Mellitus | Smoking | Oral Contracept. | Medical Management of Stroke | Stroke Intervention | Carotid Intervention | Clinical |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | ||||||||||||
| Yokoyama K [33] | 46 | M | Asian | Yes | No | No | No | - | No | tPA, MT | CEA | Residual symptoms |
| Takahashi T et al. [34] | 50 | F | Asian | Yes | No | No | No | - | No | tPA, MT Decompressive craniectomy | CEA | Partial recovery |
| Kyaw K et al. [13] | 20 | F | Caucasian | Yes | No | No | No | Yes | Yes | No | No | Poor recovery |
| Wojcik K et al. [35] | 45 | F | - | Yes, r | - | - | - | - | Yes | No | CEA | Partial recovery |
| 44 | F | - | Yes | - | - | - | - | No | tPA, MT | CAS * | Full recovery | |
| 52 | F | - | Yes | - | - | - | - | No | tPA | No | Probable recovery | |
| 47 | M | - | TIA | - | - | - | - | Yes | No | No | Full recovery | |
| 51 | F | - | Yes | - | - | - | - | No | tPA, MT | CAS | Partial recovery | |
| Martinez-Perez R et al. [36] | 47 | F | - | Yes | - | - | - | - | No | MT | CAS | Full recovery |
| Elmokadem AH et al. [37] | 36 | M | - | Yes, r | AH | No | No | - | No | MT | CAS * | Residual symptoms |
| 41 | F | - | Yes, r | No | No | No | - | No | MT | CAS * | Residual symptoms | |
| Our case | 59 | M | Caucasian | Yes | AH | No | No | - | No | MT | CAS ** | Full recovery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konieczna-Brazis, M.; Brazis, P.; Switonska, M.; Migdalski, A. Carotid Web as a Cause of Ischemic Stroke: Effective Treatment with Endovascular Techniques. J. Clin. Med. 2025, 14, 2568. https://doi.org/10.3390/jcm14082568
Konieczna-Brazis M, Brazis P, Switonska M, Migdalski A. Carotid Web as a Cause of Ischemic Stroke: Effective Treatment with Endovascular Techniques. Journal of Clinical Medicine. 2025; 14(8):2568. https://doi.org/10.3390/jcm14082568
Chicago/Turabian StyleKonieczna-Brazis, Magdalena, Pawel Brazis, Milena Switonska, and Arkadiusz Migdalski. 2025. "Carotid Web as a Cause of Ischemic Stroke: Effective Treatment with Endovascular Techniques" Journal of Clinical Medicine 14, no. 8: 2568. https://doi.org/10.3390/jcm14082568
APA StyleKonieczna-Brazis, M., Brazis, P., Switonska, M., & Migdalski, A. (2025). Carotid Web as a Cause of Ischemic Stroke: Effective Treatment with Endovascular Techniques. Journal of Clinical Medicine, 14(8), 2568. https://doi.org/10.3390/jcm14082568









