Abstract
Food cobalamin malabsorption (FCM) represents a prevalent, often underdiagnosed, etiology of vitamin B12 deficiency, particularly within an aging population. Unlike pernicious anemia, an autoimmune disorder targeting intrinsic factor, FCM stems from the impaired release of cobalamin from food proteins, primarily due to age-related gastric changes, medication-induced gastric hypochlorhydria, metformin, or non-immune atrophic gastritis. The clinical presentation of FCM mirrors that of general cobalamin deficiency, encompassing a spectrum of neurological (peripheral neuropathy, cognitive decline), hematological (megaloblastic anemia), and gastrointestinal (glossitis, anorexia) manifestations. Given the potential for irreversible neurological sequelae, early detection and intervention are paramount. High-dose oral cobalamin (125–250 mcg daily) has demonstrated efficacy, offering a convenient and cost-effective alternative to parenteral administration.
1. Introduction
Cobalamin (vitamin B12) is a vitamin found in fish, red meat, and dairy products. Vitamin B12 in animal food is bound to a protein which is subsequently broken down in the stomach by pepsin and hydrochloric acid to release free vitamin B12. This is afterwards bound to the R-protein (haptocorrin) found in saliva and gastric juice. From the enterohepatic circulation, the vitamin B12-R-protein complex is also secreted in bile. These complexes are then degraded by pancreatic enzymes to release free vitamin B12 in the duodenum. The free vitamin B12 is afterwards bound to intrinsic factor secreted by the gastric parietal cells, and then it travels undisturbed until the distal 80 cm of the ileum where it binds to mucosal cell receptors. Subsequently, vitamin B12 is carried by the transport protein transcobalamin via the portal system to all cells in the body for utilization. About 60% of vitamin B12 from food is absorbed through this pathway, and any pathophysiological changes in the stomach, pancreas, and intestine result in the disturbance of vitamin B12 absorption [1].
Cobalamin (vitamin B12) deficiency, a seemingly simple nutritional deficit, continues to challenge clinicians with its diverse clinical presentations and the complexities of its underlying etiologies [2]. While pernicious anemia (PA, also named Biermer’s disease), an autoimmune disorder, has long been recognized as a primary cause, the often-overlooked condition of food cobalamin malabsorption (FCM) deserves renewed attention [3].
FCM was first described by Carmel in 1988 [4]. FCM is a condition where the body cannot effectively extract vitamin B12 from food, leading to deficiency despite adequate dietary intake [5]. The primary problem is an inability to release vitamin B12 from dietary proteins. Thus, affected individuals can absorb vitamin B12 supplements in which the vitamin is not protein-bound, but they are less able to absorb dietary vitamin B12 bound to food proteins due to conditions that interfere with dissociation of the vitamin from food proteins [6].
2. Methods
We conducted a review on food cobalamin malabsorption syndrome. A comprehensive search was performed in the PubMed, Embase, and Cochrane Library databases up to March 2025. Keywords used included “food-cobalamin malabsorption”, “cobalamin deficiency”, and “oral cobalamin therapy”. Single case studies and unpublished works in English or French were excluded. The bibliographic references of selected articles were also examined to identify additional studies. Discrepancies were resolved by consensus or consultation with a third researcher. This methodology aims to provide a reliable synthesis of the state of the art concerning food cobalamin malabsorption.
3. Epidemiology
FCM represents a significant yet frequently underdiagnosed cause of vitamin B12 deficiency, particularly in the elderly population. Prevalence of vitamin B12 deficiency in elderly patients was 40.5% compared with 17.5% in control subjects [7]. Mild and/or subclinical vitamin B12 deficiency has been documented at relatively high frequency in older adults, with various observational studies describing a prevalence of approximately 5 to 20% depending on the population studied and the laboratory criteria used to define deficiency [7,8,9,10]. Other studies indicate that up to 60% of individuals over 65 with vitamin B12 deficiency may have FCM [11]. Otherwise, FCM has been reported to cause vitamin B12 deficiency in 40% to 70% of individuals aged 65 and above [4,12,13].
The median patient age was 66 and 67 years in two studies of 80 and 201 patients, respectively, with a predominancy of female patients [13,14].
4. Pathophysiology
PA and FCM differ in pathophysiology. PA is an autominumme autoimmune disorder characterized by the destruction of gastric parietal cells (autoimmune atrophic gastritis), leading to intrinsic factor deficiency and impaired vitamin B12 absorption. The immune system can also produce antibodies that directly target intrinsic factor, preventing it from binding to vitamin B12. Intrinsic factor is essential for vitamin B12 absorption in the terminal ileum. The lack of intrinsic factor leads to vitamin B12 malabsorption, resulting in megaloblastic anemia and potential neurological complications.
Unlike PA, FCM stems from gastric achlorhydria or non-immune atrophic gastritis, leading to impaired proteolytic digestion of food-bound cobalamin (Figure 1) despite normal intrinsic factor production. Pancreatic dysfunction can also lead to FCM. Conditions such as chronic gastritis, long-term use of acid-suppressing medications, or pancreatic insufficiency lead to the inadequate release of vitamin B12 from food proteins.
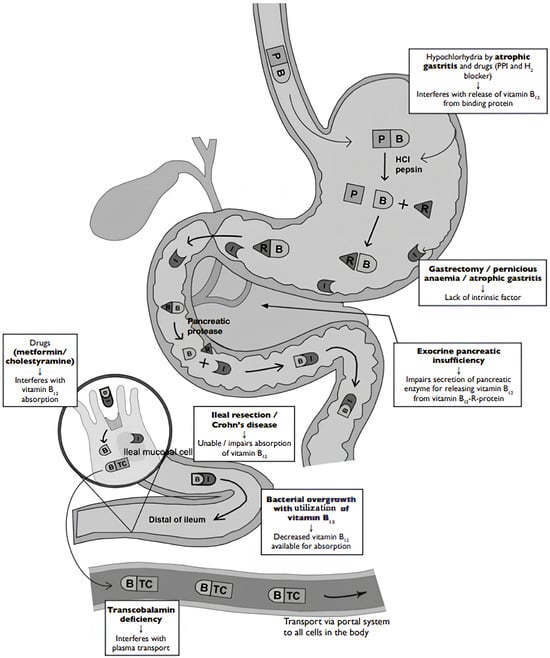
Figure 1.
Pathophysiology of pernicious anemia (Biermer’s disease) and food cobalamin malabsorption [13]. Legend: B: vitamin B12, HCI: hydrochloric acid, I: intrinsic factor, P: animal protein, PPI: proton pump inhibitor, R: protein, TC: transcobalamin.
Despite normal intrinsic factor production, vitamin B12 remains bound to food proteins and is not absorbed in the ileum. This malabsorption can cause vitamin B12 deficiency, leading to the same symptoms as PA. This distinction is important, as the diagnostic and therapeutic approaches differ significantly [15].
5. Etiology
Currently, the primary causes or associated conditions/factors of FCM mainly include advancing age, gastric disorders, Helicobacter pylori infection, and medications such as proton pump inhibitors (PPIs) and metformin (Table 1) [13,14]. A study of 80 internal medicine patients revealed that gastric atrophy was by far the most frequent cause (39%), followed by Helicobacter pylori infection (12.2%). Alcohol misuse and chronic intake of biguanides or PPIs/H2 receptor antagonists were associated with 13.7%, 10%, and 7.5%, respectively. Rarer causes such as systemic diseases (Sjögren’s syndrome and scleroderma) and pancreatic insufficiency were found in 5% and 3.8%. Finally, 12.5% of cases were idiopathic or age-related [14].

Table 1.
Causes or associated conditions/factors of food cobalamin malabsorption [13,14,15,16,17].
6. Clinical Manifestations
The clinical consequences of FCM can be insidious, mirroring those of other causes of cobalamin deficiency. However, patients with FCM may present with plain clinical and hematological manifestations, often masked by age-related comorbidities. Many individuals with food cobalamin malabsorption are not anemic and do not have other features of megaloblastic anemia, such as macrocytosis or hypersegmented neutrophils, sometimes referred to as subclinical deficiency (Table 2) [13,14,18].

Table 2.
Clinical and hematological manifestations related to cobalamin deficiency [13,14,18].
Because FCM can develop slowly, the symptoms may be very gradual and easily overlooked (subtle clinical manifestations) [4,19]. The milder symptoms seen in some patients can result from an IF-dependent transport mechanism that is still functioning when the hydrochloric acid (HCl) production is reduced.
Subtle neurological manifestations include mild paresthesia; slight sense of unsteadiness, particularly in low-light conditions or when walking on uneven surfaces; minor cognitive changes; and mood alterations with increased irritability, mild depression, or subtle changes in mood that may not be readily recognized as a medical issue [20]. Early detection and treatment are important to prevent irreversible neurological damage. Neurological manifestations are most frequent in internal medicine patients [14,20].
Subtle hematological manifestations have also been reported as mild fatigue, slight pallor, and occasional weakness [4,19]. In this context, occasional mild loss of appetite and minor digestive discomfort are noticed. In elderly populations, these subtle changes can significantly impact quality of life. Hematological manifestations, including macrocytic anemia, may be less pronounced or even absent, further complicating the diagnostic process. Other cytopenias may be found in these patients, but the discovery of a bicytopenia/pancytopenia should lead to a bone marrow aspiration or osteomedullary biopsy performance, especially in subjects over 65 years of age with no suggestive clinical context, in search of a hemopathy. Bone marrow aspiration or osteomedullary biopsy may be proposed depending on practice and country but always in conjunction with a molecular biology investigation (5q- syndrome, etc.) and a karyotype. These investigations will allow clinicians to avoid missing a myelodysplastic syndrome, which may be fortuitously associated with a B12 deficiency [14,20].
These subtle symptoms are often dismissed as normal aging or stress, leading to delayed diagnosis. Thus, it is important for clinicians to maintain a high index of suspicion for cobalamin deficiency, especially in elderly patients presenting with these vague symptoms [21].
Neuropsychiatric abnormalities can occur even in the absence of significant hematological changes [14,20]. Patients may also present no symptoms or clinical signs, 30% in our study [20].
7. Diagnosis
The diagnostic pathway for FCM requires a high index of suspicion [6,11]. While serum cobalamin levels remain the initial screening tool, they may not always accurately reflect tissue cobalamin status. Assessing methylmalonic acid (MMA) and homocysteine levels can provide valuable insights into metabolic cobalamin deficiency [22]. Importantly, intrinsic factor antibodies are typically absent, differentiating FCM from PA [5,6]. Furthermore, the Schilling test, although less frequently performed today, can aid in differentiating FCM from PA.
The Schilling test, introduced in 1953, has historically been considered the gold standard for diagnosing FCM [4]. This test involves administering a radiolabeled dose of vitamin B12 and measuring its excretion in the urine to assess absorption efficiency. However, the Schilling test and its modified versions are no longer widely available in clinical practice. Consequently, diagnosing FCM often relies on a combination of clinical evaluation, laboratory findings indicating vitamin B12 deficiency, and the exclusion of other potential causes of malabsorption [6].
While the Schilling test was once the definitive diagnostic tool for FCM, its current unavailability necessitates a comprehensive approach that integrates patient history, clinical symptoms, and laboratory assessments to accurately diagnose FCM (Table 3) [3,4,6,23].

Table 3.
Diagnostic criteria of food cobalamin malabsorption [3,4,6,23].
8. Treatment
The therapeutic approach to FCM differs from that of PA [24,25,26,27,28,29,30]. Traditionally, vitamin B12 deficiency has been treated with intramuscular injections because of the probability of low absorption in some conditions [29]. Treatment regimens are not based on robust studies and differ from center to center and country to country, but it would seem preferable to progressively increase the interval between two injections [26,27].
However, research indicates that oral cobalamin therapy is an effective alternative for managing FCM. Oral cobalamin supplementation, often at higher doses (650 µg per day), is generally effective in replenishing cobalamin stores [30]. Unlike PA, which typically requires lifelong parenteral cobalamin, oral supplementation can be a practical and cost-effective option for patients with FCM. If the symptoms are acute and potentially serious, the intramuscular form is often preferred by the majority of practitioners. The oral form is just as effective as the IM form if therapeutic compliance is good, and if the efficacy of treatment in terms of vitamin B12 and normalization of total homocysteine is monitored, even in severe forms. It may be necessary to switch from oral to intramuscular and vice versa depending on the patient’s response [31].
Multiple studies have demonstrated the effectiveness of oral vitamin B12 in treating FCM (Table 4) [21]. A systematic review showed that oral cobalamin therapy adequately corrects cobalamin deficiency in patients with FCM. In our experience, oral supplementation of FCM patients achieved satisfactory hemoglobin and vitamin B12 levels (11.5 g/dL + 1.9 g/dL over 3 months), with higher figures for IM treatment (13.2 g/dL + 3.9 g/dL) [14].

Table 4.
Cobalamin treatment: oral vs. parenteral [2,24,26].
Research suggests that a daily dose of approximately 250 micrograms of oral cyanocobalamin is sufficient for managing FCM [24,32]. Regular monitoring of vitamin B12 levels is essential to ensure therapeutic efficacy, check compliance with oral treatment, and adjust dosages as needed. While oral therapy is effective for many, individual absorption rates may vary [24]. Some patients might still require intramuscular injections, especially if they have conditions affecting intestinal absorption [2].
9. Conclusions
FCM is a common, yet commonly missed, disorder that may be masked by many other conditions. The challenge lies in the under-recognition of FCM. The subtle clinical presentation, the overlap with age-related conditions, and the potential for normal or borderline serum cobalamin levels can lead to diagnostic delays. Increased awareness among clinicians regarding the prevalence and clinical manifestations of FCM is important as well as iatrogenic causes such as PPI treatment.
Funding
This research received no external funding.
Conflicts of Interest
The authors declared no conflicts of interest.
References
- Guéant, J.L.; Guéant-Rodriguez, R.M.; Alpers, D.H. Chapter Nine—Vitamin B12 absorption and malabsorption. In Vitamins and Hormones; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 119, pp. 241–274. Available online: https://www.sciencedirect.com/science/article/pii/S0083672922000164 (accessed on 27 March 2025).
- NICE. CKS Anaemia—B12 and Folate Deficiency. 2023. Available online: https://cks.nice.org.uk/topics/anaemia-b12-folate-deficiency (accessed on 29 December 2023).
- Jajoo, S.S.; Zamwar, U.M.; Nagrale, P. Etiology, Clinical Manifestations, Diagnosis, and Treatment of Cobalamin (Vitamin B12) Deficiency. Cureus 2024, 16, e52153. [Google Scholar] [CrossRef] [PubMed]
- Carmel, R.; Sinow, R.M.; Siegel, M.E.; Samloff, I.M. Food cobalamin malabsorption occurs frequently in patients with unexplained low serum cobalamin levels. Arch. Intern. Med. 1988, 148, 1715–1719. [Google Scholar] [PubMed]
- Pardo-Cabello, A.J.; Manzano-Gamero, V.; Puche-Cañas, E. Vitamin B12: For more than just the treatment of megaloblastic anemia? Rev. Clin. Esp. 2023, 223, 114–119. [Google Scholar]
- Green, R.; Miller, J.W. Vitamin B12 deficiency. Vitam. Horm. 2022, 119, 405–439. [Google Scholar]
- Lindenbaum, J.; Rosenberg, I.H.; Wilson, P.W.; Stabler, S.P.; Allen, R.H. Prevalence of cobalamin deficiency in the Framingham elderly population. Am. J. Clin. Nutr. 1994, 60, 2. [Google Scholar]
- Wolffenbuttel, B.H.R.; Wouters, H.J.C.M.; Heiner-Fokkema, M.R.; van der Klauw, M.M. The Many Faces of Cobalamin (Vitamin B12) Deficiency. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 200–214. [Google Scholar] [PubMed]
- Figlin, E.; Chetrit, A.; Shahar, A.; Shpilberg, O.; Zivelin, A.; Rosenberg, N.; Brok-Simoni, F.; Gadoth, N.; Sela, B.-A.; Seligsohn, U. High prevalences of vitamin B12 and folic acid deficiency in elderly subjects in Israel. Br. J. Haematol. 2003, 123, 696. [Google Scholar] [CrossRef]
- Clarke, R.; Grimley Evans, J.; Schneede, J.; Nexo, E.; Bates, C.; Fletcher, A.; Prentice, A.; Johnston, C.; Ueland, P.M.; Refsum, H.; et al. Vitamin B12 and folate deficiency in later life. Age Ageing 2004, 33, 34. [Google Scholar] [CrossRef]
- van Asselt, D.Z.; de Groot, L.C.; van Staveren, W.A.; Blom, H.J.; Wevers, R.A.; Biemond, I.; Hoefnagels, W.H. Role of cobalamin intake and atrophic gastritis in mild cobalamin deficiency in older Dutch subjects. Am. J. Clin. Nutr. 1998, 68, 328. [Google Scholar] [CrossRef]
- Allen, L.H. How common is vitamin B-12 deficiency? Am. J. Clin. Nutr. 2009, 89, 693S–696S. [Google Scholar]
- Andrès, E.; Affenberger, S.; Zimmer, J.; Vinzio, S.; Grosu, D.; Pistol, G.; Maloisel, F.; Noel, E.; Kaltenbach, G.; Blickle, J.F. Current hematological findings in cobalamin deficiency. A study of 201 consecutive patients with documented cobalamin deficiency. Clin. Lab. Haematol. 2006, 28, 50–56. [Google Scholar] [CrossRef]
- Andrès, E.; Perrin, A.E.; Demangeat, C.; Kurtz, J.E.; Vinzio, S.; Grunenberger, F.; Goichot, B.; Schlienger, J.L. The syndrome of food-cobalamin malabsorption revisited in a Department of Internal Medicine. A monocentric cohort study of 80 patients. Eur. J. Intern. Med. 2003, 14, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Carmel, R. Subclinical cobalamin deficiency. Curr. Opin. Gastroenterol. 2012, 28, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Tas Kilic, D.; Akdogan, A.; Kilic, L.; Sari, A.; Erden, A.; Armagan, B.; Kilickaya, M.; Kalyoncu, U.; Turhan, T.; Kiraz, S.; et al. Evaluation of Vitamin B12 Deficiency and Associated Factors in Patients With Systemic Sclerosis. J. Clin. Rheumatol. 2018, 24, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Urbanski, G.; Chabrun, F.; Schaepelynck, B.; May, M.; Loiseau, M.; Schlumberger, E.; Delattre, E.; Lavigne, C.; Lacombe, V. Association of Primary Sjögren’s Syndrome and Vitamin B12 Deficiency: A Cross-Sectional Case-Control Study. J. Clin. Med. 2020, 9, 4063. [Google Scholar] [CrossRef]
- Andrès, E.; Zulfiqar, A.A.; Vogel, T. State of the art review: Oral and nasal vitamin B12 therapy in the elderly. QJM 2020, 113, 5–15. [Google Scholar] [CrossRef]
- Andrès, E.; Noel, E.; Kaltenbach, G.; Perrin, A.E.; Vinzio, S.; Goichot, B.; Schlienger, J.L.; Blickle, J.F. Carences en vitamine B12 avec test de Schilling normal ou syndrome de non-dissociation de la vitamine B12 de ses protéines porteuses chez le sujet âgé. Etude de 60 patients. Rev. Med. Interne 2003, 24, 218–223. [Google Scholar] [CrossRef]
- Hunt, A.; Harrington, D.; Robinson, S. Vitamin B12 deficiency. BMJ 2014, 349, g5226. [Google Scholar] [CrossRef]
- Wolffenbuttel, B.H.; Owen, P.J.; Ward, M.; Green, R. Vitamin B12. BMJ 2023, 383, e071725. [Google Scholar] [CrossRef]
- Wong, C.W. Vitamin B12 deficiency in the elderly: Is it worth screening? Hong Kong Med. J. 2015, 21, 155–164. [Google Scholar] [CrossRef]
- Hvas, A.M.; Morkbak, A.L.; Hardlei, T.F.; Nexo, E. The vitamin B12 absorption test, CobaSorb, identifies patients not requiring vitamin B12 injection therapy. Scand. J. Clin. Lab. Investig. 2011, 71, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Wolffenbuttel, B.H.R.; McCaddon, A.; Ahmadi, K.R.; Green, R. A Brief Overview of the Diagnosis and Treatment of Cobalamin (B12) Deficiency. Food Nutr. Bull. 2024, 45 (Suppl. S1), S40–S49. [Google Scholar] [CrossRef]
- Sukumar, N.; Saravanan, P. Investigating vitamin B12 deficiency. BMJ 2019, 365, l1865. [Google Scholar] [PubMed]
- Wang, H.; Li, L.; Qin, L.L.; Song, Y.; Vidal-Alaball, J.; Liu, T.H. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency. Cochrane Database Syst. Rev. 2018, 3, CD004655. [Google Scholar]
- Masucci, L.; Goeree, R. Vitamin B12 intramuscular injections versus oral supplements: A budget impact analysis. Ont. Health Technol. Assess. Ser. 2013, 13, 1–24. [Google Scholar] [PubMed]
- Abdelwahab, O.A.; Abdelaziz, A.; Diab, S.; Khazragy, A.; Elboraay, T.; Fayad, T.; Diab, R.A.; Negida, A. Efficacy of different routes of vitamin B12 supplementation for the treatment of patients with vitamin B12 deficiency: A systematic review and network meta-analysis. Ir. J. Med. Sci. 2024, 193, 1621–1639. [Google Scholar]
- Sanz-Cuesta, T.; Escortell-Mayor, E.; Cura-Gonzalez, I.; Martin-Fernandez, J.; Riesgo-Fuertes, R.; Garrido-Elustondo, S.; Mariño-Suárez, J.E.; Álvarez-Villalba, M.; Gómez-Gascón, T.; González-García, I.; et al. Oral versus intramuscular administration of vitamin B12 for vitamin B12 deficiency in primary care: A pragmatic, randomized, non-inferiority clinical trial (OB12). BMJ Open 2020, 10, e033687. [Google Scholar] [CrossRef]
- Bensky, M.J.; Ayalon-Dangur, I.; Ayalon-Dangur, R.; Naamany, E.; Gafter-Gvili, A.; Koren, G.; Shiber, S. Comparison of sublingual vs. intramuscular administration of vitamin B12 for the treatment of patients with vitamin B12 deficiency. Drug Deliv. Transl. Res. 2019, 9, 625–630. [Google Scholar]
- Tank, N.; Wood, C.; Ahankari, A.; Mackenna, B.; Walker, A.; Lemanska, A. Vitamin B12 prescribing from 2015 to 2024 in English general practice: An observational study to investigate the switch from injections to tablets. BMJ Open 2025, 15, e093748. [Google Scholar] [CrossRef]
- Andrès, E.; Kurtz, J.E.; Perrin, A.E.; Maloisel, F.; Demangeat, C.; Goichot, B.; Schlienger, J.L. Oral cobalamin therapy for the treatment of patients with food-cobalamin malabsorption. Am. J. Med. 2001, 111, 126–129. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).