The Efficacy of 532/755 nm Laser Therapy for Facial Pigmented and Vascular Lesions: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Inclusion Criteria
- hemangiomas
- vascular malformations
- hyperpigmentation
- freckles
- chloasma/ostuda/melasma
- nevoid hypermelanosis
- ephilides
- lentigines
- port wine stain
- nevus of Ota-like macules
2.2. Data Extraction and Outcomes
2.3. Data Synthesis and Statistical Analyses
2.4. Risk of Bias
3. Results
3.1. Search Results
3.2. Study and Subject Characteristics
3.3. Clinical Efficacy of 532 and 755 Lasers Compared to Other Lasers
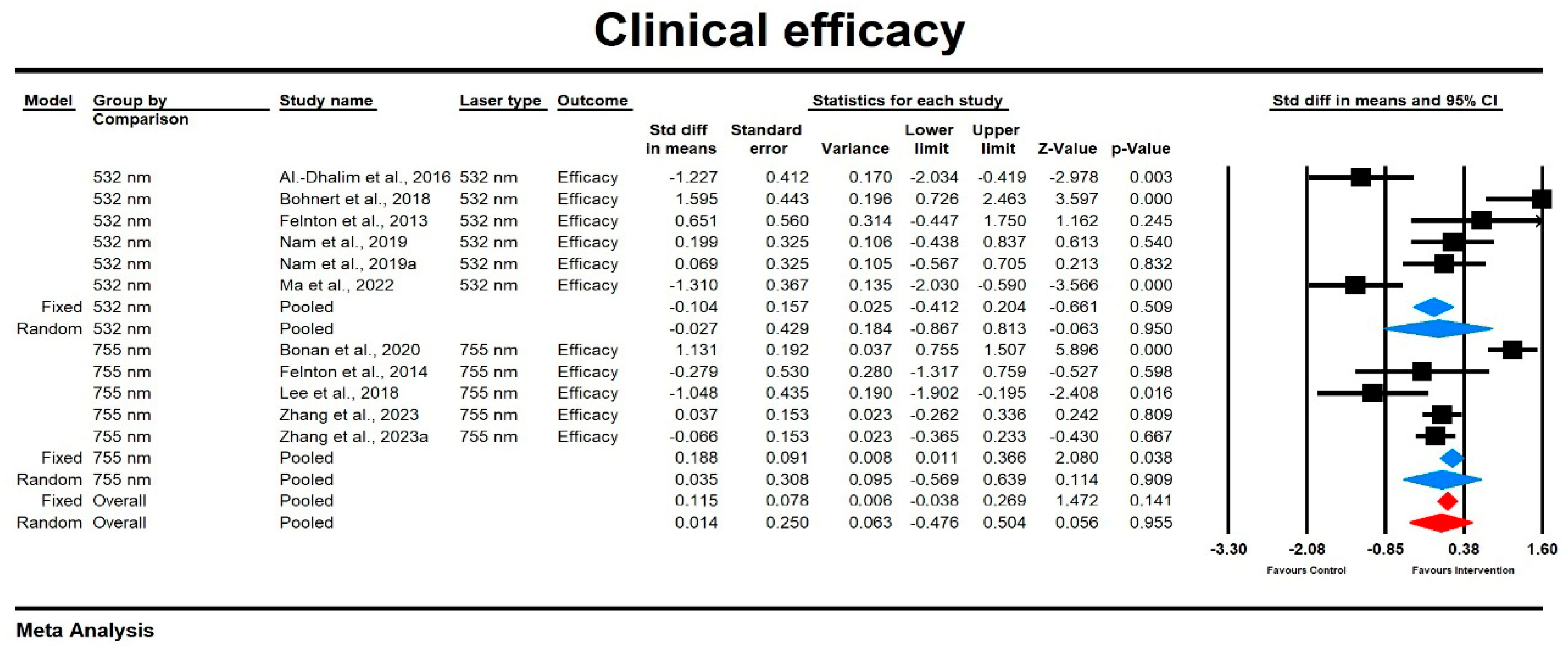
3.3.1. Improvement
Improvement Score
Relative Risk for Improvement
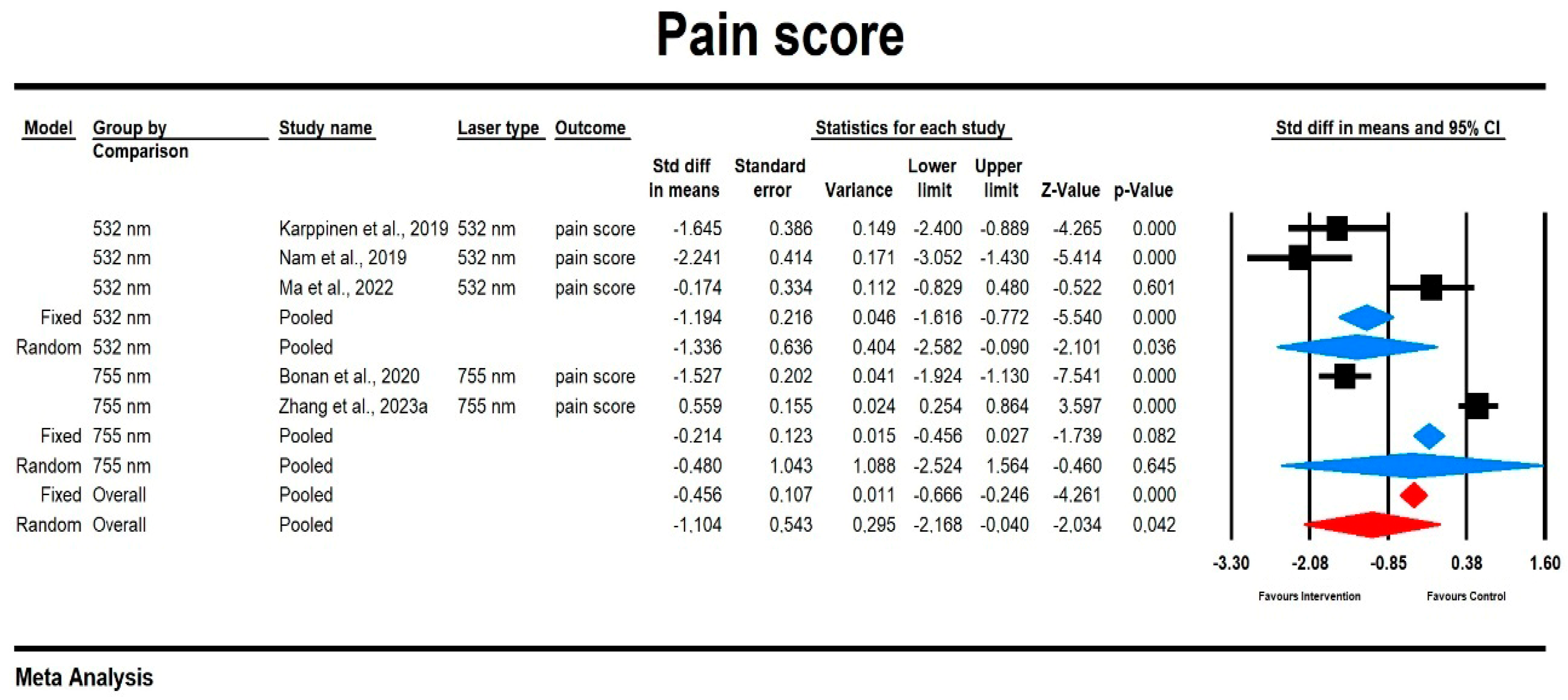
3.3.2. Pain
Pain Score
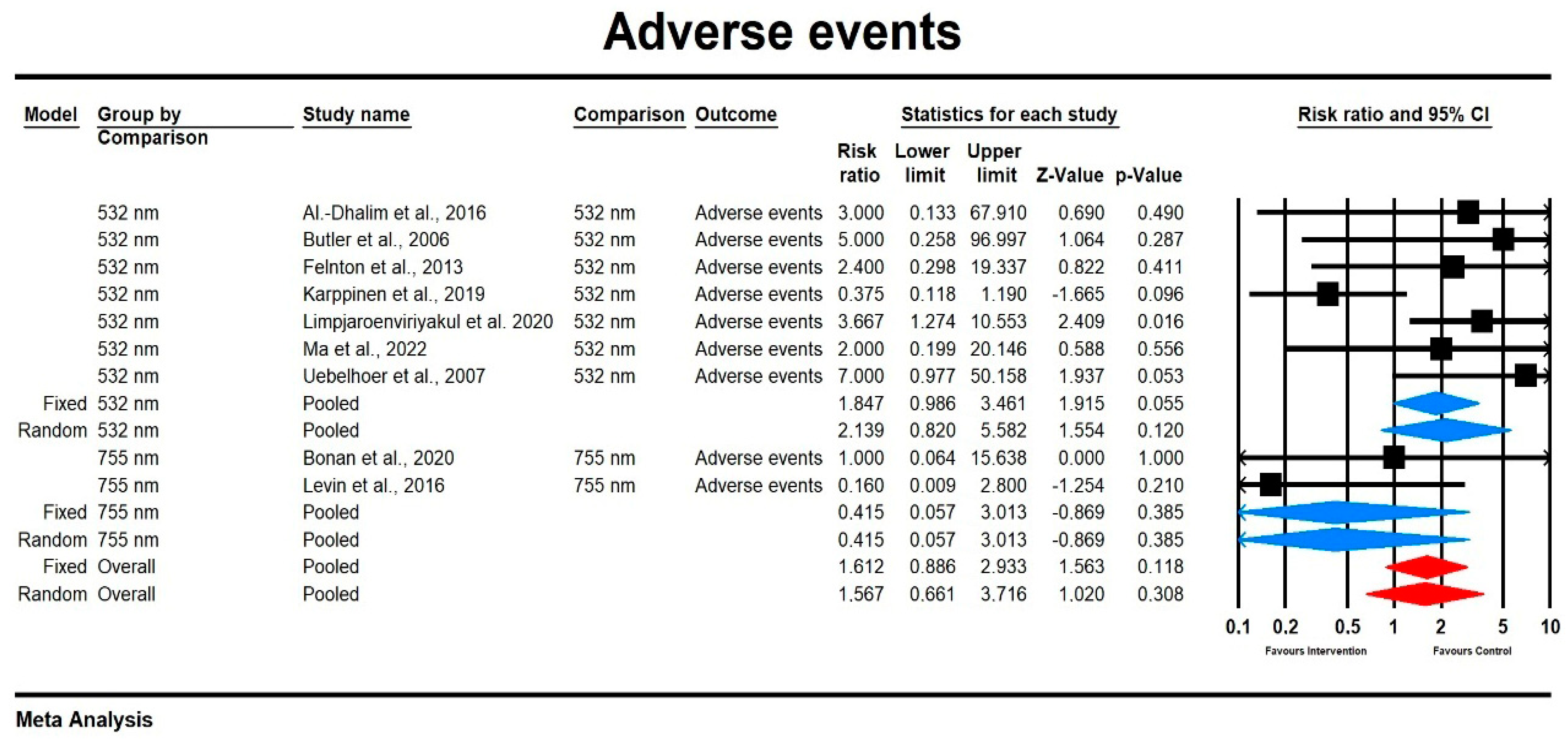
3.3.3. Adverse Events
Relative Risk for Adverse Events
3.3.4. Patient Satisfaction
Patient Satisfaction Score and Relative Risk
3.4. Meta-Regression Analyses
3.5. Risk of Bias
- Random Sequence Generation (Selection Bias): All studies demonstrated a “Low Risk” of selection bias, suggesting that the randomization process was robust.
- Allocation Concealment (Selection Bias): Most studies maintained a low risk by ensuring allocation concealment, though a few studies were rated as “Unclear” due to insufficient reporting.
- Blinding of Participants and Personnel (Performance Bias): While blinding was achieved in several studies, approximately one-third had a “High Risk” due to lack of blinding of participants and personnel, which could potentially impact performance bias.
- Blinding of Outcome Assessment (Detection Bias): Most studies ensured low risk for outcome assessment blinding, although some had “Unclear” or “High Risk” ratings due to partial or no blinding of the outcome assessors.
- Incomplete Outcome Data (Attrition Bias): Nearly all studies were rated as “Low Risk”, indicating good data completeness. Only one study had unclear attrition data, which may affect short-term outcomes.
- Selective Reporting (Reporting Bias): All studies showed a low risk of reporting bias, suggesting consistency in reporting all specified outcomes.
- Other Bias: Only one study presented a potential source of other bias due to a device manufacturer affiliation, while all others were free from additional noted biases.
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Confidence Interval |
| Cochran Risk-of-Bias Tool | Cochrane Risk-of-Bias Tool for Randomized Trials |
| CINAHL | Cumulative Index to Nursing and Allied Health Literature |
| Embase | Excerpta Medica Database |
| IPL | Intense Pulsed Light |
| I2 | I-Squared Statistic |
| KTP laser | Potassium Titanyl Phosphate Laser |
| MMASI | Melasma Area and Severity Index |
| Nd: YAG laser | Neodymium-Doped Yttrium Aluminum Garnet Laser |
| Nd laser | Neodymium Laser |
| OC | Observed Cases |
| PIH | Post-inflammatory Hyperpigmentation |
| PDL | Pulsed Dye Laser |
| PL | Photolysis |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| Q Statistic, Q-value | Q Statistic |
| Q-switched laser | Q-switched Laser |
| RCTs | Randomized Controlled Trials |
| RR | Risk Ratio |
| ROB | Risk of Bias |
| SE | Standard Error |
| SMD | Standardized Mean Difference |
| SP | Standard Pulsed |
| τ2 | Between-Study Variance |
| USA | United States of America |
| VAS | Visual Analog Scale |
| WHO | World Health Organization |
| n | Number |
| nm | Nanometer |
| p | p-value |
| MoveoPL mode | Moveo Photo Light Mode |
| PubMed | Public/Publisher MEDLINE |
References
- Barone, M.; De Bernardis, R.; Persichetti, P. Aesthetic Medicine Across Generations: Evolving Trends and Influences. Aesthetic Plast. Surg. 2024, 1–3. [Google Scholar] [CrossRef]
- Khavkin, J.; Ellis, D.A.F. Aging Skin: Histology, Physiology, and Pathology. Facial Plast. Surg. Clin. N. Am. 2011, 19, 229–234. [Google Scholar] [CrossRef]
- Cestari, T.F.; Dantas, L.P.; Boza, J.C. Acquired Hyperpigmentations. An. Bras. Dermatol. 2014, 89, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak-Stefańska, V.; Maleszka, R.; Boer, M.; Kiedrowicz, M. Erythematotelangiectatic skin–diagnostic difficulties. Ann. Acad. Med. Stetin. 2009, 55, 58–65, discussion 65. [Google Scholar] [PubMed]
- Zhou, Y.; Hamblin, M.R.; Wen, X. An Update on Fractional Picosecond Laser Treatment: Histology and Clinical Applications. Lasers Med. Sci. 2023, 38, 45. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.R.; Kaufman, J.; Metelitsa, A.I.; Green, J.B. Picosecond Lasers: The next Generation of Short-Pulsed Lasers. Semin. Cutan. Med. Surg. 2014, 33, 164–168. [Google Scholar] [CrossRef]
- Haykal, D.; Cartier, H.; Maire, C.; Mordon, S. Picosecond Lasers in Cosmetic Dermatology: Where Are We Now? An Overview of Types and Indications. Lasers Med. Sci. 2023, 39, 8. [Google Scholar] [CrossRef]
- Zawodny, P.; Stój, E.; Kulig, P.; Skonieczna-Żydecka, K.; Sieńko, J. VISIA Skin Analysis System as a Tool to Evaluate the Reduction of Pigmented Skin and Vascular Lesions Using the 532 Nm Laser. CCID 2022, 15, 2187–2195. [Google Scholar] [CrossRef]
- Zawodny, P.; Malec, W.; Gill, K.; Skonieczna-Żydecka, K.; Sieńko, J. Assessment of the Effectiveness of Treatment of Vascular Lesions within the Facial Skin with a Laser with a Wavelength of 532 Nm Based on Photographic Diagnostics with the Use of Polarized Light. Sensors 2023, 23, 1010. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- RoB 2: A Revised Cochrane Risk-of-Bias Tool for Randomized Trials|Cochrane Bias. Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 1 November 2024).
- Al-Dhalimi, M.A.; Al-Janabi, M.H. Split Lesion Randomized Comparative Study between Long Pulsed Nd:YAG Laser 532 and 1064 Nm in Treatment of Facial Port-Wine Stain. Lasers Surg. Med. 2016, 48, 852–858. [Google Scholar] [CrossRef]
- Bohnert, K.; Dorizas, A.; Sadick, N. A Prospective, Randomized, Double-Blinded, Split-Face Pilot Study Comparing Q-Switched 1064-Nm Nd:YAG versus 532-Nm Nd:YAG Laser for the Treatment of Solar Lentigines. J. Cosmet. Laser Ther. 2018, 20, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Butler, E.G.; McClellan, S.D.; Ross, E.V. Split Treatment of Photodamaged Skin with KTP 532 Nm Laser with 10 Mm Handpiece versus IPL: A Cheek-to-Cheek Comparison. Lasers Surg. Med. 2006, 38, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Felton, S.J.; Al-Niaimi, F.; Ferguson, J.E.; Madan, V. Our Perspective of the Treatment of Naevus of Ota with 1,064-, 755- and 532-Nm Wavelength Lasers. Lasers Med. Sci. 2014, 29, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Karppinen, T.; Kantola, E.; Karppinen, A.; Rantamäki, A.; Kautiainen, H.; Mordon, S.; Guina, M. Treatment of Telangiectasia on the Cheeks with a Compact Yellow (585 Nm) Semiconductor Laser and a Green (532 Nm) KTP Laser: A Randomized Double-Blinded Split-Face Trial. Lasers Surg. Med. 2019, 51, 223–229. [Google Scholar] [CrossRef]
- Limpjaroenviriyakul, N.; Jurairattanaporn, N.; Kamanamool, N.; Rojhirunsakool, S.; Kanokrungsee, S.; Udompataikul, M. Low-Fluence Q-Switched Nd:YAG 1064-Nm Laser versus Q-Switched Nd:YAG 532-Nm Laser in the Treatment of Hyperpigmented Lips: A Prospective, Randomized, Controlled, Evaluator-Blinded Trial. Lasers Med. Sci 2020, 35, 165–171. [Google Scholar] [CrossRef]
- Nam, C.H.; Kim, M.H.; Hong, S.P.; Park, B.C. Fractional 532-Nm KTP Diode Laser and 595-Nm Pulsed Dye Laser in Treatment of Facial Telangiectatic Erythema. J. Cosmet. Dermatol. 2019, 18, 783–787. [Google Scholar] [CrossRef]
- Ma, S.Y.; Gong, Y.Q.; Zhang, W.J.; Liang, B.H.; Li, Y.M.; Xie, Z.M.; Zhu, H.L. Split-Face Comparison of the Efficacy of Picosecond 532 Nm Nd:YAG Laser and Q-Switched 755 Nm Alexandrite Laser for Treatment of Freckles. J. Cosmet. Laser Ther. 2022, 24, 22–27. [Google Scholar] [CrossRef]
- Uebelhoer, N.S.; Bogle, M.A.; Stewart, B.; Arndt, K.A.; Dover, J.S. A Split-Face Comparison Study of Pulsed 532-Nm KTP Laser and 595-Nm Pulsed Dye Laser in the Treatment of Facial Telangiectasias and Diffuse Telangiectatic Facial Erythema. Dermatol. Surg. 2007, 33, 441. [Google Scholar] [PubMed]
- West, T.B.; Alster, T.S. Comparison of the Long-Pulse Dye (590–595 Nm) and KTP (532 Nm) Lasers in the Treatment of Facial and Leg Telangiectasias. Dermatol. Surg. 1998, 24, 221. [Google Scholar] [CrossRef] [PubMed]
- Bonan, P.; Troiano, M.; Bruscino, N.; Verdelli, A. Treatment of Benign Hyperpigmentations and Pigmented Scars by 755 Alexandrite Laser Comparing the Single Pass versus MultiPass (MoveoPL) Emission in Skin Types I-IV. Dermatol. Ther. 2021, 34, e14819. [Google Scholar] [CrossRef]
- Fabi, S.G.; Friedmann, D.P.; Niwa Massaki, A.B.; Goldman, M.P. A Randomized, Split-Face Clinical Trial of Low-Fluence Q-Switched Neodymium-Doped Yttrium Aluminum Garnet (1064 Nm) Laser versus Low-Fluence Q-Switched Alexandrite Laser (755 Nm) for the Treatment of Facial Melasma. Lasers Surg. Med. 2014, 46, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-C.; Lin, Y.-F.; Hu, S.; Huang, Y.-L.; Chang, S.-L.; Cheng, C.-Y.; Chang, C.-S. A Split-Face Study: Comparison of Picosecond Alexandrite Laser and Q-Switched Nd:YAG Laser in the Treatment of Melasma in Asians. Lasers Med. Sci. 2018, 33, 1733–1738. [Google Scholar] [CrossRef]
- Lee, Y.B.; Shin, J.Y.; Cheon, M.S.; Oh, S.T.; Cho, B.K.; Park, H.J. Photorejuvenation Using Long-Pulsed Alexandrite and Long-Pulsed Neodymium:Yttrium-Aluminum-Garnet Lasers: A Pilot Study of Clinical Outcome and Patients’ Satisfaction in Koreans. J. Dermatol. 2012, 39, 425–429. [Google Scholar] [CrossRef]
- Levin, M.K.; Ng, E.; Bae, Y.C.; Brauer, J.A.; Geronemus, R.G. Treatment of Pigmentary Disorders in Patients with Skin of Color with a Novel 755 Nm Picosecond, Q-Switched Ruby, and Q-Switched Nd:YAG Nanosecond Lasers: A Retrospective Photographic Review. Lasers Surg. Med. 2016, 48, 181–187. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, H.; Ge, Y.; Yang, Y.; Guo, L.; Wu, Q.; Zeng, R.; Shi, H.; Huang, Y.; Zhao, W.; et al. Comparison of the Efficacy and Safety of a 730 Nm Picosecond Titanium Sapphire Laser and a 755 Nm Picosecond Alexandrite Laser for the Treatment of Freckles in Asian Patients: A Two-Center Randomized, Split-Face, Controlled Trial. Lasers Surg. Med. 2023, 55, 636–641. [Google Scholar] [CrossRef]
- Zawodny, P.; Wahidi, N.; Zawodny, P.; Duchnik, E.; Stój, E.; Malec, W.R.; Kulaszyńska, M.; Skonieczna-Żydecka, K.; Sieńko, J. Evaluation of the Efficacy of the 755 Nm Picosecond Laser in Eliminating Pigmented Skin Lesions after a Single Treatment Based on Photographic Analysis with Polarised Light. J. Clin. Med. 2024, 13, 304. [Google Scholar] [CrossRef]
- Pigment—Detailed Explanation. Available online: https://cambridgelaserclinic.com/laser-treatments/pigment/detailed-explanation/ (accessed on 3 January 2025).
- Laser and Light-Based Treatments for Pigmented Lesions. Available online: https://www.thepmfajournal.com/features/features/post/laser-and-light-based-treatments-for-pigmented-lesions (accessed on 3 January 2025).
- Alster, T.S.; Tanzi, E.L. Laser Surgery in Dark Skin. Skinmed 2003, 2, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.R.; Parrish, J.A. Selective Photothermolysis: Precise Microsurgery by Selective Absorption of Pulsed Radiation. Science 1983, 220, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Tanghetti Md, E.; Jennings, J. A Comparative Study with a 755 Nm Picosecond Alexandrite Laser with a Diffractive Lens Array and a 532 Nm/1064 Nm Nd:YAG with a Holographic Optic. Lasers Surg. Med. 2018, 50, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.V. Light-Based Treatment of Pigmented Lesions. Cosmet. Dermatol. 2011, 24, 515–522. [Google Scholar]
- Tanghetti, E.A. The Histology of Skin Treated with a Picosecond Alexandrite Laser and a Fractional Lens Array. Lasers Surg. Med. 2016, 48, 646–652. [Google Scholar] [CrossRef]
- Chan, H.H.L.; Kono, T. The Use of Lasers and Intense Pulsed Light Sources for the Treatment of Pigmentary Lesions. Skin. Therapy Lett. 2004, 9, 5–7. [Google Scholar]


| Study Characteristics | Intervention | Comparator | Sample Characteristics | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Publication Year/Country | Sponsorship/Blinding/ Study Type | N Total Randomized/Analyzed | Laser 1 Name | Length of Wave [nm] | Fluence [J/cm2] | Spot Size [mm] | Frequency [Hz] | Pulse Duration [ps] | Anesthetic Cream Applied (Y/N) | Laser 2 Name | Length of Wave [nm] | Fluence [J/cm2] | Spot Size [mm] | Frequency [Hz] | Pulse Duration [ps} | Intervention Duration | Age: Mean/SD | N Males | Type of Lesion | Fitzpatrick Type (List Type with n) |
| Al-Dhalim et al., 2016/Iraq [15] | A/None/SF | 19/14 | Nd: YAG | 532 | 30 | 3 | 1 | 25 [ms] | Y | Nd: YAG | 1064 | 300 | 3 | 2 | 20 [ms] | 6 every 4 weeks | 22.07/9.003 | 1 | Facial port wine stain | III-3, IV-11 |
| Bohnert et al., 2018/USA [16] | A/DB/SF | 10/10 | KTP/Nd: YAG | 532/1064 | 4; 4.5 | 3; 6 | 10 | ND | Y | Nd: YAG | 1064 | 4 | 6 | 10 | ND | 6 every 2–3 weeks | 55/(40–63) range | 0 | Mild-to-severe facial mottled pigmentation | I-1, II-7, III-2 |
| Butler et al., 2006/USA [17] | Ind/DB/SF | 19/19 | KTP | 532 | 7–10 | 10 | ND | 15–30 [ms] | N | IPL (Starlux) | IPL | 25–36 | 10 × 15 | ND | 20 [ms] | ND | Over 18 years old/ND | ND | Pigmented and/or vascular dyschromias | ND |
| Felton et al., 2014/England [18] | A/None/Other participants | 21/15 (11 for 532 and 755) | Nd: YAG-532 nm | 532 | 0.5–5 | 2–5 | ND | 50 [ns] | Y | QS alexandrite (Alex)—755 nm | 755 | 4.2–18 | 2–4 | ND | 100 [ns] | Median 8 (4–13) | 24 (median)/15–61 (range) | 5 | Nevus of Ota (NO) | II-2, IV-5, V-8 |
| Karppinen et al., 2019/Multicenter [19] | A/DB/SF | 24/18 | KTP | 532 | 20–30 | 1 | ND | 10 [ms] | N | Yellow laser—PhotoLase (585 nm) | 585 | 5.6–8.1 | 1.4 | ND | 25 [ms] | 1–2 every 1–2 months | 48/27–62 (range) | 2 | Telangiectasia | I-6, II-8, III-4 |
| Limpjaroenviriyakul et al., 2020/England [20] | Ind/SB/Other participants | 30/30 | Q-switched Nd: YAG 532 nm laser (QS 532-nm) | 532 | 1 | 2 | 3 | ND | Y | Low-fluence Q-switched Nd: YAG 1064 nm laser (LFQS 1064 nm) | 1064 | 2.4 | 6 | 5 | ND | 1064 nm: 5 every 2 weeks, 532 nm: once | 27.73/7.91 | 8 | Idiopathic hyperpigmented lip | ND |
| Nam et al., 2019/Korea [21] | A/DB/SF | 20/19 | Fractional 532 nm KTP laser | 532 | 3.86 | 20 × 20 [mm2] | ND | 5 [ms] | ND | 595 nm pulsed dye laser (PDL) | 595 | 7.5 | 10 | ND | 6 [ms] | 3 every 4 weeks | 41.5/21–59 (range) | 5 | Facial erythema and telangiectasia | ND |
| Ma et al., 2022/China [22] | A/DB/SF | 18/18 | QSAL-treated group or picosecond 532 nm Nd: YAG | 532 | 0.5–0.6 | 3 | 2 [MHz] | 375 | Y | Q-switched alexandrite laser | 755 | 5–6 | 3 | 2 [MHz] | 100 ± 10 [ns] | 1 | 27.11/4.81 | 3 | Freckles | III-14, IV-4 |
| Uebelhoer et al., 2007/USA [23] | Ind/SB/SF | 15/14 | 532 nm KTP laser (Gemini, Laserscope) | 532 | 10; 9 | 5; 10 | ND | 23 [ms] | ND | 595 nm flashlamp-pumped, long-pulsed PDL (V-Beam, Candela) | 595 | 7.5 | 10 | 1 | 10 [ms] | 3 every 3 weeks | 52.4/35–70 (range) | 8 | Facial telangiectasias, diffuse telangiectatic facial erythema | I-6, II-6, III-2, IV-1 |
| West and Alster 1998/USA [24] | A/None/Other participants | 20/17 | KTP (532 nm) laser (Aura; Laserscope, San Jose, CA, USA) | 532 | 15 | 1 | ND | 10 [ms] | ND | 590 or 595 nm long-pulse dye laser (ScleroPlus Laser; Candela Corporation, Wayland, MA, USA) | 590, 595 | 15; 20 | 2 × 7, 2 × 7 | ND | 1.5 [ms] | 1 or 2 (8-week interval) | 40/23–69 | 0 | Facial or leg telangiectasia | I-Nd, II-Nd, III-Nd |
| Bonan et al., 2021/Italy [25] | Ind/SB/SF | 63/63 | MoveoPL 755 alexandrite laser | 755 | 18–25 | ND | 2.5–3.5 | ND | ND | Standard SP emission | SP | 18–25 | 5–10 | 1–1.5 | ND | 2 every 50 days | 57/12 | 9 | Benign hyperpigmentation, pigmentation scars | I-16, II-12, III-19, IV-16 |
| Fabi et al., 2014/USA [26] | Ind/DB/SF | 20/16 | Low-fluence QSAL | 755 | 2.0, 1.8, 1.2, 1.1 | 8 | 5 | ND | ND | Low-fluence Q-switched Nd: YAG | 1064 | 2.0 | 8 | 5 | ND | 6 every 1 week | 43.4/32–64 (range) | 1 | Melasma | II-2, III-7, IV-11 |
| Lee et al., 2018/England [27] | Ind/None/SF | 12/12 | 755 nm picosecond alexandrite laser | 755 | 0.88–1.18 | 4.4–5.1 | ND | 650 | ND | 1064 nm QS-Nd: YAG | 1064 | 2.0, 3.5, 3.2 | 8, 6, 4 | ND | ND | 4 every 1 month | ND/32–52 (range) | 1 | Melasma | III-Nd, IV-Nd |
| Lee et al., 2012/Korea [28] | A/None/Two groups based on type of lesions on the face ((i) those with pigmentation; and (ii) those with facial flushing, skin laxity or some pigmentation.) | 116/116 | Long-pulsed 755 nm alexandrite | 755 | 125 | 1.5–18 | ND | 0.25–0.5 [ms] | Y | Long-pulsed 1064 nm Nd: YAG | 1064 | 40–50 | 10 | ND | 0.25–300 [ms] | 1 | 40.73/2.35 | 6 | Facial flushing and telangiectasia | III-Nd, IV-Nd |
| Levin et al., 2016/USA [29] | Ind/TB/Side-by-side | 42/42 | 755 nm alexandrite picosecond laser | 755 | 0.71–4.07 | 2.5–6 | ND | 750–900 | ND | Q-switched frequency-doubled 532 nm Nd: YAG nanosecond, Q-switched 694 nm ruby nanosecond | 532, 694, 1064 | ND | ND | ND | ND | picosecond: 4.12; nanosecond: 5.46 treatments | 37.1/1–71 (range) | 6 | Nevus of Ota | III-16, IV-11, V-14, VI-1 |
| Zhang et al., 2023/China [30] | A/SB/SF | 86/86 | Picosecond alexandrite laser (PicoSure; Cynosure) | 755 | 3.77–4.80 | 2.3–2.6 | 1 | 550–750 | ND | Ti:sapphire laser (PicoWay; Syneron-Candela) | 730 | 3.75 | 2-3 | 1 | 250 | 1 | 30.7/21–62 (range) | 7 | Freckles | III-16, IV-70 |
| Improvement/Clinical Efficacy Endpoint | |||||||
|---|---|---|---|---|---|---|---|
| Publication Year | Name of Tool | Laser 1 Ø | Laser 1 ± | Laser 1 n | Laser 2 Ø | Laser 2 ± | Laser 2 n |
| 532 nm | |||||||
| Al-Dhalim et al., 2016 [15] | VAS 5-point visual analog scale assessment for halves of PWS with photos | 2.28 | 1.43 | 14 | 3.71 | 0.82 | 14 |
| Bohnert et al., 2018 [16] | Global Aesthetic Improvement scale (1–5) with CR VISIA | 1.35 | 0.37 | 19 | 0.76 | 0.37 | 10 |
| Felton et al., 2014 [18] | ND | 90 | 6.32 | 5 | 74.28 | 28.7 | 10 |
| Nam et al., 2019 [21] | Colorimetric Endpoint Analysis: CR-400 device (Minolta, Tokyo, Japan) | 12.77 | 2.77 | 19 | 12.22 | 2.75 | 19 |
| Nam et al., 2019 [21] | Clinical photography endpoint analysis: 10-point Percent Change Scale | 2.89 | 1.71 | 19 | 2.79 | 1.13 | 19 |
| Ma et al., 2022 [22] | Photographic Endpoint Analysis: Five-grade Percent Change Scale | 3.11 | 0.583 | 18 | 3.78 | 0.428 | 18 |
| Uebelhoer et al., 2007 [23] | Photographic Endpoint Analysis: Five-grade Percent Change Scale | 85 | nd | 8 | 75 | nd | 8 |
| West and Alster 1998 [24] | Photographic Evaluation: assessed by a physician and nurse. | 1.45 | nd | 20 | 3 | nd | 20 |
| 755 nm | |||||||
| Bonan et al., 2020 [25] | Grading score (0–4) using LifeViz digital imaging system | 3.6 | 0.6 | 63 | 2.8 | 0.8 | 63 |
| Lee et al., 2018 [27] | VAS-5-point visual analog scale assessment | 1.38 | 0.48 | 12 | 2.04 | 0.75 | 12 |
| Levin et al., 2016 [29] | VAS 5-point visual analog scale assessment | 2.44 | nd | 8 | 2.57 | nd | 22 |
| Zhang et al., 2023 [30] | Photographic Evaluation via VISIA-CR camera (Canfield Scientific) | 69.27 | 7.75 | 86 | 68.99 | 7.42 | 86 |
| Zhang et al., 2023 [30] | Global Aesthetic Improvement Scale (GAIS) score (5-point scale) | 4.02 | 0.3 | 86 | 4.04 | 0.31 | 86 |
| Felton et al., 2014 [18] | Endpoint Analysis of Improvement: percentage-based evaluation | 75 | 18.02 | 6 | 82.22 | 29.73 | 9 |
| Author | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) (Mortality) | Incomplete Outcome Data Addressed (Attrition Bias) (Short-Term Outcomes) | Selective Reporting (Reporting Bias) | Other Bias |
|---|---|---|---|---|---|---|---|
| Al-Dhalimi and Al-Janabi (2016) [15] | Low Risk—Randomization by simple draw | Low Risk—Half-lesion treatment | High Risk—Not blinded | High Risk—Outcome assessors not blinded | Unclear—Attrition not fully explained (5 dropouts) | Low Risk | None noted |
| Bohnert et al. (2018) [16] | Low Risk—Randomized split-face study | Low Risk—Side randomization | Low Risk—Double-blinded | Low Risk—Blinded investigator assessments | Low Risk—All participants completed | Low Risk | None noted |
| Bonan et al. (2021) [25] | Low Risk—Randomization conducted | Unclear Risk—Allocation details unclear | Low Risk—Controlled and blinded | Low Risk—Outcome assessments were blinded | Low Risk—High completion rate | Low Risk | None noted |
| Butler et al. (2006) [17] | Low Risk—Randomized, split-face design | Low Risk—Clear allocation | Low Risk—Blinding maintained | Low Risk—Blinded assessors | Low Risk—All participants completed | Low Risk | None noted |
| Fabi et al. (2014) [26] | Low Risk—Randomization applied | Low Risk—Allocation well managed | Low Risk—Double-blind protocol | Low Risk—Independent evaluations | Low Risk—High follow-up rate for each group | Low Risk | None noted |
| Felton et al. (2014) [18] | Low Risk—Randomization via pre-test patches | Low Risk—Allocated by laser response | Unclear Risk—No personnel blinding details | Low Risk—Independent evaluations | Low Risk—High follow-up completion | Low Risk | None noted |
| Karppinen et al. (2019) [19] | Low Risk—Randomized, double-blinded | Low Risk—Blinded side allocation | Low Risk—Double-blinded design | Low Risk—Blinded assessors | Low Risk—All participants completed | Low Risk | Device bias (manufacturer affiliation) |
| Limpjaroenviriyakul et al. (2020) [20] | Low Risk—Randomized controlled study | Low Risk—Allocation by block | High Risk—No blinding reported | Unclear Risk—No specific blinding for outcome assessors | Low Risk—All patients completed study | Low Risk | None noted |
| Zhang et al. (2023) [30] | Low Risk—Randomized, split-face controlled | Low Risk—Controlled side allocation | Low Risk—Blinded split-face trial | Low Risk—Blinded outcome assessments | Low Risk—Complete dataset | Low Risk | None noted |
| Lee et al. (2012) [28] | Low Risk—Randomized split treatment | Unclear Risk—Allocation not specified | High Risk—No blinding | Low Risk—Blinded assessors | Low Risk—Full data presented | Low Risk | None noted |
| Lee et al. (2018) [27] | Low Risk—Randomized, split-face design | Unclear Risk—Random allocation unclear | High Risk—Blinding not reported | Unclear-No blinding for outcome assessment | Low Risk—High retention rate | Low Risk | None noted |
| Levin et al. (2016) [29] | Low Risk—Retrospective analysis | Unclear Risk—Allocation not described | High Risk—No blinding for personnel | Low Risk—Blinded evaluation of outcomes | Low Risk—Complete data presented | Low Risk | Retrospective design |
| Ma et al. (2022) [22] | Low Risk—Randomized split-face study | Low Risk—Clear allocation | High Risk—No blinding | Low Risk—Blinded outcome assessors | Low Risk—All participants completed | Low Risk | None noted |
| Nam et al. (2019) [21] | Low Risk—Randomized controlled study | Unclear Risk—Allocation methods unclear | High Risk—No blinding for personnel | Low Risk—Outcome assessment blinded | Low Risk—High completion rate | Low Risk | None noted |
| Uebelhoer et al. (2007) [23] | Low Risk—Randomized, split-face design | Low Risk—Clear allocation | Low Risk—Single-blinded for participants | Low Risk—Blinded outcome assessments | Low Risk—High retention rate | Low Risk | None noted |
| West and Alster (1998) [24] | Low Risk—Randomized, split-face comparison | Low Risk—Clear allocation | Low Risk—Single-blinding for patients | Low Risk—Independent blinded evaluation | Low Risk—All data complete | Low Risk | None noted |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawodny, P.; Zawodny, P.; Kulaszyńska, M.; Stój, E.; Knap-Czechowska, A.; Skonieczna-Żydecka, K.; Sieńko, J. The Efficacy of 532/755 nm Laser Therapy for Facial Pigmented and Vascular Lesions: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 2546. https://doi.org/10.3390/jcm14082546
Zawodny P, Zawodny P, Kulaszyńska M, Stój E, Knap-Czechowska A, Skonieczna-Żydecka K, Sieńko J. The Efficacy of 532/755 nm Laser Therapy for Facial Pigmented and Vascular Lesions: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(8):2546. https://doi.org/10.3390/jcm14082546
Chicago/Turabian StyleZawodny, Piotr, Paweł Zawodny, Monika Kulaszyńska, Elżbieta Stój, Anna Knap-Czechowska, Karolina Skonieczna-Żydecka, and Jerzy Sieńko. 2025. "The Efficacy of 532/755 nm Laser Therapy for Facial Pigmented and Vascular Lesions: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 8: 2546. https://doi.org/10.3390/jcm14082546
APA StyleZawodny, P., Zawodny, P., Kulaszyńska, M., Stój, E., Knap-Czechowska, A., Skonieczna-Żydecka, K., & Sieńko, J. (2025). The Efficacy of 532/755 nm Laser Therapy for Facial Pigmented and Vascular Lesions: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(8), 2546. https://doi.org/10.3390/jcm14082546







