1. Introduction
Inflammatory bowel disease (IBD) is a chronic immune-mediated inflammatory disorder, characterized by alternating episodes of flares and remissions. The pathogenesis of IBD is complex, involving genetic predisposition, gut dysbiosis, loss of intestinal barrier integrity, and an abnormal immune response to microbial or environmental antigens. The two main types of IBD are ulcerative colitis (UC) and Crohn’s disease (CD), which differ significantly in terms of location and extent of lesions, as well as extraintestinal manifestations, possible complications, and the effectiveness of therapeutic approaches. The prevalence of IBD is increasing worldwide as the disease is more commonly diagnosed in patients between the ages of 18 and 35, as well as in the pediatric population. Therefore, considering the rising prevalence and the younger age at diagnosis, early detection is crucial to prevent the development of severe disease and potential complications [
1,
2,
3,
4]. At the same time, IBD diagnosis is often delayed due to the heterogeneity of clinical manifestations—especially in Crohn’s disease—and the presence of non-specific symptoms, which may lead to misdiagnosis and further progression of the disease. Reliable and sensitive biomarkers may not only support early IBD diagnosis, but also help to predict its development several years before the onset of clinical symptoms. The diagnosis of IBD is currently based on invasive methods, such as endoscopic examination, while non-invasive ones are mainly limited to measurements of C-reactive protein (CRP) and fecal calprotectin. Elevated fecal calprotectin is observed not only in IBD but also in gastrointestinal infections and colorectal cancer, as well as dietary allergies and celiac disease [
1,
4]. Therefore, none of the currently studied biomarkers enable IBD diagnosis on their own, let alone effective differentiation between UC and CD. The management of UC and CD requires a more personalized approach and the use of biomarkers that could complement endoscopic examinations in the diagnostic process, as well as facilitate close monitoring of disease progression in order to minimize intestinal damage [
5,
6,
7]. Consequently, there is a pressing need to identify new non-invasive biomarkers that could aid in the diagnosis of IBD and facilitate the differentiation between UC and CD.
The pathogenesis of IBD is related to the increased activation of immune cells, with neutrophils being among the first cells infiltrating the intestinal tissue during disease progression. At the site of inflammation, neutrophils neutralize pathogens through the generation of reactive oxygen species and the release of neutrophil extracellular traps (NETs) containing proteases such as neutrophil elastase (NE).
NE is not only the most abundant serine protease released from neutrophils, but also the most active one, as it accounts for approximately 80% of the proteolytic activity in the human body. The key role of NE is the neutralization of pathogens; however, it has also been suggested to play a role in modulating the inflammatory response by upregulating pro-inflammatory cytokines and thereby enhancing the inflammatory process. NE is also engaged, both directly and indirectly, via the activation of matrix metalloproteinases (MMPs), in the remodeling of the extracellular matrix (ECM) through the cleavage of collagen, laminin, fibronectin, and elastin, thereby contributing to the intestinal damage observed in IBD. Moreover, NE is implicated in another key aspect of IBD pathogenesis, namely the impairment of intestinal barrier integrity, as it can cleave tight junction proteins, such as E-cadherin [
8,
9,
10,
11]. Therefore, NE is actively engaged in the development of IBD by amplifying inflammatory processes, damaging intestinal tissue, and impairing intestinal barrier integrity. Considering the multifactorial involvement of NE in IBD pathogenesis, its measurement may be useful not only for the diagnosis of IBD but also as a marker of disease activity. Consequently, in this study the urinary profile of NE will be measured in patients with IBD (both CD and UC) as well as in healthy individuals.
Urine was chosen as the biological material for our study due to its non-invasive collection, allowing for easy and repeated sampling. Additionally, urine can accumulate proteolytic enzymes and their inhibitors, providing insight into the long-term activity of inflammatory processes occurring in IBD. Both NE (≈30 kDa) and elafin (≈6–12 kDa) have relatively small molecular weights, allowing them to freely pass through the glomerular filtration barrier. Unlike serum, where NE is tightly regulated by endogenous inhibitors, especially α1-antytrypsin, urine may better reflect its actual proteolytic activity and imbalances between NE and elafin.
Another biomarker analyzed in our study is elafin, one of the inhibitors of NE. Elafin is a serine protease inhibitor expressed in epithelial cells throughout the entire gastrointestinal tract, as well as in macrophages and neutrophils. Its anti-protease activity is relatively narrow, as it inhibits only neutrophil elastase and neutrophil proteinase 3; however, it can bind to both proteases with equally high affinity. Apart from its elastase inhibitor domain, elafin also possesses transglutaminase substrate domain, which enables its binding to ECM components, thereby protecting ECM from excessive proteolysis. Moreover, this protein demonstrates anti-inflammatory properties as it may inhibit the pro-inflammatory NF-κB pathway, thereby suppressing the inflammatory process [
12,
13]. During IBD, elafin may not only suppress the proteolytic activity of NE, but also attenuate the inflammatory response. Consequently, a disruption in the NE/elafin balance may lead to intestinal tissue damage, compromised intestinal barrier integrity, and an excessive inflammatory response—features characteristic for IBD. Therefore, in our study, we will evaluate not only the urinary profiles of NE and elafin but also the NE/elafin ratio to elucidate the proteolytic–anti-proteolytic balance in IBD patients. The aim of our study is to assess the utility of NE, elafin, and the NE/elafin urinary profile in the diagnosis of IBD, as well as in differential diagnoses between UC and CD. Additionally, the utility of these biomarkers in evaluating disease activity will also be evaluated.
4. Discussion
The identification of reliable biomarkers for IBD is crucial for early and accurate diagnosis, which not only guides treatment strategies but also helps to predict the disease course. The accurate and early identification of UC or CD using reliable biomarkers may not only limit the extent of intestinal tissue injury, but also allows for prompt initiation of therapy, thereby increasing the likelihood of achieving clinical remission within the first few months of treatment [
6]. Although fecal calprotectin has been identified as a diagnostic biomarker of IBD, its clinical utility remains restricted due to its lack of specificity in distinguishing IBD from other inflammatory gastrointestinal disorders. Therefore, in our research we aimed to identify new biomarkers that could support the diagnostic process for IBD and aid in differentiating UC from CD. The biomarkers assessed in this study (NE, elafin, NE/elafin ratio) were selected based on their active role in key processes related to the pathogenesis of IBD, including intestinal barrier disruption, excessive immune response, and intestinal tissue damage. Urine was chosen as the biological material for analysis due to its accessibility, non-invasive collection, and ability to reflect biochemical changes that occur during disease progression. In serum, NE activity is strictly regulated by endogenous inhibitors such as α1-antitrypsin (α1AT). However, in urine, the NE-α1-antitrypsin complex is typically absent or present only in trace amounts due to the high molecular weight of α1AT. Therefore, urine may provide a more accurate representation of NE’s actual proteolytic activity while also reflecting the balance between NE and elafin, as both have low molecular weights.
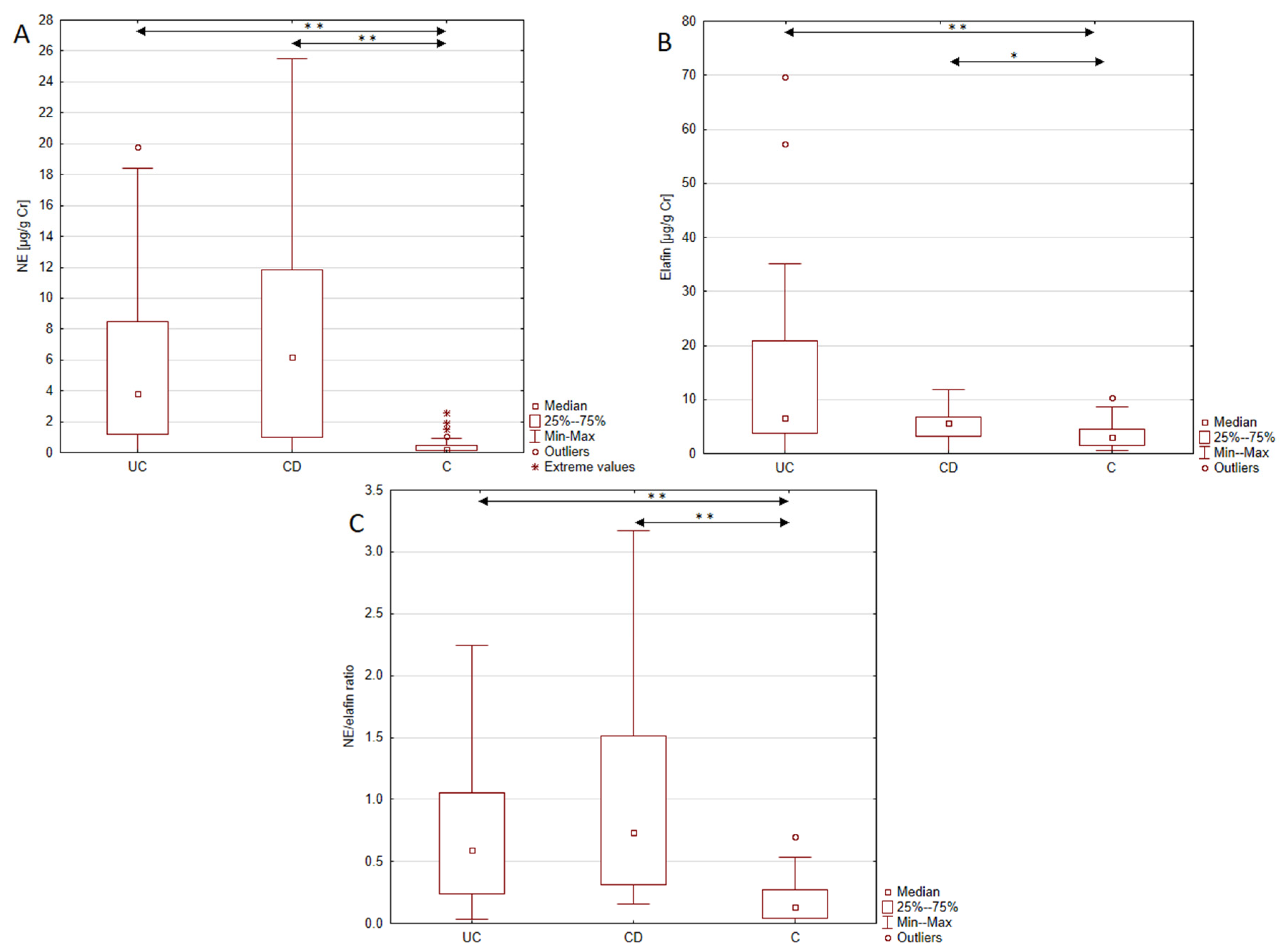
To our knowledge, this is the first study demonstrating increased urinary excretion of NE in patients with IBD (both UC and CD) compared to healthy individuals. The observed increase in NE excretion reached 17-fold in UC and 28-fold in CD patients compared to the control group. The demonstrated upregulation of NE is in line with the results of Kuno et al. [
15], who noted elevated expression of NE in mucosal biopsies from UC patients compared to controls. Researchers have also noted differences in NE levels between inflamed and non-inflamed tissues from UC patients, while no such difference was observed between non-inflamed UC tissue and control samples. Moreover, the level of NE correlated positively with the number of neutrophils and mononuclear cells, indicating a local inflammation-driven increase in NE during UC. Additionally, in a study conducted by Curciarello et al. [
16], the NE activity in the mucosal tissue of IBD patients was elevated and increased over time, whereas it remained stable in the control group, suggesting both enhanced and prolonged NE activity in UC patients. NE also plays a role in ECM proteolysis, releasing pro-inflammatory cytokines deposited within the matrix and modulating the inflammatory response via Toll-like receptor 4 and the NF-κB pathway, thereby contributing to the development of inflammatory processes. Ginzberg et al. [
17] demonstrated in a cellular model of the intestinal epithelium that NE migration across the epithelial barrier was associated with E-cadherin degradation, detachment of the epithelial monolayer, and the disruption of adherens junction integrity. These actions of NE may lead to increased intestinal and ulcer formation—key features of IBD. Additionally, some researchers indicate that NE may limit epithelial proliferation and induce epithelial-to-mesenchymal transition, resulting in impaired mucosal repair. Taken together, these findings highlight NE as a protease with a substantial contribution to intestinal tissue damage progression. This causal role of NE in the development of UC may explain its increased urinary excretion in UC patients [
9,
15,
18]. In contrast, reports regarding NE expression in CD patients remain inconsistent. In our study, NE excretion in CD patients was significantly higher than in healthy individuals. However, in Kuno et al.’s [
15] study, NE expression did not differ between CD patients and controls. The observed inconsistency may be related to differences in treatment and disease duration, as our study included only newly diagnosed, treatment-naïve patients, whereas the study by Kuno et al. involved CD patients diagnosed 15 months to 18 years prior to the study, many of whom received treatment with 5-amino salicylic acid or prednisolone. Conversely, Langhorst et al. [
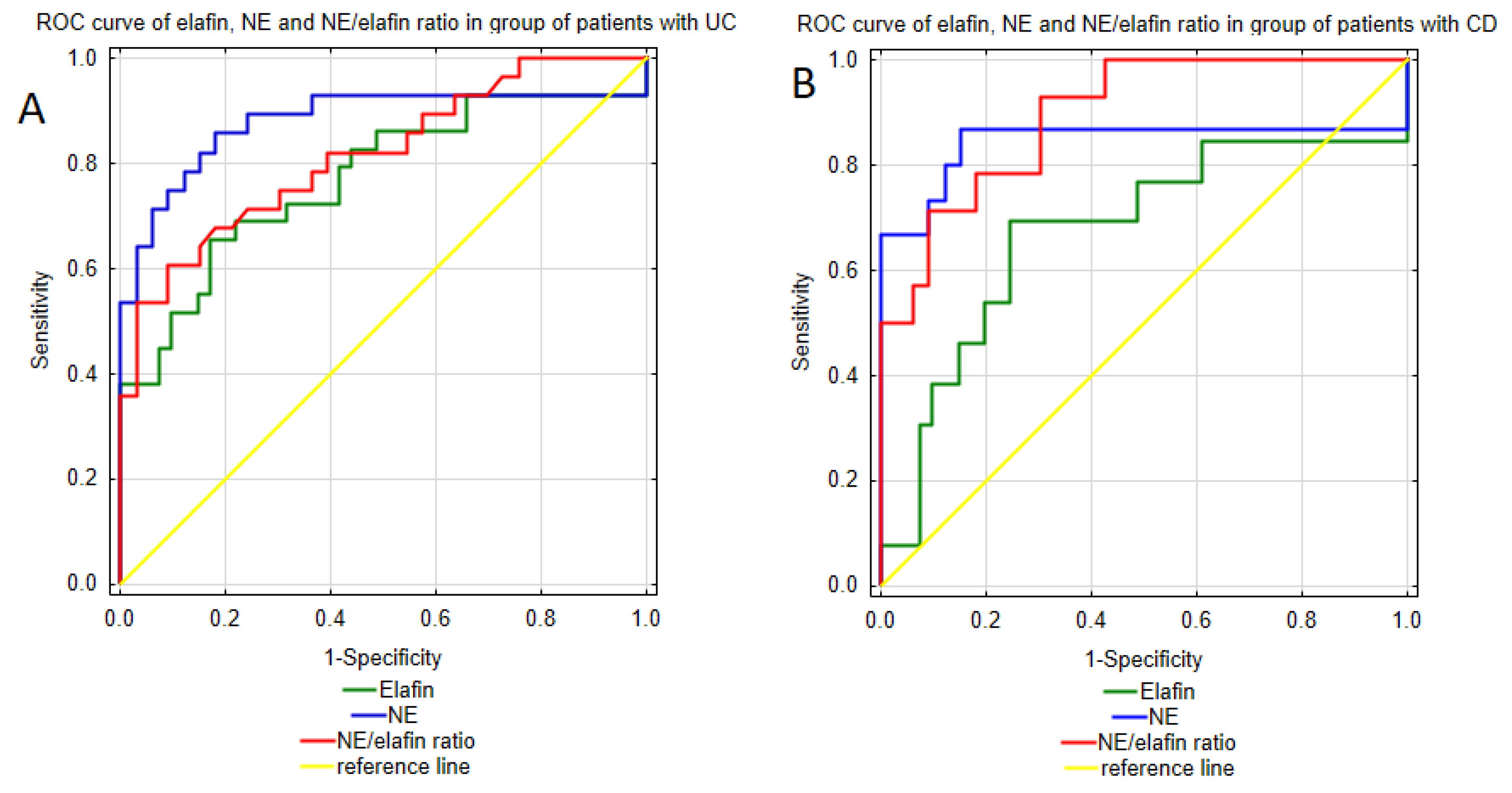
19] reported an increased expression of fecal NE in both CD and UC patients compared to patients with irritable bowel syndrome (IBS), identifying NE as a superior marker for IBD compared to CRP. Moreover, measurements of fecal NE allow active IBD to be differentiated from inactive IBD, indicating its potential role in monitoring the disease activity. Among the analyzed parameters, neutrophil elastase emerged as a promising biomarker for IBD diagnosis. Conducted ROC curve analysis demonstrated excellent discriminative ability of urinary NE in distinguishing Crohn’s disease patients and healthy individuals. Moreover, the analysis revealed high sensitivity and specificity of NE urinary profile, supporting its potential as a diagnostic biomarker for CD. Considering the higher NPV compared to the PPV (93.32% vs. 72.2%), this test may overdiagnose CD. The ROC curve analysis of urinary NE in UC patients also yielded encouraging results, demonstrating a strong ability to differentiate patients with UC from healthy individuals with high specificity and sensitivity. These findings, together with the strong values of both the positive and negative test results, highlight the potential clinical application of urinary NE measurements in the diagnosis of UC.
Furthermore, this study demonstrated increased urinary excretion of elafin in both UC and CD patients compared to the control group. The results obtained are in line with results of Wang et al.’s [
20] study, who noted increased serum elafin levels in IBD patients. Similar results were presented in Krawiec et al.’s [
21] study, which was conducted in a pediatric IBD population. The up-regulation of elafin in IBD was also presented in a study by Schmid et al. [
22], which reported increased elafin expression in inflamed intestinal tissue compared to non-inflamed tissue from IBD patients. The increase in elafin levels observed in IBD may be related to the inflammatory process, as this protein is constitutively expressed in intestinal epithelial cells, but its expression increases in response to inflammatory stimuli. Key triggers of elafin up-regulation include the IL-1β and TNF-α-two cytokines, which are known to play a pivotal role in IBD pathogenesis [
23,
24]. Beyond its role as an NE inhibitor, elafin is involved in maintaining intestinal barrier integrity and exerts anti-inflammatory effects. This protective function of elafin was demonstrated in the study conducted by Motta et al. [
24] using both cellular and animal models of IBD. In that study, elafin not only inhibited TNFα-induced intestinal barrier permeability, but also improved the organization of tight junction proteins. Moreover, elafin administration suppressed the inflammatory response by reducing the levels of IBD-related pro-inflammatory cytokines including IL-6, IL-8, IL-17, TNF-α, as well as down-regulating the NF-κB pathway [
24,
25,
26]. These findings suggest that despite its protective role against colitis and its up-regulated expression in IBD, the effect of elafin may not be sufficient to suppress the pro-inflammatory processes underlying IBD. Nevertheless, given its involvement in the key pathological mechanisms of IBD—namely intestinal barrier integrity and inflammatory process—we assessed the diagnostic utility of elafin measurements in IBD. The urinary elafin profile proved useful in diagnosing both UC and CD, with a better diagnostic performance in UC patients. Urinary elafin measurements effectively distinguished UC patients from healthy individuals with high specificity and satisfactory sensitivity. Moreover, the urinary elafin measurements demonstrated a strong PPV and NPV, indicating a low incidence of false positive and false negative diagnoses. In CD patients, the elafin urinary profile also showed a strong ability to differentiate CD patients from healthy individuals with a slightly better sensitivity but a lower specificity, suggesting a higher susceptibility to false positive diagnoses. Additionally, in CD patients, urinary elafin levels exhibited a lower variability, suggesting greater stability of this biomarker in CD patients.
In this study, the increase in NE levels was accompanied by an elevation of elafin expression; however, this up-regulation of elafin may not counteract NE activity in IBD. Consequently, the protease–anti-protease balance may remain disrupted, contributing to intestinal tissue damage. These findings are supported by the study of Curciarello et al. [
16], which assessed the protease–anti-protease balance in the mucosal tissue of IBD patients and healthy individuals. Despite increased elafin expression, patients with IBD exhibited enhanced NE activity, which was further reflected by a decreased elastin level, an extracellular matrix component degraded by NE. To exclude a reduced suppressive effect of elafin on NE, the researchers evaluated NE’s sensitivity to elafin-mediated inhibition. Upon exposure to elafin, elastin proteolysis was diminished, confirming that NE remained responsive to elafin’s inhibitory effects. These findings emphasize that measurements of NE and elafin concentrations alone may be insufficient to assess the proteolytic–anti-proteolytic balance during IBD. Assessments of the NE/elafin ratio may provide a more comprehensive reflection of the protease—anti-protease imbalance, which could directly contribute to the degradation of the extracellular matrix and subsequent damage of intestinal tissue observed in both UC and CD. Moreover, in a previously mentioned study by Curciarello et al. [
16], an imbalance between NE and elafin was related to a loss of response to biological treatment in UC patients, likely due to NE’s ability to neutralize anti-TNF-α agents. At the same time, this effect was mitigated by the administration of exogenous elafin. These findings suggest that the disruption of the NE/elafin balance may not only contribute to disease pathogenesis, but also to a loss of response to anti-inflammatory biological treatment in IBD patients. Therefore, in this study we additionally assessed the NE/elafin ratio to better illustrate the proteolytic–anti-proteolytic balance in UC and CD patients compared to healthy individuals. The results revealed a 4.5-fold increase in the NE/elafin ratio in patients with UC and a 5.6-fold increase in CD patients compared to healthy individuals, indicating an imbalance in proteolytic and anti-proteolytic activity in IBD. These findings correspond with the results obtained in the studies conducted by Motta et al. [
25], Barry et al. [
9], and Schmid et al. [
21], which also demonstrated a disrupted proteolytic–anti-proteolytic balance during IBD and highlighted its causative role in intestinal tissue damage. Moreover, enhanced NE activity in IBD may not only drive inflammation but also contribute to abdominal pain. NE has been shown to cleave protease-activated receptor 2 (PAR2), leading to the sensitization of nociceptive neurons and subsequent development of neurogenic inflammation and pain [
27,
28]. Despite the recognized role of the protease–anti-protease balance in IBD pathogenesis, the NE/elafin ratio has not yet been considered as a potential diagnostic biomarker. Therefore, in this study, we assessed the NE/elafin excretion ratio to explore its potential utility in diagnosing UC and CD. Among the analyzed biomarkers, the NE/elafin ratio demonstrated the best potential in distinguishing patients with Crohn’s disease from healthy individuals, with a 92.9% of sensitivity, indicating a low risk of CD misdiagnosis. Similarly, in UC patients, the NE/elafin ratio effectively distinguished UC patients from healthy individuals, with 90.9% specificity and 60.7% sensitivity. These results suggest that the assessment of the NE/elafin ratio in UC patients is more prone to omit UC patients in population. Comparable results were observed considering NE urine profile, which biomarker effectively distinguished both UC and CD patients with high sensitivity and specificity. Regarding the AUC values of the analyzed biomarkers, the NE/elafin ratio and NE provided better diagnostic values compared to elafin measurements in urine. Given the potential role of NE/elafin as an indicator of treatment response—as suggested by Curciarello et al. [
16]—measurements of this ratio might prove useful in monitoring therapeutic efficacy during biological treatment. Since the study cohort consisted exclusively of newly diagnosed patients who had not yet received biological treatment, the potential of this ratio as a treatment monitoring tool should be further analyzed in future studies.
The biomarkers—elafin, NE, and the NE/elafin ratio—evaluated in this study demonstrated potential for the early detection of both UC and CD, which could ultimately contribute to improved clinical outcomes through earlier intervention. A key strength of our study lies in the inclusion of only newly diagnosed UC and CD patients, allowing us to assess the diagnostic value of selected biomarkers. Additionally, the use of urine samples offers the advantage of a non-invasive, easily repeatable method of biomarker assessment. Moreover, due to the high molecular weight of α1-antitrypsin, its filtration into urine is significantly limited, which restricts the formation of NE-α1–antitrypsin complexes in urinary samples. As a result, urinary measurements of free NE may more accurately reflect its true proteolytic potential and the imbalance between NE and its physiological inhibitor, elafin. In this study, NE and elafin concentrations were normalized to the urinary creatinine level in each subject to minimize the effect of urine concentration variability. This standardization allows us to assess each biomarker in a single urine sample, reducing the need for 24 h urine collection. At the same time, referencing the urinary creatinine level helps to decrease the error related to intra- and inter-individual variability in the NE and elafin profiles among tested subjects. Nonetheless, this study has some limitations, among which the relatively small size of study groups may limit the ability to generalize the obtained results to a larger population of IBD patients. Consequently, the findings reported here should be interpreted with caution and validated in a larger independent cohort of patients with different disease manifestations, especially in the case of patients with Crohn’s disease, to ensure greater variability in and the external validity of the analyzed biomarkers. Additionally, external validation would allow for the assessment of both intra-individual and inter-individual variability, which may influence the accuracy and reproducibility of urinary elafin and NE measurements in IBD patients.