Exploring Net Immunosuppressive Status with Torque Teno Virus Viral Load in Kidney Transplant Recipients with High Molecular Injury
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AbMR | antibody-mediated rejection |
| CMV | cytomegalovirus |
| dd-cfDNA | donor-derived circulating free DNA |
| EBV | Epstein–Barr virus |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| HSV | Herpes simplex virus |
| IQR | interquartile range |
| MPA | mycophenolic acid |
| KTx | kidney transplant recipients |
| TTV-load | Torque Teno virus load |
| VZV | Varicella zoster virus |
References
- Dugast, E.; Soulillou, J.; Foucher, Y.; Papuchon, E.; Guerif, P.; Paul, C.; Riochet, D.; Chesneau, M.; Cesbron, A.; Renaudin, K.; et al. Failure of Calcineurin Inhibitor (Tacrolimus) Weaning Randomized Trial in Long-Term Stable Kidney Transplant Recipients. Am. J. Transplant. 2016, 16, 3255–3261. [Google Scholar] [CrossRef]
- Briggs, J.D. Causes of death after renal transplantation. Nephrol. Dial. Transplant. 2001, 16, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Buell, J.F.; Gross, T.G.; Woodle, E.S. Malignancy after transplantation. Transplantation 2005, 80 (Suppl. S2), S254–S264. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.M.; Li, Y.; De Winter, B.C.M.; Shi, Y.Y.; Baan, C.C.; Van Gelder, T.; Hesselink, D.A. Pharmacokinetic considerations related to therapeutic drug monitoring of tacrolimus in kidney transplant patients. Expert Opin. Drug. Metab. Toxicol. 2017, 13, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lopez, A.; Calderon-Zapata, A.; Gomez-Montero, A.; Lozano-Suarez, N.; Giron-Luque, F. The Value of Protocol Biopsy in Kidney Transplantation on Monitoring Transplant Outcomes: A Systematic Review and Meta-Analysis. Transplant. Proc. 2024, 56, 1231–1240. [Google Scholar] [CrossRef]
- Jaksch, P.; Görzer, I.; Puchhammer-Stöckl, E.; Bond, G. Integrated Immunologic Monitoring in Solid Organ Transplantation: The Road Toward Torque Teno Virus-guided Immunosuppression. Transplantation 2022, 106, 1940–1951. [Google Scholar]
- Van Rijn, A.L.; Roos, R.; Dekker, F.W.; Rotmans, J.I.; Feltkamp, M. Torque teno virus load as marker of rejection and infection in solid organ transplantation–A systematic review and meta-analysis. Rev. Med. Virol. 2023, 33, e2393. [Google Scholar] [CrossRef]
- Zeng, J.; Tang, Y.; Lin, T.; Song, T. Torque-teno virus for the prediction of graft rejection and infection disease after kidney transplantation: A systematic review and meta-analysis. J. Med. Virol. 2023, 95, e28677. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Vaitla, P.; Craici, I.M.; Leeaphorn, N.; Hansrivijit, P.; Salim, S.A.; Bathini, T.; Rivera, F.H.C.; Cheungpasitporn, W. The Use of Donor-Derived Cell-Free DNA for Assessment of Allograft Rejection and Injury Status. J. Clin. Med. 2020, 9, 1480. [Google Scholar] [CrossRef]
- Wijtvliet, V.P.; Plaeke, P.; Abrams, S.; Hens, N.; Gielis, E.M.; Hellemans, R.; Massart, A.; Hesselink, D.A.; De Winter, B.Y.; Abramowicz, D.; et al. Donor-derived cell-free DNA as a biomarker for rejection after kidney transplantation: A systematic review and meta-analysis. Transplant. Int. 2020, 33, 1626–1642. [Google Scholar] [CrossRef]
- Xing, Y.; Guo, Q.; Wang, C.; Shi, H.; Zheng, J.; Jia, Y.; Li, C.; Hao, C. Donor-derived cell-free DNA as a diagnostic marker for kidney-allograft rejection: A systematic review and meta-analysis. Biomol. Biomed. 2024, 24, 731. [Google Scholar] [CrossRef]
- González-López, E.; Ocejo-Vinyals, J.G.; Renuncio-García, M.; Roa-Bautista, A.; Arribas, D.S.S.; Escagedo, C.; García-Saiz, M.d.M.; Valero, R.; García-Berbel, P.; Millán, J.C.R.S.; et al. Donor-derived cell-free DNA at 1 month after kidney transplantation relates to HLA Class II eplet mismatch load. Biomed 2023, 11, 2741. [Google Scholar] [CrossRef]
- Mayer, K.A.; Omic, H.; Weseslindtner, L.; Doberer, K.; Reindl-Schwaighofer, R.; Viard, T.; Tillgren, A.; Haindl, S.; Casas, S.; Eskandary, F.; et al. Levels of donor-derived cell-free DNA and chemokines in BK polyomavirus-associated nephropathy. Clin. Transplant. 2022, 36, e14785. [Google Scholar] [CrossRef]
- Bloom, R.D.; Bromberg, J.S.; Poggio, E.D.; Bunnapradist, S.; Langone, A.J.; Sood, P.; Matas, A.J.; Mehta, S.; Mannon, R.B.; Sharfuddin, A.; et al. Circulating Donor-Derived Cell-Free DNA in Blood for Diagnosing Active Rejection in Kidney Transplant Recipients (DART) Study Investigators. Cell-Free DNA and Active Rejection in Kidney Allografts. J. Am. Soc. Nephrol. 2017, 28, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Cañamero, L.; Benito-Hernández, A.; González, E.; Escagedo, C.; Rodríguez-Vidriales, M.; García-Saiz, M.d.M.; Valero, R.; Belmar, L.; de Cos, M.A.; Francia, M.V.; et al. Torque teno virus load predicts opportunistic infections after kidney transplantation but is not associated with maintenance immunosuppression exposure. Biomedicines 2023, 11, 1410. [Google Scholar] [CrossRef] [PubMed]
- Görzer, I.; Haupenthal, F.; Maggi, F.; Gelas, F.; Kulifaj, D.; Brossault, L.; Puchhammer-Stöckl, E.; Bond, G. Validation of plasma Torque Teno viral load applying a CE-certified PCR for risk stratification of rejection and infection post kidney transplantation. J. Clin. Virol. 2023, 158, 105348. [Google Scholar] [CrossRef]
- Schinstock, C.A.; Mannon, R.B.; Budde, K.; Chong, A.S.; Haas, M.; Knechtle, S.; Lefaucheur, C.; Montgomery, R.A.; Nickerson, P.; Tullius, S.G.; et al. Recommended treatment for antibody-mediated rejection after kidney transplantation: The 2019 expert consensus from the transplantion society working group. Transplantation 2020, 104, 911–922. [Google Scholar] [CrossRef]
- Tharmaraj, D.; Mulley, W.R.; Dendle, C. Current and emerging tools for simultaneous assessment of infection and rejection risk in transplantation. Front. Immunol. 2024, 15, 1490472. [Google Scholar] [CrossRef]
- Kim, H.D.; Bae, H.; Kang, H.; Lee, H.; Eum, S.H.; Yang, C.W.; Choi, Y.J.; Chung, B.H.; Oh, E.-J. Donor-derived cell-free DNA predicted allograft rejection and severe microvascular inflammation in kidney transplant recipients. Front. Immunol. 2024, 15, 1433918. [Google Scholar] [CrossRef]
- Akifova, A.; Budde, K.; Amann, K.; Buettner-Herold, M.; Choi, M.; Oellerich, M.; Beck, J.; Bornemann-Kolatzki, K.; Schütz, E.; Bachmann, F.; et al. Donor-derived cell-free DNA monitoring for early diagnosis of antibody-mediated rejection after kidney transplantation: A randomized trial. Nephrol. Dial. Transplant. 2025, in press. [Google Scholar] [CrossRef]
- Mayer, K.A.; Schrezenmeier, E.; Diebold, M.; Halloran, P.F.; Schatzl, M.; Schranz, S.; Haindl, S.; Kasbohm, S.; Kainz, A.; Eskandary, F.; et al. A randomized phase 2 trial of felzartamab in antibody-mediated rejection. N. Eng. J. Med. 2024, 391, 122–132. [Google Scholar] [CrossRef]
- Wen, J.; Sun, R.; Yang, H.; Ran, Q.; Hou, Y. Detection of BK polyomavirus-associated nephropathy using plasma graft-derived cell-free DNA: Development of a novel algorithm from programmed monitoring. Front. Immunol. 2022, 13, 1006970. [Google Scholar] [CrossRef] [PubMed]
- Jaksch, P.; Kundi, M.; Görzer, I.; Muraközy, G.; Lambers, C.; Benazzo, A.; Hoetzenecker, K.; Klepetko, W.; Puchhammer-Stöckl, E. Torque teno virus as a novel biomarker targeting the efficacy of immunosuppression after lung transplantation. J. Infect. Dis. 2018, 218, 1922–1928. [Google Scholar] [CrossRef] [PubMed]
- Görzer, I.; Haloschan, M.; Jaksch, P.; Klepetko, W.; Puchhammer-Stöckl, E. Plasma DNA levels of Torque teno virus and immunosuppression after lung transplantation. J. Heart Lung Transplant. 2014, 33, 320–323. [Google Scholar] [CrossRef]
- Görzer, I.; Jaksch, P.; Kundi, M.; Seitz, T.; Klepetko, W.; Puchhammer-Stöckl, E. Pre-transplant plasma Torque Teno virus load and increase dynamics after lung transplantation. PLoS ONE 2015, 10, e0122975. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Macera, L.; Pistello, M.; Maggi, F. Torque Teno virus viremia correlates with intensity of maintenance immunosuppression in adult orthotopic liver transplant. J. Infect. Dis. 2014, 210, 667–668. [Google Scholar] [CrossRef]
- Schiemann, M.; Puchhammer-Stöckl, E.; Eskandary, F.; Kohlbeck, P.; Rasoul-Rockenschaub, S.; Heilos, A.; Kozakowski, N.; Görzer, I.; Kikić, Ž.; Herkner, H.; et al. Torque Teno Virus Load-Inverse Association with Antibody-Mediated Rejection After Kidney Transplantation. Transplantation 2017, 101, 360–367. [Google Scholar] [CrossRef]
- Frye, B.C.; Bierbaum, S.; Falcone, V.; Köhler, T.C.; Gasplmayr, M.; Hettich, I.; Dürk, T.; Idzko, M.; Zissel, G.; Hengel, H.; et al. Kinetics of Torque Teno Virus-DNA Plasma Load Predict Rejection in Lung Transplant Recipients. Transplantation 2019, 103, 815–822. [Google Scholar] [CrossRef]
- Solis, M.; Velay, A.; Gantner, P.; Bausson, J.; Filipputtu, A.; Freitag, R.; Moulin, B.; Caillard, S.; Fafi-Kremer, S. Torqueteno-virus viremia for early prediction of graft rejection after kidney transplantation. J. Infect. 2019, 79, 56–60. [Google Scholar] [CrossRef]
- Ruiz, P.; Martínez-Picola, M.; Santana, M.; Muñoz, J.; Pérez-Del-Pulgar, S.; Koutsoudakis, G.; Sastre, L.; Colmenero, J.; Crespo, G.; Navasa, M. Torque Teno Virus Is Associated with the State of Immune Suppression Early After Liver Transplantation. Liver Transpl. 2019, 25, 302–310. [Google Scholar] [CrossRef]
- Berg, R.; Clemmensen, T.S.; Petersen, M.S.; Mogensen, L.J.; Christiansen, M.; Rolid, K.; Nytrøen, K.; Møller, B.K.; Gullestad, L.; Eiskjær, H.; et al. Kinetics of Torque Teno virus in heart transplant patients. Human Immunol. 2023, 84, 110720. [Google Scholar] [CrossRef] [PubMed]
- Reineke, M.; Speer, C.; Bundschuh, C.; Klein, J.A.F.; Loi, L.; Sommerer, C.; Zeier, M.; Schnitzler, P.; Morath, C.; Benning, L. Impact of induction agents and maintenance immunosuppression on Torque Teno virus loads and year-one complications after kidney transplantation. Front. Immunol. 2024, 13, 1492611. [Google Scholar] [CrossRef]
- Regele, F.; Haupenthal, F.; Doberer, K.; Görzer, I.; Kapps, S.; Strassl, R.; Bond, G. The kinetics of Torque Teno virus plasma load following calcineurin inhibitor dose change in kidney transplant recipients. J. Med. Virol. 2024, 96, e29554. [Google Scholar] [CrossRef] [PubMed]
- Benning, L.; Morath, C.; Kühn, T.; Bartenschlager, M.; Kim, H.; Beimler, J.; Buylaert, M.; Nusshag, C.; Kälble, F.; Reineke, M.; et al. Humoral response to SARS-CoV-2 mRNA vaccination in previous non-responder kidney transplant recipients after short-term withdrawal of mycophenolic acid. Front. Med. 2022, 9, 958293. [Google Scholar] [CrossRef]
- Regele, F.; Heinzel, A.; Hu, K.; Raab, L.; Eskandary, F.; Faé, I.; Zelzer, S.; Böhmig, G.A.; Bond, G.; Fischer, G.; et al. Stopping of Mycophenolic Acid in Kidney Transplant Recipients for 2 Weeks Peri-Vaccination Does Not Increase Response to SARS-CoV-2 Vaccination-A Non-randomized, Controlled Pilot Study. Front. Med. 2022, 9, 914424. [Google Scholar] [CrossRef]
- Reineke, M.; Morath, C.; Speer, C.; Rudek, M.; Bundschuh, C.; Klein, J.A.; Mahler, C.F.; Kälble, F.; Nusshag, C.; Beimler, J.; et al. Dynamics of torque teno virus load in kidney transplant recipients with indication biopsy and therapeutic modifications of immunosuppression. Front. Med. 2024, 11, 1337367. [Google Scholar] [CrossRef]
- Hill, P.; Cross, N.B.; Barnett, A.N.; Palmer, S.C.; Webster, A.C. Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients. Cochrane Database Syst. Rev. 2017, 1, CD004759. [Google Scholar] [CrossRef]
- Webster, A.C.; Ruster, L.P.; McGee, R.G.; Matheson, S.L.; Higgins, G.Y.; Willis, N.S.; Chapman, J.R.; Craig, J.C. Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database Syst. Rev. 2010, 2010, CD003897. [Google Scholar] [CrossRef]
- Fernández-Ruiz, M.; Albert, E.; Giménez, E.; Ruiz-Merlo, T.; Parra, P.; López-Medrano, F.; San Juan, R.; Polanco, N.; Andrés, A.; Navarro, D.; et al. Monitoring of alphatorquevirus DNA levels for the prediction of immunosuppression-related complications after kidney transplantation. Am. J. Transplant. 2019, 19, 1139–1149. [Google Scholar] [CrossRef]
- Maggi, F.; Focosi, D.; Statzu, M.; Bianco, G.; Costa, C.; Macera, L.; Spezia, P.G.; Medici, C.; Albert, E.; Navarro, D.; et al. Early post-transplant torquetenovirus viremia predicts cytomegalovirus reactivations in solid organ transplant recipients. Sci. Rep. 2018, 8, 15490. [Google Scholar] [CrossRef]
- Handala, L.; Descamps, V.; Morel, V.; Castelain, S.; François, C.; Duverlie, G.; Helle, F.; Brochot, E. No correlation between Torque Teno virus viral load and BK virus replication after kidney transplantation. J. Clin. Virol. 2019, 116, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Macera, L.; Boggi, U.; Nelli, L.C.; Maggi, F. Short-term kinetics of torque teno virus viraemia after induction immunosuppression confirm T lymphocytes as the main replication-competent cells. J. Gen. Virol. 2015, 96 Pt 1, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Strassl, R.; Schiemann, M.; Doberer, K.; Görzer, I.; Puchhammer-Stöckl, E.; Eskandary, F.; Kikić, Ž.; Gualdoni, G.A.; Vossen, M.G.; Rasoul-Rockenschaub, S.; et al. Quantification of Torque Teno Virus Viremia as a Prospective Biomarker for Infectious Disease in Kidney Allograft Recipients. J. Infect. Dis. 2018, 218, 1191–1199. [Google Scholar] [CrossRef]
- Doberer, K.; Schiemann, M.; Strassl, R.; Haupenthal, F.; Dermuth, F.; Görzer, I.; Eskandary, F.; Reindl-Schwaighofer, R.; Kikić, Ž.; Puchhammer-Stöckl, E.; et al. Torque teno virus for risk stratification of graft rejection and infection in kidney transplant recipients—A prospective observational trial. Am. J. Transplant. 2020, 20, 2081–2090. [Google Scholar] [CrossRef]
- Querido, S.; Martins, C.; Gomes, P.; Pessanha, M.A.; Arroz, M.J.; Adragão, T.; Casqueiro, A.; Oliveira, R.; Costa, I.; Azinheira, J.; et al. Kinetics of torque Teno virus viral load is associated with infection and de novo donor specific antibodies in the first year after kidney transplantation: A prospective cohort study. Viruses 2023, 15, 1464. [Google Scholar] [CrossRef]
- Mafi, S.; Essig, M.; Rerolle, J.-P.; Lagathu, G.; Crochette, R.; Brodard, V.; Schvartz, B.; Gouarin, S.; Bouvier, N.; Engelmann, I.; et al. Torque teno virus viremia and QuantiFERON®-CMV assay in prediction of cytomegalovirus reactivation in R+ kidney transplant recipients. Front. Med. 2023, 10, 1180769. [Google Scholar] [CrossRef]
- van Rijn, A.L.; Wunderink, H.F.; Sidorov, I.A.; de Brouwer, C.S.; Kroes, A.C.; Putter, H.; de Vries, A.P.; Rotmans, J.I.; Feltkamp, M.C. Torque teno virus loads after kidney transplantation predict allograft rejection but not viral infection. J. Clin. Virol. 2021, 140, 104871. [Google Scholar] [CrossRef]
- Chauvelot, L.; Barba, T.; Saison, C.; Siska, E.; Kulifaj, D.; Bakker, S.J.L.; Koenig, A.; Rabeyrin, M.; Buron, F.; Picard, C.; et al. Longitudinal monitoring of Torque Teno virus DNAemia in kidney transplant recipients correlates with long-term complications of inadequate immunosuppression. J. Med. Virol. 2024, 96, e29806. [Google Scholar] [CrossRef]

| Number of Patients | 11 | 31 | p |
|---|---|---|---|
| Recipient age (years) | 60.0 [48.0–73.0] | 59.0 [47.0–66.0] | 0.572 |
| Recipient sex (male) | 63.6% | 80.6% | 0.255 |
| Diabetic nephropathy | 18.2% | 25.8% | 0.610 |
| Time in renal replacement therapy (months) | 24.5 [10.5–108.7] | 10.7 [0.0–32.6] | 0.062 |
| Retransplant | 36.4% | 12.9% | 0.089 |
| Pre-emptive transplantation | 18.2% | 22.6% | 0.760 |
| Donor age (years) | 61.0 [47.0–65.0] | 58.0 [47.0–65.0] | 0.910 |
| Antigen HLA Class-I and -II mismatches | 8.0 [7.0–9.0] | 7.0 [5.0–9.0] | 0.233 |
| Cold ischaemia time (h) | 20.0 [18.0–24.0] | 22.0 [17.0–24.0] | 0.822 |
| Induction | 72.7% | 64.5% | 0.620 |
| Thymoglobulin (%) | 45.5% | 16.1% | 0.050 |
| Basiliximab (%) | 27.3% | 48.4% | 0.224 |
| Delayed graft function (%) | 36.4% | 22.6% | 0.372 |
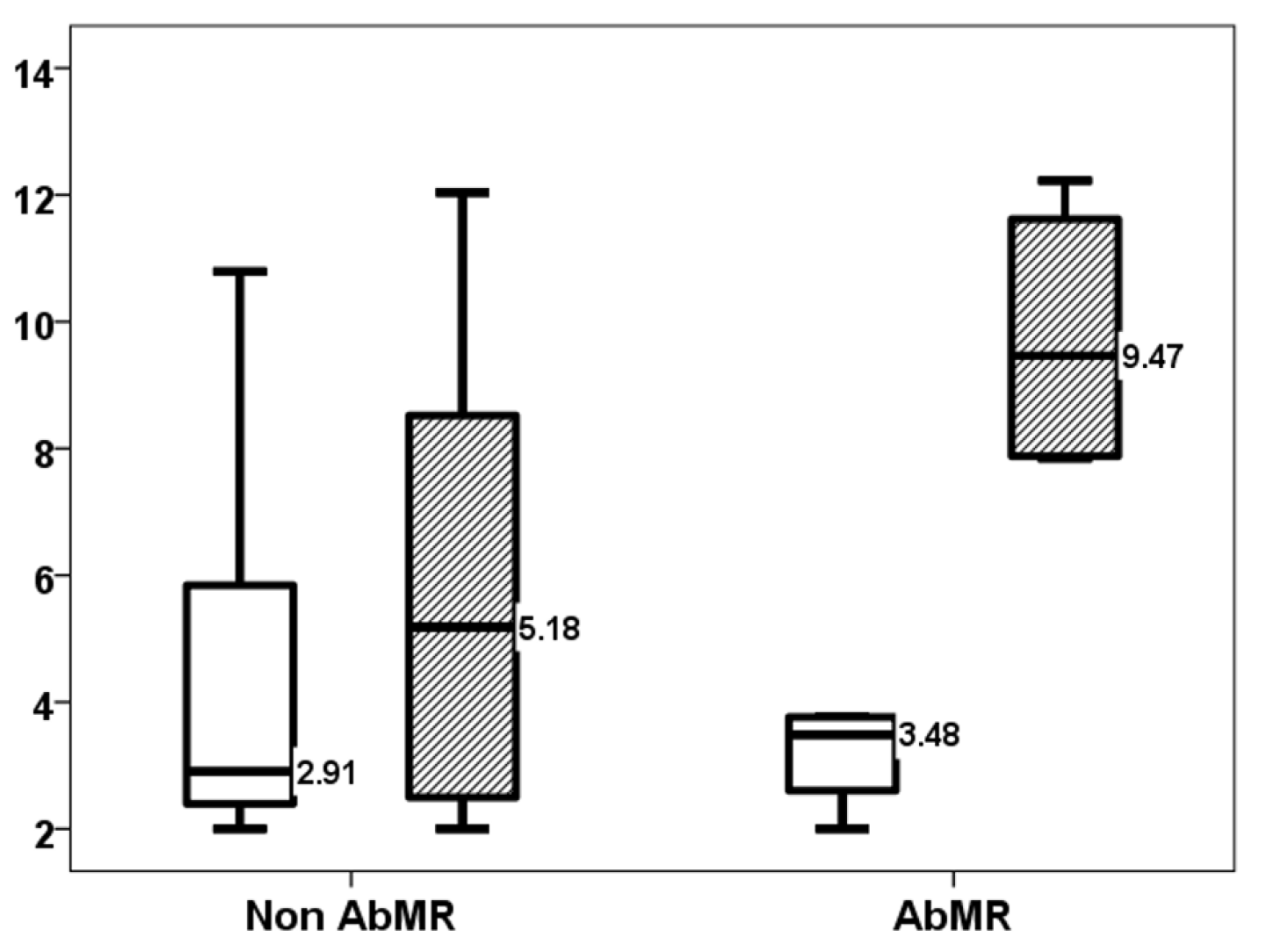
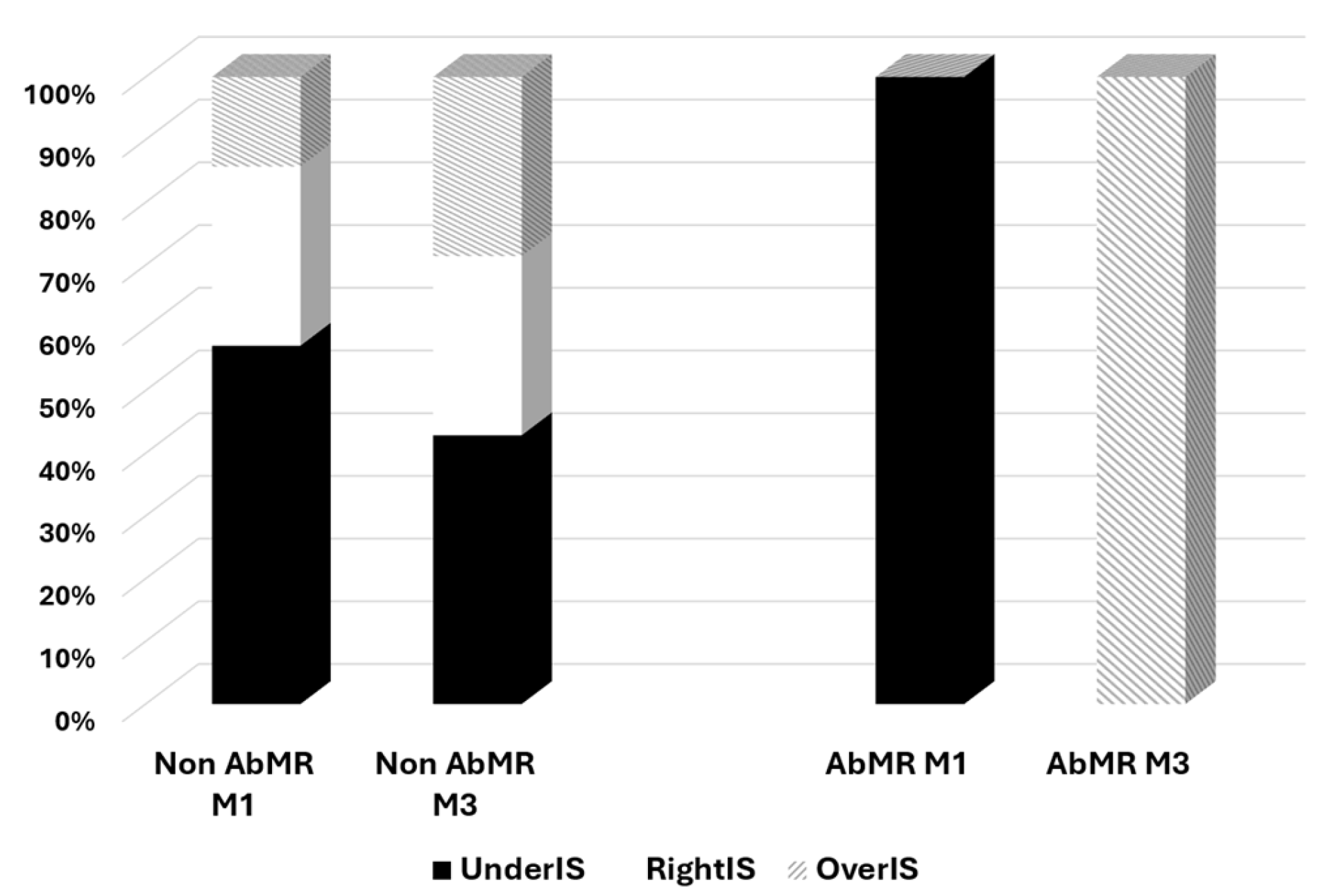
| 1-month AbMR | 36.4% | 3.2% | 0.004 |
| Infection from months 1 to 3 | 36.4% | 41.9% | 0.746 |
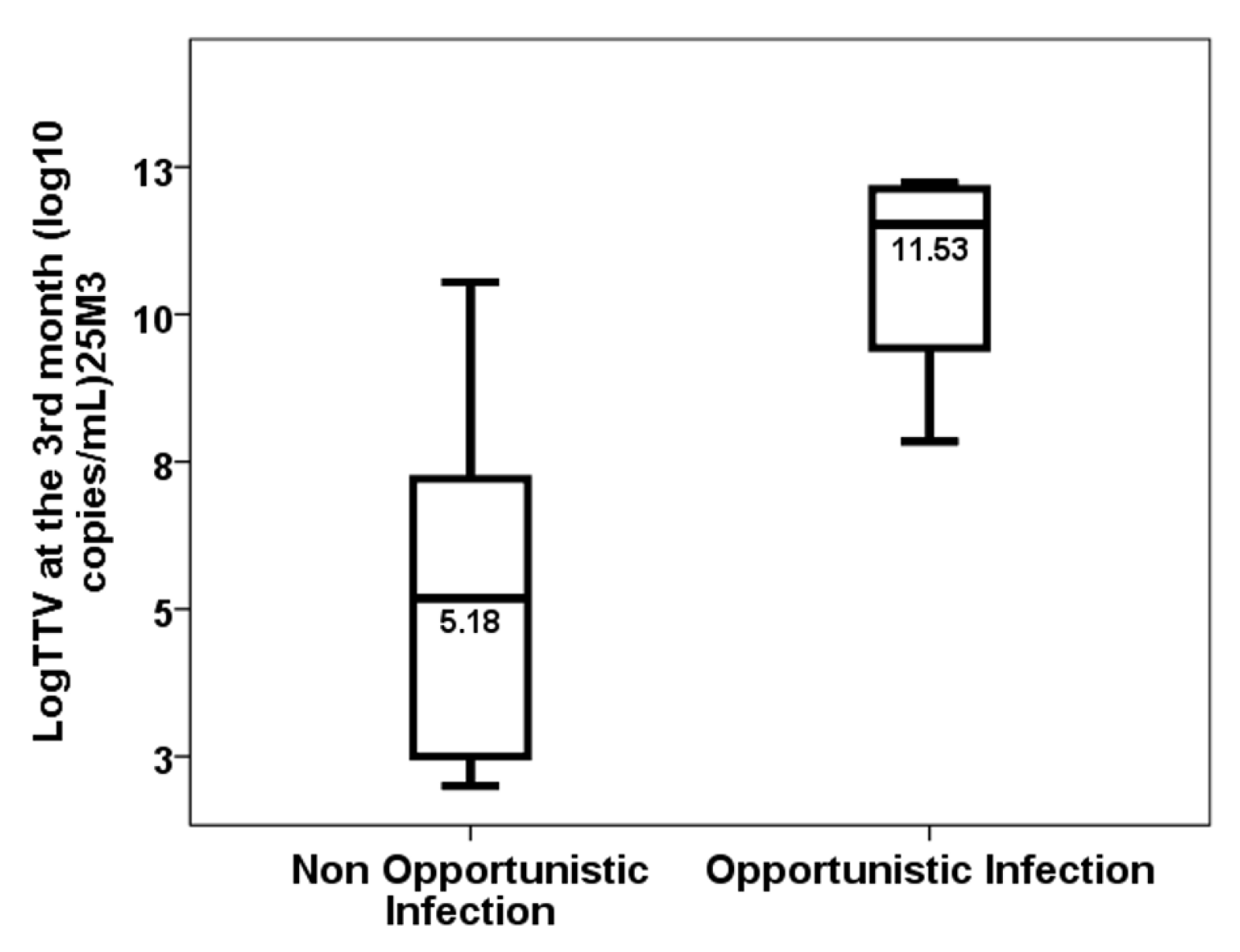
| Opportunistic infection from months 1 to 3 | 27.3% | 12.9% | 0.272 |
| First-month eGFR (mL/min/1.73 m2) | 43.0 [28.0–54.0] | 51.0 [42.0–72.0] | 0.138 |
| 1-year eGFR (mL/min/1.73 m2) | 45.0 [38.0–49.0] | 50.0 [38.0–71.8] | 0.514 |
| First-month mean Tacrolimus trough level (ng/mL) | 12.5 [11.4–14.3] | 12.3 [11.2–14.2] | 0.822 |
| Tacrolimus trough level at month 1 (ng/mL) | 13.0 [10.0–15.0] | 12.0 [9.0–14.0] | 0.233 |
| Mycophenolic acid trough level at month 1 (ng/mL) | 2.0 [1.8–4.0] | 2.0 [1.0–3.0] | 0.569 |
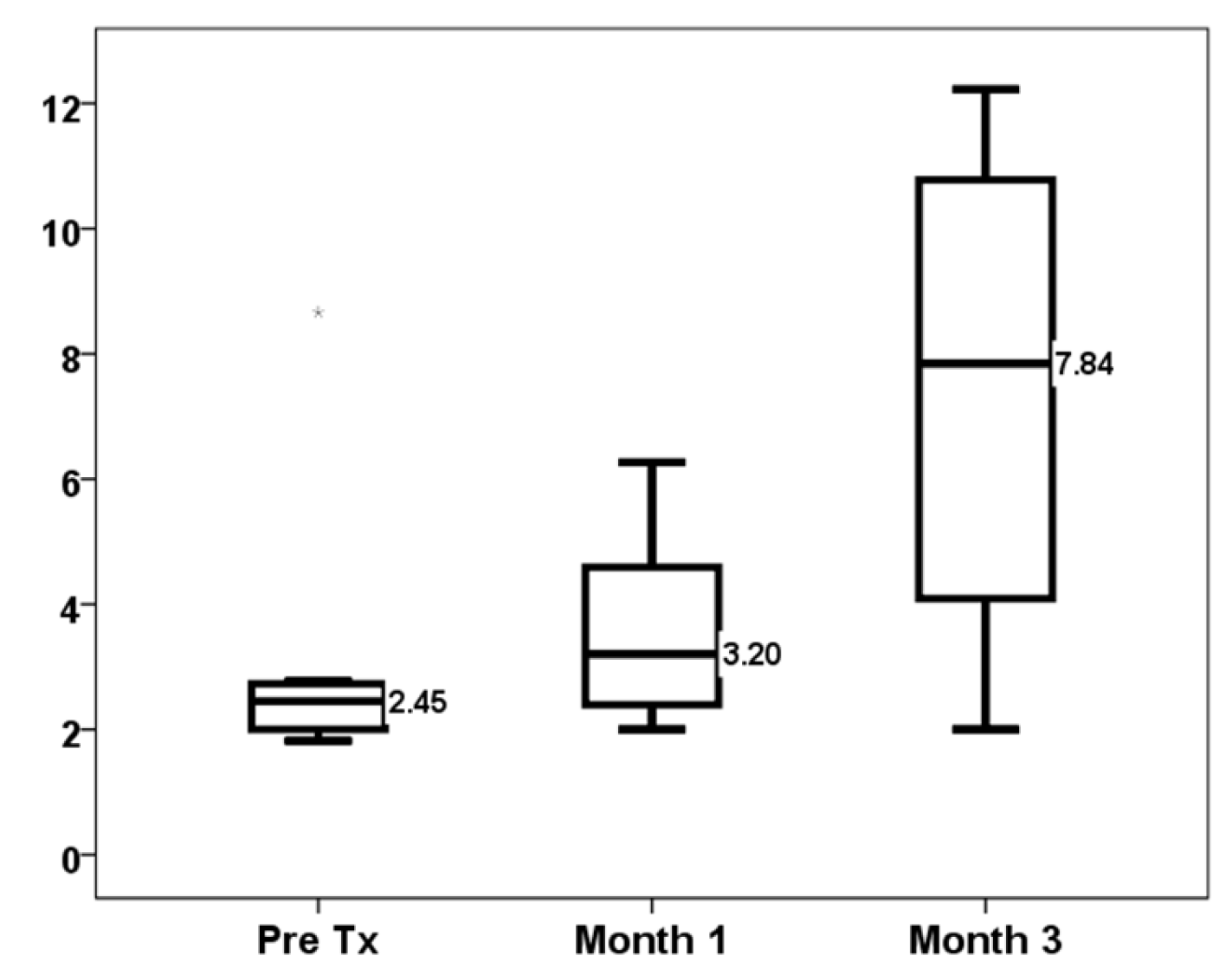
| First-month TTV load (log10 copies/mL) | 3.20 [2.09–5.41] | 2.23 [1.18–3.28] | 0.117 |
| Third-month TTV load (log10 copies/mL) | 7.84 [3.00–11.01] | 5.72 [2.85–8.59] | 0.295 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigo, E.; González-López, E.; Ocejo-Vinyals, J.G.; Pasache, E.; García-Majado, C.; López del Moral, C.; García-Santiago, A.; Benito-Hernández, A.; Francia, M.V.; Ruiz, J.C. Exploring Net Immunosuppressive Status with Torque Teno Virus Viral Load in Kidney Transplant Recipients with High Molecular Injury. J. Clin. Med. 2025, 14, 2417. https://doi.org/10.3390/jcm14072417
Rodrigo E, González-López E, Ocejo-Vinyals JG, Pasache E, García-Majado C, López del Moral C, García-Santiago A, Benito-Hernández A, Francia MV, Ruiz JC. Exploring Net Immunosuppressive Status with Torque Teno Virus Viral Load in Kidney Transplant Recipients with High Molecular Injury. Journal of Clinical Medicine. 2025; 14(7):2417. https://doi.org/10.3390/jcm14072417
Chicago/Turabian StyleRodrigo, Emilio, Elena González-López, Javier Gonzalo Ocejo-Vinyals, Enrique Pasache, Cristina García-Majado, Covadonga López del Moral, Ana García-Santiago, Adalberto Benito-Hernández, María Victoria Francia, and Juan Carlos Ruiz. 2025. "Exploring Net Immunosuppressive Status with Torque Teno Virus Viral Load in Kidney Transplant Recipients with High Molecular Injury" Journal of Clinical Medicine 14, no. 7: 2417. https://doi.org/10.3390/jcm14072417
APA StyleRodrigo, E., González-López, E., Ocejo-Vinyals, J. G., Pasache, E., García-Majado, C., López del Moral, C., García-Santiago, A., Benito-Hernández, A., Francia, M. V., & Ruiz, J. C. (2025). Exploring Net Immunosuppressive Status with Torque Teno Virus Viral Load in Kidney Transplant Recipients with High Molecular Injury. Journal of Clinical Medicine, 14(7), 2417. https://doi.org/10.3390/jcm14072417






