Clinical Outcome of Endoscopic and Endoscopic-Assisted Microscopic Removal of Glomus Tympanicum: A Multicenter Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Selection
2.2. Glomus Tympanicum Evaluation
2.3. Surgical Technique
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
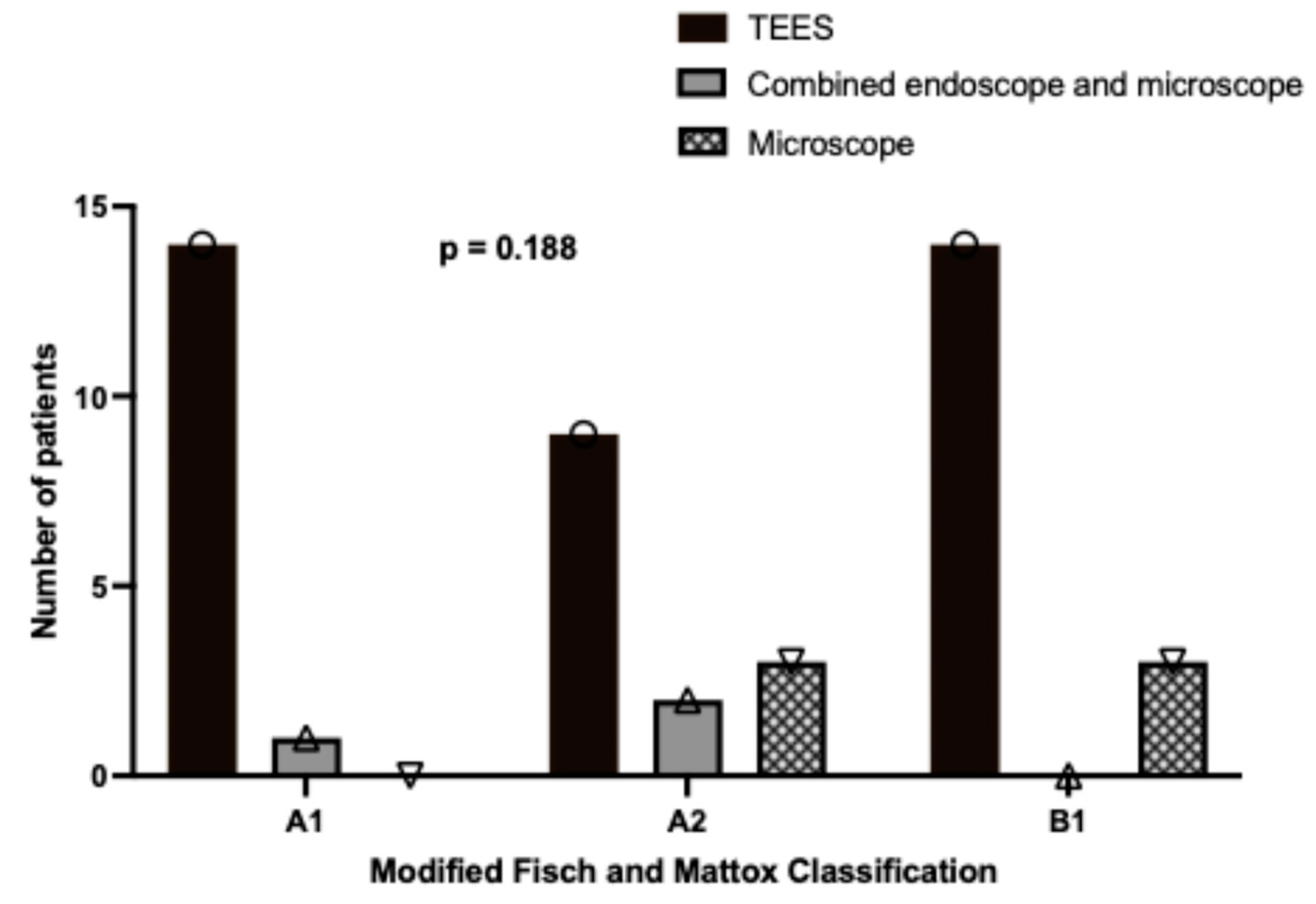
3.2. Relationship Between Disease Stage and Surgical Approach
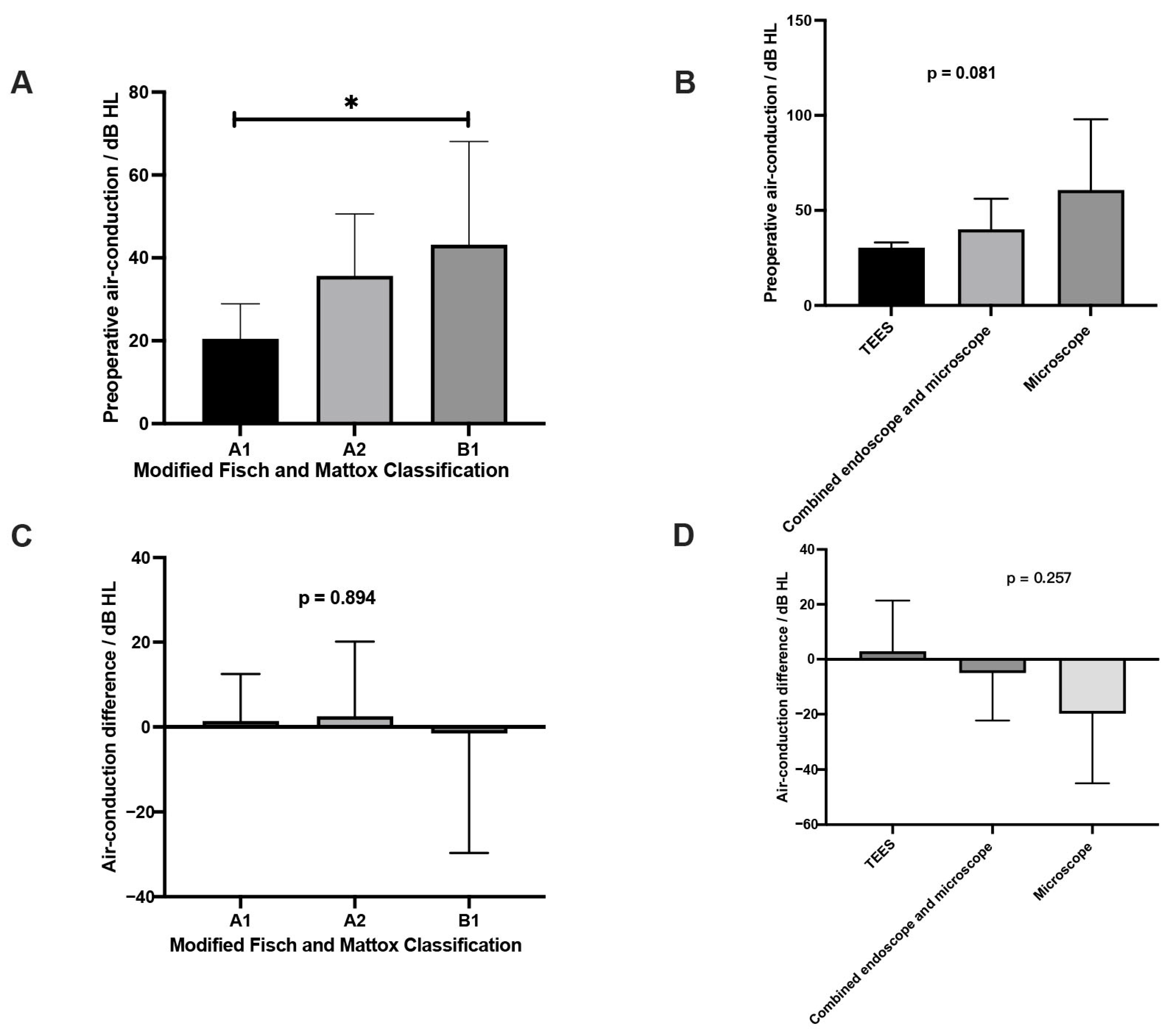
3.3. Comparisons of Characteristics Between Preoperative and Postoperative
3.4. Recurrence of Paraganglioma
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boedeker, C.C.; Ridder, G.J.; Schipper, J. Paragangliomas of the head and neck: Diagnosis and treatment. Fam. Cancer 2005, 4, 55–59. [Google Scholar] [PubMed]
- Sanna, M.; Fois, P.; Pasanisi, E.; Russo, A.; Bacciu, A. Middle ear and mastoid glomus tumors (glomus tympanicum): An algorithm for the surgical management. Auris Nasus Larynx 2010, 37, 661–668. [Google Scholar] [CrossRef]
- Sahoo, P.R.; Sahu, M.; Khan, I.W.; Samantaray, K. Glomus tympanicum removal using transcanal endoscopic assisted surgery: An experience with six cases. World J. Otorhinolaryngol.-Head Neck Surg. 2023, 9, 302–307. [Google Scholar] [CrossRef]
- Ghate, G.; Bhatnagar, A.; Mukhtar, S. Post-Embolization Excision of Glomus Tympanicum: A Case Report. Cureus 2022, 14, e21414. [Google Scholar]
- Fisch, U.; Mattox, D.E. Microsurgery of the Skull Base; George Thieme Verlag: Stuttgart, Germany, 1988. [Google Scholar]
- Jackson, C.G.; Glasscock, M.E., 3rd; Harris, P.F. Glomus Tumors. Diagnosis, classification, and management of large lesions. Arch. Otolaryngol. 1982, 108, 401–410. [Google Scholar] [PubMed]
- Kunnath, A.J.; Freeman, M.H.; Witcher, R.; Patro, A.; Lindquist, N.R.; Tawfik, K.O. Endoscopic Versus Microscopic Transcanal Resection of Glomus Tympanicum: A Retrospective Comparative Study. Otol. Neurotol. 2024, 45, 426–429. [Google Scholar] [CrossRef]
- Fermi, M.; Ferri, G.; Ebaied, T.B.; Alicandri-Ciufelli, M.; Bonali, M.; El-Dine, M.B.; Presutti, L. Transcanal Endoscopic Management of Glomus Tympanicum: Multicentric Case Series. Otol. Neurotol. 2021, 42, 312–318. [Google Scholar] [PubMed]
- Killeen, D.E.; Wick, C.C.; Hunter, J.B.; Rivas, A.; Wanna, G.B.; Nogueira, J.F.; Kutz, J.W.J.; Isaacson, B. Endoscopic Management of Middle Ear Paragangliomas: A Case Series. Otol. Neurotol. 2017, 38, 408–415. [Google Scholar]
- Kaul, V.F.; Filip, P.; Schwam, Z.G.; Wanna, G.B. Nuances in transcanal endoscopic surgical technique for glomus tympanicum tumors. Am. J. Otolaryngol. 2020, 41, 102562. [Google Scholar]
- Quick, M.E.; Acharya, A.; Friedland, P.; Kong, J.H.K.; Saxby, A.J.; Patel, N.P.; Kadhim, L. Endoscopic Management of Early Stage Middle Ear Paragangliomas—An Australian Case Series. Otol. Neurotol. 2021, 42, e1677–e1682. [Google Scholar] [CrossRef]
- Surmelioglu, O.; Bajin, M.D.; Kaya, I.; Okuyucu, S.; Ozturk, K.; Orhan, K.S.; Karlıdag, T.; Ardıc, F.N.; Ozdek, A.; Yorgancılar, E.; et al. Transcanal Endoscopic Management of Middle Ear Paragangliomas. Otol. Neurotol. 2023, 44, 798–803. [Google Scholar]
- Nogueira, J.F.; de Sousa Lobo Ferreira Querido, R.; Gonçalves da Silva Leite, J.; Cabral da Costa, T. Future of Endoscopic Ear Surgery. Otolaryngol. Clin. N. Am. 2021, 54, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wu, J.; Lyu, J.; Chen, B.; Wang, W.; Chi, F.; Yuan, Y.; Ren, D. Microscopic Versus Endoscopic Ear Surgery for Early-Stage Glomus Tympanicum Tumors. Ear Nose Throat J. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Alaani, A.; Chavda, S.V.; Irving, R.M. The crucial role of imaging in determining the approach to glomus tympanicum tumours. Eur. Arch. Otorhinolaryngol. 2009, 266, 827–831. [Google Scholar]
- Sweeney, A.D.; Carlson, M.L.; Wanna, G.B.; Bennett, M.L. Glomus tympanicum tumors. Otolaryngol. Clin. N. Am. 2015, 48, 293–304. [Google Scholar]
- House, J.W.; Brackmann, D.E. Facial nerve grading system. Otolaryngol. Head Neck Surg. 1985, 93, 146–147. [Google Scholar]
- Patnaik, U.; Prasad, S.; Medina, M.; Al-Qahtani, M.; D’orazio, F.; Falcioni, M.; Piccirillo, E.; Russo, A.; Sanna, M. Long term surgical and hearing outcomes in the management of tympanomastoid paragangliomas. Am. J. Otolaryngol. 2015, 36, 382–389. [Google Scholar] [CrossRef]
- Yilala, M.H.; Fancello, G.; Fancello, V.; Lauda, L.; Sanna, M. Long-Term Surgical Outcome of Class A and B Tympanomastoid Paragangliomas. Cancers 2024, 16, 1466. [Google Scholar] [CrossRef]
- Liscak, R.; Urgosik, D.; Chytka, T.; Simonova, G.; Novotny, J.; Vymazal, J.; Guseynova, K.; Vladyka, V. Leksell Gamma Knife radiosurgery of the jugulotympanic glomus tumor: Long-term results. J. Neurosurg. 2014, 121 (Suppl. S2), 198–202. [Google Scholar]
- Lee, C.-C.; Pan, D.H.-C.; Wu, J.-C.; Chung, W.-Y.; Wu, H.-M.; Yang, H.-C.; Liu, K.-D.; Guo, W.-Y.; Shih, Y.-H. Gamma knife radiosurgery for glomus jugulare and tympanicum. Stereotact. Funct. Neurosurg. 2011, 89, 291–298. [Google Scholar]
- Carlson, M.L.; Sweeney, A.D.; Pelosi, S.; Wanna, G.B.; Glasscock, M.E., 3rd; Haynes, D.S. Glomus tympanicum: A review of 115 cases over 4 decades. Otolaryngol. Head Neck Surg. 2015, 152, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Ridge, S.E.; Shetty, K.R.; Lee, D.J. Current trends and applications in endoscopy for otology and neurotology. World J. Otorhinolaryngol.-Head Neck Surg. 2021, 7, 101–108. [Google Scholar] [CrossRef]
- Tarabichi, M.; Nogueira, J.F.; Marchioni, D.; Presutti, L.; Pothier, D.D.; Ayache, S. Transcanal endoscopic management of cholesteatoma. Otolaryngol. Clin. N. Am. 2013, 46, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Marchioni, D.; Alicandri-Ciufelli, M.; Gioacchini, F.M.; Bonali, M.; Presutti, L. Transcanal endoscopic treatment of benign middle ear neoplasms. Eur. Arch. Otorhinolaryngol. 2013, 270, 2997–3004. [Google Scholar] [CrossRef] [PubMed]
- Fountarlis, A.L.; Hajiioannou, J.; Lachanas, V.; Tsitiridis, I.; Saratziotis, A.; Alagianni, A.; Skoulakis, C. Endoscopic Management of Glomus Tympanicum Tumor: Report of Three Cases and Review of the Literature. J. Audiol. Otol. 2023, 27, 145–152. [Google Scholar] [CrossRef]
- Chaushu, H.; Butrus, F.; Oron, Y.; Handzel, O.; Abu-Eta, R.; Muhanna, N.; Ungar, O.J. Success and safety of endoscopic versus microscopic resection of temporal bone paraganglioma: A meta-analysis. Eur. Arch. Otorhinolaryngol. 2024, 281, 5119–5127. [Google Scholar] [CrossRef]
- Su, P.; Wrobel, B.B.; Zada, G.; Mack, W.J.; Ge, M.; Kim, J.; Ference, E.D. Thermal Conductance Through the Skull Base From Endoscopic Surgery Cauterization Instruments: Cadaver and Goat Model. Otolaryngol. Head Neck Surg. 2021, 165, 899–904. [Google Scholar] [CrossRef]
- Remacha, J.; Pujol, L.; Caballero-Borrego, M.; Sandoval, M.; Viza, I.; Codina, A.; Bernal-Sprekelsen, M.; Larrosa, F. Transcanal endoscopic carbon dioxide laser resection of early-stage (A1-B1) glomus tympanicum tumours: Single-centre case series. J. Laryngol. Otol. 2024, 138, 782–786. [Google Scholar] [CrossRef]
- Alkheder, A.; Ghareeb, A.; Almasalmeh, M.S.; Yousfan, A. Effective surgical management of glomus tympanicum tumor using diode laser: A case report study. Int. J. Surg. Case Rep. 2023, 107, 108356. [Google Scholar] [CrossRef]
- Molony, N.C.; Salto-Tellez, M.; Grant, W.E. KTP laser assisted excision of glomus tympanicum. J. Laryngol. Otol. 1998, 112, 956–958. [Google Scholar] [CrossRef]
- Robinson, P.J.; Grant, H.R.; Bown, S.G. NdYAG laser treatment of a glomus tympanicum tumour. J. Laryngol. Otol. 1993, 107, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; Ghoneim, M.; Ahmed, S.S.; Elsobki, A.; Elzhzahy, A.A.; Hemdan, A. Endoscopic transcanal coblation excision of glomus tympanicum: A novel technique. Eur. Arch. Otorhinolaryngol. 2024, 281, 4657–4664. [Google Scholar] [CrossRef] [PubMed]
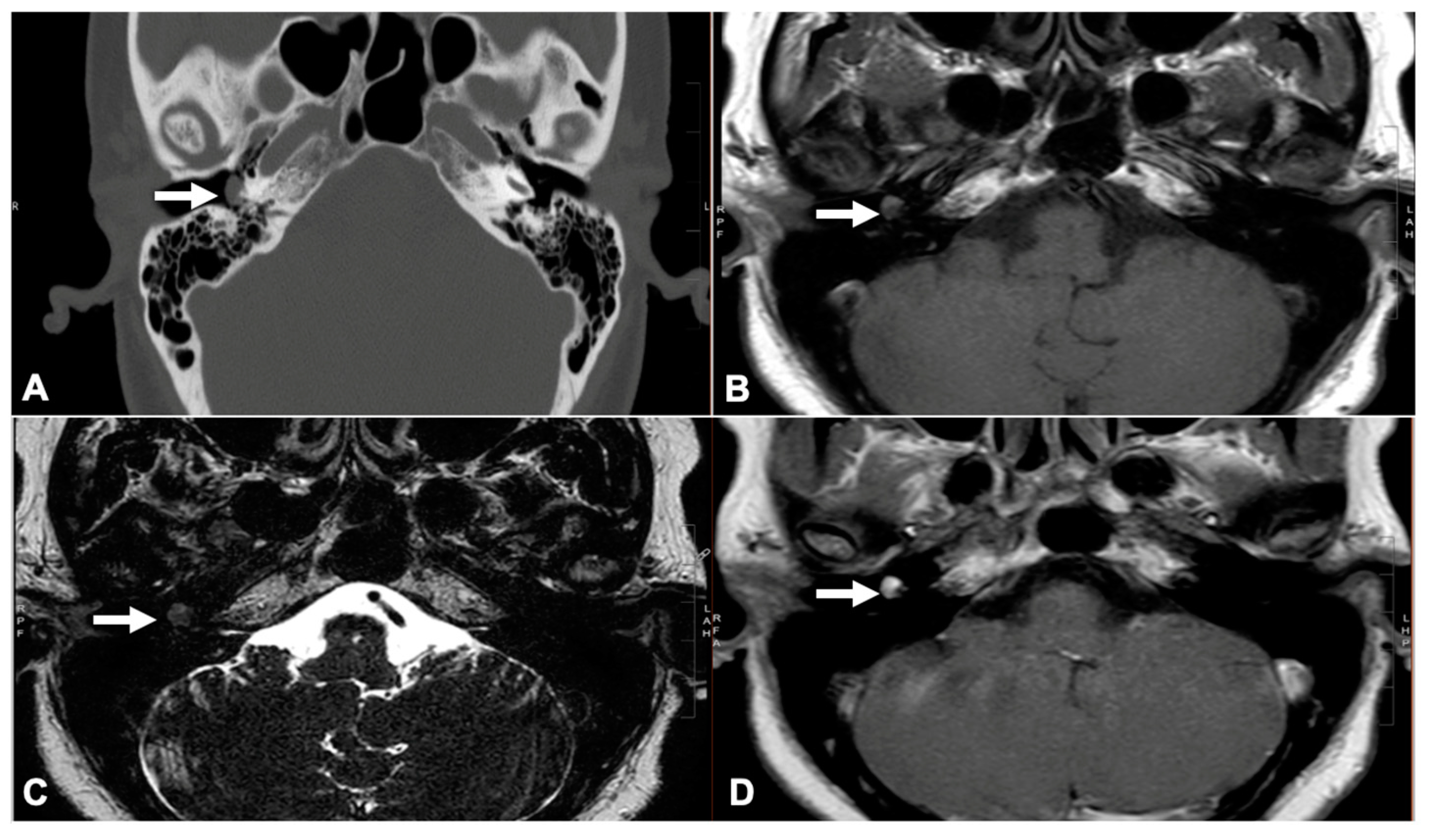

| Characteristic | N = 46, %/Median (Range) |
|---|---|
| Age (years) | 52 (22–83) |
| Gender | |
| Female | 82.6% (38/46) |
| Male | 17.4% (8/46) |
| Side | |
| Left | 54.3% (25/46) |
| Right | 45.7% (21/46) |
| Center | |
| Hong Kong, China | 17.4% (8/46) |
| Mainland, China | 30.4% (14/46) |
| Paris, France | 10.9% (5/46) |
| Egypt | 37.0% (17/46) |
| Oman | 4.3% (2/46) |
| Stage (Modified Fisch and Mattox Classification) | |
| A1 | 32.6% (15/46) |
| A2 | 30.4% (14/46) |
| B1 | 37.0% (17/46) |
| Surgical approach | |
| TEES | 80.4% (37/46) |
| Endoscopic-assisted microscope | 6.5% (3/46) |
| Microscope | 13.0% (6/46) |
| Follow-up time (years) | 4 (1–11) |
| Surgical Approach | Recurrence Rate | p-Value |
|---|---|---|
| TEES | 2.7% (1/37) | >0.05 ab, ac |
| Endoscopic-assisted microscope | 0% (0/3) | >0.05 ab, bc |
| Microscope | 0% (0/6) | >0.05 ac, bc |
| Stage | Recurrence Rate | p-Value |
|---|---|---|
| A1 | 0% (0/15) | >0.05 ab, ac |
| A2 | 0% (0/14) | >0.05 ab, bc |
| B1 | 5.9% (1/17) | >0.05 ac, bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.; Chen, X.; Badr-El-Dine, M.; Al Zaabi, K.; Cai, X.; Wang, Q.; Cornu, N.; Kania, R.; Tong, M.C.F. Clinical Outcome of Endoscopic and Endoscopic-Assisted Microscopic Removal of Glomus Tympanicum: A Multicenter Retrospective Study. J. Clin. Med. 2025, 14, 2388. https://doi.org/10.3390/jcm14072388
Chang W, Chen X, Badr-El-Dine M, Al Zaabi K, Cai X, Wang Q, Cornu N, Kania R, Tong MCF. Clinical Outcome of Endoscopic and Endoscopic-Assisted Microscopic Removal of Glomus Tympanicum: A Multicenter Retrospective Study. Journal of Clinical Medicine. 2025; 14(7):2388. https://doi.org/10.3390/jcm14072388
Chicago/Turabian StyleChang, Waitsz, Xiaoxin Chen, Mohamed Badr-El-Dine, Khalid Al Zaabi, Xinzhang Cai, Qi Wang, Nicolas Cornu, Romain Kania, and Michael Chi Fai Tong. 2025. "Clinical Outcome of Endoscopic and Endoscopic-Assisted Microscopic Removal of Glomus Tympanicum: A Multicenter Retrospective Study" Journal of Clinical Medicine 14, no. 7: 2388. https://doi.org/10.3390/jcm14072388
APA StyleChang, W., Chen, X., Badr-El-Dine, M., Al Zaabi, K., Cai, X., Wang, Q., Cornu, N., Kania, R., & Tong, M. C. F. (2025). Clinical Outcome of Endoscopic and Endoscopic-Assisted Microscopic Removal of Glomus Tympanicum: A Multicenter Retrospective Study. Journal of Clinical Medicine, 14(7), 2388. https://doi.org/10.3390/jcm14072388








