Development of a YOLOv3-Based Model for Automated Detection of Thoracic Ossification of the Posterior Longitudinal Ligament and the Ligamentum Flavum on Plain Radiographs
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
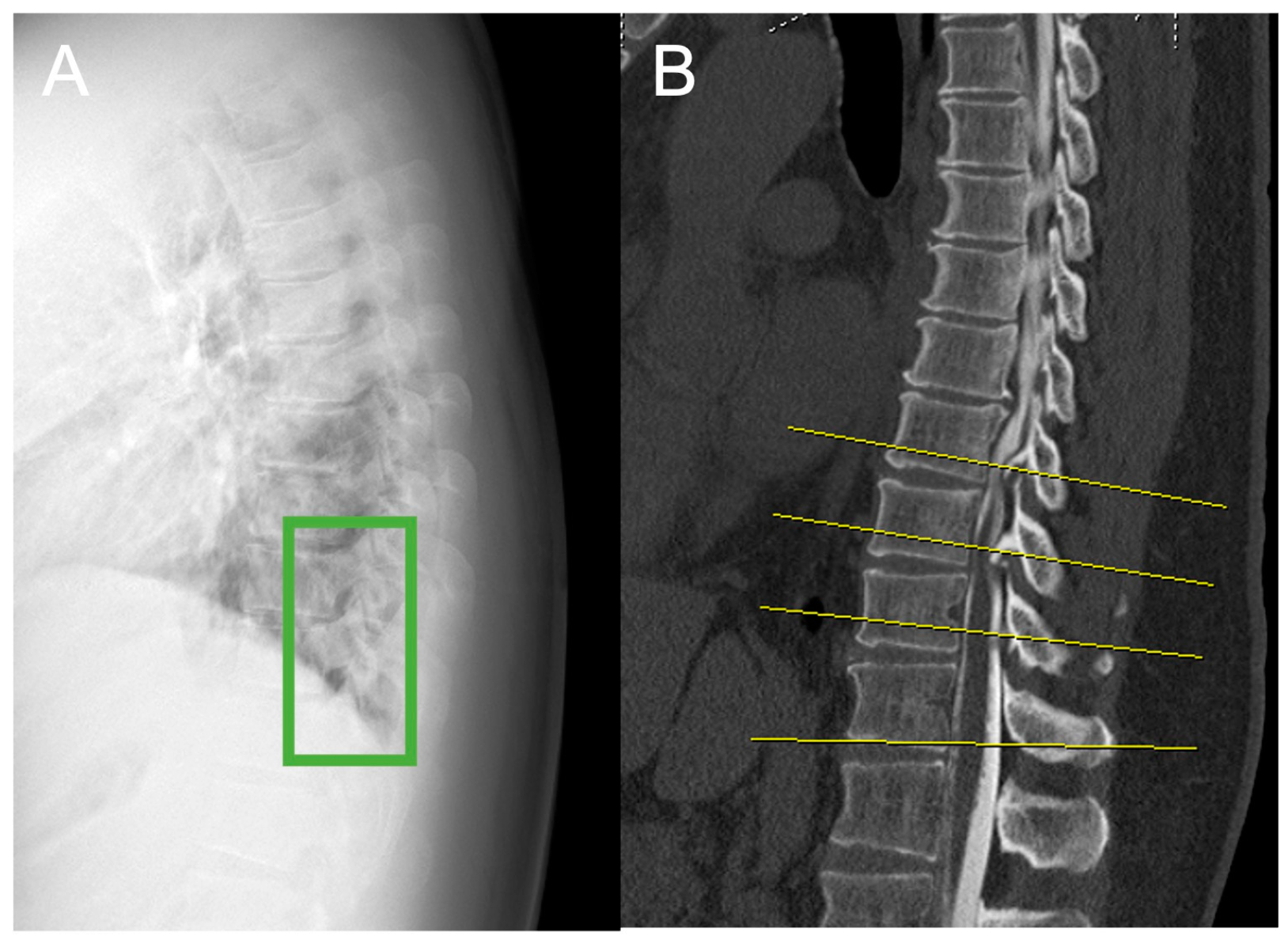
2.2. Plain Radiograph Dataset
2.3. Image Preparation and Annotation
2.4. Object Detection
2.5. Performance Evaluation
2.6. Image Assessment by Doctors
2.7. Statistical Analysis
3. Results
3.1. Comparative Analysis of True and False Outcomes
3.2. Overall Model Performance
3.3. Detection Performance Based on Ossification Type
3.4. Performance Across Thoracic Spine Levels
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Miyasaka, K.; Kaneda, K.; Sato, S.; Iwasaki, Y.; Abe, S.; Takei, H.; Tsuru, M.; Tashiro, K.; Abe, H.; Fujioka, Y. Myelopathy due to ossification or calcification of the ligamentum flavum: Radiologic and histologic evaluations. AJNR Am. J. Neuroradiol. 1983, 4, 629–632. [Google Scholar] [PubMed]
- Ando, K.; Imagama, S.; Kaito, T.; Takenaka, S.; Sakai, K.; Egawa, S.; Shindo, S.; Watanabe, K.; Fujita, N.; Matsumoto, M.; et al. Outcomes of surgery for thoracic myelopathy owing to thoracic ossification of the ligamentum flavum in a nationwide multicenter prospectively collected study in 223 patients: Is instrumented fusion necessary? Spine (Phila Pa 1976) 2020, 45, E170–E178. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.f.; Liu, F.-J.; Zhang, Y.z.; Shen, Y.; Ding, W.; Xu, J.x. Clinical results and intramedullary signal changes of posterior decompression with transforaminal interbody fusion for thoracic myelopathy caused by combined ossification of the posterior longitudinal ligament and ligamentum flavum. Chin. Med. J. 2013, 126, 3822. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Meng, H.; Du, J.; Tao, H.; Luo, Z.; Wang, Z. Management of thoracic myelopathy caused by ossification of the posterior longitudinal ligament combined with ossification of the ligamentum flavum-a retrospective study. Spine J. Off. J. N. Am. Spine Soc. 2012, 12, 1093–1102. [Google Scholar] [CrossRef]
- Onishi, E.; Yasuda, T.; Yamamoto, H.; Iwaki, K.; Ota, S. Outcomes of surgical treatment for thoracic myelopathy: A single-institutional study of 73 patients. Spine 2016, 41, E1356–E1363. [Google Scholar] [CrossRef]
- Miyazawa, N.; Akiyama, I. Ossification of the ligamentum flavum of the cervical spine. J. Neurosurg. Sci. 2007, 51, 139–144. [Google Scholar]
- Hou, X.; Sun, C.; Liu, X.; Liu, Z.; Qi, Q.; Guo, Z.; Li, W.; Zeng, Y.; Chen, Z. Clinical features of thoracic spinal stenosis-associated myelopathy: A retrospective analysis of 427 cases. Clin. Spine Surg. 2016, 29, 86–89. [Google Scholar] [CrossRef]
- Fujimori, T.; Le, H.; Hu, S.S.; Chin, C.; Pekmezci, M.; Schairer, W.; Tay, B.K.; Hamasaki, T.; Yoshikawa, H.; Iwasaki, M. Ossification of the posterior longitudinal ligament of the cervical spine in 3161 patients: A ct-based study. Spine (Phila Pa 1976) 2015, 40, E394–E403. [Google Scholar] [CrossRef]
- Choucha, A.; Travaglini, F.; De Simone, M.; Evin, M.; Farah, K.; Fuentes, S. The Da Vinci Robot, a promising yet underused minimally invasive tool for spine surgery: A scoping review of its current role and limits. Neurochirurgie 2025, 71, 101624. [Google Scholar] [CrossRef]
- Le, H.V.; Wick, J.B.; Van, B.W.; Klineberg, E.O. Ossification of the Posterior Longitudinal Ligament: Pathophysiology, Diagnosis, and Management. J. Am. Acad. Orthop. Surg. 2022, 30, 820–830. [Google Scholar] [CrossRef]
- Hirabayashi, S. Ossification of the ligamentum flavum. Spine Surg. Relat. Res. 2017, 1, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Nakashima, H.; Segi, N.; Ouchida, J.; Oda, M.; Yamauchi, I.; Oishi, R.; Miyairi, Y.; Mori, K.; Imagama, S. Automated detection of the thoracic ossification of the posterior longitudinal ligament using deep learning and plain radiographs. BioMed Res. Int. 2023, 2023, 8495937. [Google Scholar] [CrossRef]
- Ito, S.; Nakashima, H.; Segi, N.; Ouchida, J.; Oda, M.; Yamauchi, I.; Oishi, R.; Miyairi, Y.; Mori, K.; Imagama, S. Automated detection and diagnosis of spinal schwannomas and meningiomas using deep learning and magnetic resonance imaging. J. Clin. Med. 2023, 12, 5075. [Google Scholar] [CrossRef]
- Tzutalin, D. LabelImg. Git Code. 2015. Available online: https://github.com/tzutalin/labelImg (accessed on 26 March 2025).
- Tamai, K.; Terai, H.; Hoshino, M.; Yabu, A.; Tabuchi, H.; Sasaki, R.; Nakamura, H. A deep learning algorithm to identify cervical ossification of posterior longitudinal ligaments on radiography. Sci. Rep. 2022, 12, 2113. [Google Scholar] [CrossRef]
- Bochkovskiy, A.; Wang, C.-Y.; Liao, H.-Y.M. YOLOv4: Optimal speed and accuracy of object detection. arXiv 2020, arXiv:2004.10934. [Google Scholar]
- Joseph Redmon, A.F. Yolov3: An incremental improvement. arXiv 2018, arXiv:1804.02767. [Google Scholar]
- Okada, G.; Hosoi, S.; Kato, K.; Ohta, K.; Tachi, Y.; Sonoda, J.; Yada, H. Case report 779. Carbonate apatite calcification of ligamentum flavum. Skelet. Radiol. 1993, 22, 211–213. [Google Scholar] [CrossRef]
- Lee, S.E.; Jahng, T.A.; Kim, H.J. Surgical outcomes in patients with mild symptoms, but severely compressed spinal cord from cervical ossification of the posterior longitudinal ligament. J. Clin. Neurosci. 2016, 33, 163–168. [Google Scholar] [CrossRef]
- Ando, K.; Imagama, S.; Ito, Z.; Hirano, K.; Muramoto, A.; Kato, F.; Yukawa, Y.; Kawakami, N.; Sato, K.; Matsubara, Y.; et al. Predictive factors for a poor surgical outcome with thoracic ossification of the ligamentum flavum by multivariate analysis: A multicenter study. Spine (Phila Pa 1976) 2013, 38, E748–E754. [Google Scholar] [CrossRef]
- Hioki, A.; Miyamoto, K.; Hosoe, H.; Shimizu, K. Two-staged decompression for thoracic paraparesis due to the combined ossification of the posterior longitudinal ligament and the ligamentum flavum: A case report. Arch. Orthop. Trauma Surg. 2008, 128, 175–177. [Google Scholar] [CrossRef]
- Tomita, K. Total decompression of the spinal cord for combined ossification of posterior longitudinal ligament and yellow ligament in the thoracic spine. Arch. Orthop. Trauma Surg. 1990, 109, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Hafiz, M.S.; Jim, J.R.; Kabir, M.M.; Mridha, M.F. A systematic review of deep learning data augmentation in medical imaging: Recent advances and future research directions. Healthc. Anal. 2024, 5, 100340. [Google Scholar] [CrossRef]
- De Simone, M.; Choucha, A.; Ciaglia, E.; Conti, V.; Pecoraro, G.; Santurro, A.; Puca, A.A.; Cascella, M.; Iaconetta, G. Discogenic low back pain: Anatomic and pathophysiologic characterization, clinical evaluation, biomarkers, AI, and treatment options. J. Clin. Med. 2024, 13, 5915. [Google Scholar] [CrossRef] [PubMed]
- Turlip, R.; Khela, H.; Dagli, M.M.; Chauhan, D.; Ghenbot, Y.; Ahmad, H.; Yoon, J. Redefining precision: The current and future roles of artificial intelligence in spine surgery. Artif. Intell. Surg. 2024, 4, 324–330. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, J.; Ou, X.; Ying, H.; Hu, C.; Zhang, Z.; Hu, W. The impact of training sample size on deep learning-based organ auto-segmentation for head-and-neck patients. Phys. Med. Biol. 2021, 66, 185012. [Google Scholar] [CrossRef]
- Maki, S.; Furuya, T.; Katsumi, K.; Nakajima, H.; Honjoh, K.; Watanabe, S.; Kaito, T.; Takenaka, S.; Kanie, Y.; Iwasaki, M.; et al. Multimodal deep learning-based radiomics approach for predicting surgical outcomes in patients with cervical ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976) 2024, 49, 1561–1569. [Google Scholar] [CrossRef]

| Patients | Controls | ||
|---|---|---|---|
| N | 176 | 180 | |
| Sex (M/F) | 90/86 | 90/90 | |
| Age (years) | 54.9 ± 14.6 | 55.7 ± 17.6 | |
| Height (cm) | 161.3 ± 10.0 | 158.3 ± 12.1 | |
| Weight (kg) | 79.9 ± 21.1 | 59.6 ± 16.2 | |
| Level of thoracic spine | Upper | 112 | n.a. |
| Middle | 97 | n.a. | |
| Lower | 63 | n.a. | |
| Type of Ossification | OPLL | 95 | n.a. |
| OLF | 30 | n.a. | |
| OPLL+OLF | 51 | n.a. | |
| Detection (n) | ||||
|---|---|---|---|---|
| TP | FP | FN | TN | |
| Object detection | 136 | 58 | 12 | 150 |
| Spine surgeon 1 | 124 | 43 | 37 | 156 |
| Spine surgeon 2 | 122 | 54 | 42 | 144 |
| AC (%) | PR (%) | RR (%) | F (%) | |
|---|---|---|---|---|
| Object detection | 80.6 | 70.3 | 92.6 | 79.9 |
| Spine surgeon 1 | 78.1 | 74.9 | 77.2 | 76.0 |
| Spine surgeon 2 | 73.8 | 69.5 | 75.0 | 72.1 |
| OPLL Type | Accuracy (%) | ||
|---|---|---|---|
| Object Detection | Surgeon 1 | Surgeon 2 | |
| OPLL | 81.1 | 74.7 | 72.6 |
| OLF | 53.3 | 50.0 | 53.3 |
| OPLL+OLF | 86.3 | 76.5 | 74.5 |
| Level of the Thoracic Spine | Accuracy (%) | ||
|---|---|---|---|
| Object Detection | Surgeon 1 | Surgeon 2 | |
| Upper | 91.1 | 84.8 | 83.0 |
| Middle | 87.6 | 78.4 | 75.3 |
| Lower | 63.5 | 63.5 | 58.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, S.; Nakashima, H.; Segi, N.; Ouchida, J.; Yamauchi, I.; Hirai, T.; Oda, M.; Mori, K.; Yamazaki, M.; Yoshii, T.; et al. Development of a YOLOv3-Based Model for Automated Detection of Thoracic Ossification of the Posterior Longitudinal Ligament and the Ligamentum Flavum on Plain Radiographs. J. Clin. Med. 2025, 14, 2389. https://doi.org/10.3390/jcm14072389
Ito S, Nakashima H, Segi N, Ouchida J, Yamauchi I, Hirai T, Oda M, Mori K, Yamazaki M, Yoshii T, et al. Development of a YOLOv3-Based Model for Automated Detection of Thoracic Ossification of the Posterior Longitudinal Ligament and the Ligamentum Flavum on Plain Radiographs. Journal of Clinical Medicine. 2025; 14(7):2389. https://doi.org/10.3390/jcm14072389
Chicago/Turabian StyleIto, Sadayuki, Hiroaki Nakashima, Naoki Segi, Jun Ouchida, Ippei Yamauchi, Takashi Hirai, Masahiro Oda, Kensaku Mori, Masashi Yamazaki, Toshitaka Yoshii, and et al. 2025. "Development of a YOLOv3-Based Model for Automated Detection of Thoracic Ossification of the Posterior Longitudinal Ligament and the Ligamentum Flavum on Plain Radiographs" Journal of Clinical Medicine 14, no. 7: 2389. https://doi.org/10.3390/jcm14072389
APA StyleIto, S., Nakashima, H., Segi, N., Ouchida, J., Yamauchi, I., Hirai, T., Oda, M., Mori, K., Yamazaki, M., Yoshii, T., & Imagama, S. (2025). Development of a YOLOv3-Based Model for Automated Detection of Thoracic Ossification of the Posterior Longitudinal Ligament and the Ligamentum Flavum on Plain Radiographs. Journal of Clinical Medicine, 14(7), 2389. https://doi.org/10.3390/jcm14072389





