Utility of the Fragility Score (FS) Determined Through Radiofrequency Ecographic Multi-Spectrometry (REMS) in the Follow-Up of Patients with Axial Spondyloarthritis (AxSpA)
Abstract
1. Introduction
- Secondary hyperparathyroidism: Inadequate vitamin D reduces intestinal calcium absorption, leading to hypocalcemia. The resulting compensatory increase in PTH (secondary hyperparathyroidism) stimulates osteoclast activity to mobilize calcium from bone, chronically driving excessive bone resorption and increasing fracture risk [8].
- Impaired bone matrix formation: Deficient VDR activation reduces the transcription of genes responsible for synthesizing key bone matrix proteins, resulting in a suboptimal collagen framework and poor-quality bone that is more susceptible to fractures [9].
- Altered osteoblast–osteoclast coupling: Insufficient calcitriol impairs osteoblast function, while unopposed PTH action enhances osteoclast-mediated resorption, contributing to the progression from osteopenia to osteoporosis [10].
- Baseline assessment: Evaluate serum 25(OH)D, calcium, and PTH levels before initiating supplementation to tailor the dose to the patient’s needs.
- Monitoring and adjustments: Regular monitoring of vitamin D levels, bone turnover markers, and inflammatory indices (e.g., CRP, ESR) allows for dosage adjustments to optimize outcomes [18].
- Adjunctive lifestyle modifications: Weight-bearing exercise, adequate sunlight exposure, and a diet rich in calcium and vitamin D are essential to support bone health [18].
- Vitamin D is integrally involved in both skeletal and immune health. Its active metabolite, calcitriol, is vital for optimal calcium absorption, bone matrix integrity, and the modulation of inflammatory processes. In conditions such as osteopenia, osteoporosis, and axial spondyloarthritis, vitamin D deficiency can trigger secondary hyperparathyroidism and impaired bone remodeling, thereby increasing fracture risk and contributing to abnormal bone formation. Future research should focus on the following:
- Mechanistic studies: Detailed explorations of VDR activation, NF-κB inhibition, and the modulation of the RANK/RANKL/OPG axis in both normal and inflamed bone tissue.
- Randomized controlled trials: Well-designed studies to determine optimal dosing; the long-term benefits of combination therapy with vitamin D, calcium, and vitamin K2; and impacts on clinical outcomes such as fracture rates and disease activity in axSpA.
- Personalized supplementation strategies: Developing individualized treatment protocols based on genetic predispositions, baseline vitamin D status, and inflammatory profiles to maximize benefits and minimize risks [19].
2. Materials and Methods
- Having a positive AxSpA diagnosis based on the ASAS classification criteria (2009) [25];
- Having completed standard bloodwork specific for AxSpA (must include inflammatory markers like ESR and CRP);
- Having completed lumbar spine X-rays (at least lateral incidence);
- Having completed blood tests detecting the presence of HLA-B27 at any time in the past;
- Not diagnosed with any other pathology that could influence bone metabolism (e.g., diabetes mellitus, hyperparathyroidism, hypothyroidism);
- Not taking medication that reduces bone density (glucocorticoid therapy orally, intravenously, or intramuscularly);
- Having read, understood, and signed the informed consent form;
- Having a recent test result for 25-OH-Vitamin D, or performing the test within a month of the first visit;
- Agreeing to biannual follow-up visits for 18 months.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanis, J.A. Assessment of Osteoporosis at the Primary Health Care Level; WHO Collaborating Centre for Metabolic Bone Diseases, University of Sheffield: Sheffield, UK, 2007. [Google Scholar]
- Machado, P.; Landewe, R.; Braun, J.; Van Der Heijde, D.; Ramiro, S.; Baraliakos, X.; van den Bosch, F.; Sepriano, A.; Regel, A.; Ciurea, A.; et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann. Rheum. Dis. 2017, 76, 978–991. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Vitamin D physiology. Prog. Biophys. Mol. Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef] [PubMed]
- Lips, P.; van Schoor, N.M. The effect of vitamin D on bone and osteoporosis. Best Pr. Res. Clin. Endocrinol. Metab. 2011, 25, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef]
- Gómez-García, I.; Ladehesa-Pineda, M.L.; Diaz-Tocados, J.M.; López-Medina, C.; Abalos-Aguilera, M.C.; Ruiz-Vilches, D.; Paz-Lopez, G.; Gonzalez-Jimenez, A.; Ranea, J.A.G.; Escudero-Contreras, A.; et al. Bone metabolism and inflammatory biomarkers in radiographic and non-radiographic axial spondyloarthritis patients: A comprehensive evaluation. Front. Endocrinol. 2024, 15, 1227196. [Google Scholar] [CrossRef]
- van der Heijde, D.; Dougados, M. New bone formation in spondyloarthritis. Curr. Opin. Rheumatol. 2013, 25, 461–467. [Google Scholar] [CrossRef]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and the immune system: New perspectives on an old theme. Rheum. Dis. Clin. N. Am. 2010, 39, 365–379. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Cortet, B.; Dennison, E.; Diez-Perez, A.; Locquet, M.; Muratore, M.; Nogués, X.; Crespo, D.O.; Quarta, E.; Brandi, M.L. Radiofrequency echographic multi spectrometry (REMS) for the diagnosis of osteoporosis in a European multicenter clinical context. Bone 2021, 143, 115786. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, M.; Gatti, D.; Viapiana, O.; Cianferotti, L.; Cavalli, L.; Caffarelli, C.; Conversano, F.; Quarta, E.; Pisani, P.; Girasole, G.; et al. Radiofrequency echographic multi spectrometry compared with dual-energy X-ray absorptiometry for osteoporosis diagnosis on lumbar spine and femoral neck. Osteoporos. Int. 2019, 30, 391–402. [Google Scholar] [CrossRef]
- Al Refaie, A.; Baldassini, L.; Mondillo, C.; Giglio, E.; De Vita, M.; Tomai Pitinca, M.D.; Gonnelli, S.; Caffarelli, C. Radiofrequency Echographic Multi Spectrometry (R.E.M.S.): New Frontiers for Ultrasound Use in the Assessment of Bone Status—A Current Picture. Diagnostics 2023, 13, 1666. [Google Scholar] [CrossRef]
- Pisani, P.; Conversano, F.; Muratore, M.; Adami, G.; Brandi, M.L.; Caffarelli, C.; Casciaro, E.; Di Paola, M.; Franchini, R.; Gatti, D.; et al. Fragility Score: A REMS-based indicator for the prediction of incident fragility fractures at 5 years. Aging Clin. Exp. Res. 2023, 35, 763–773. [Google Scholar] [CrossRef]
- AAOS Now. Radiofrequency Echographic Multi-Spectrometry Delivers a New Form of Ultrasound to Assess Bone Density and Quality. Available online: https://www.aaos.org/aaosnow/2024/dec/clinical/clinical02/ (accessed on 14 December 2024).
- Koçyiğit, B.F.; Akyol, A. Vitamin D levels in patients with ankylosing spondylitis: Is it related to disease activity? Pak. J. Med Sci. 2018, 34, 1209–1214. [Google Scholar] [CrossRef]
- Badea, I.-A.; Bojinca, M.; Milicescu, M.; Vutcanu, O.; Ilina, A.-R. Literature review of Radiofrequency Echographic Multi-Spectrometry (REMS) in the diagnosis of osteoporosis and bone fragility. Romanian J. Rheumatol. 2022, 31, 18–21. [Google Scholar] [CrossRef]
- Pitinca, M.T.; Caffarelli, C.; Gonnelli, S. THU0636-HPR REMS TECHNOLOGY APPLIED TO RHEUMATIC DISEASE. Ann. Rheum Dis. 2021, 79, 563. [Google Scholar]
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef]
- Shevchuk, S.; Pavliuk, O.; Shevchuk, O.; Zviahina, O. AB1046 Level of Vitamin D in Men with Ankylosing Spondylitis, Connection with Disease Activity and Seasonality. Ann. Rheum. Dis. 2023, 82, 1745. [Google Scholar]
- Briot, K.; Roux, C. Inflammation, bone loss and fracture risk in spondyloarthritis. RMD Open. 2015, 1, e000052. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Żuchowski, P.; Dura, M.; Jeka, D.; Wojciechowski, R.; Bierwagen, M.; Kułakowski, M. Osteoporosis in axial radiographic spondyloarthritis: Diagnostic limitations of bone mineral density and the need for comprehensive fracture risk assessment. Reumatologia. 2024, 62, 466–474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deng, S.; He, Y.; Nian, X.; Sun, E.; Li, L. Relationship between Vitamin D levels and pain and disease activity in patients with newly diagnosed axial spondyloarthritis. Int. J. Nurs. Sci. 2019, 7, 54–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rejnmark, L. Effects of vitamin d on muscle function and performance: A review of evidence from randomized controlled trials. Ther. Adv. Chronic Dis. 2011, 2, 25–37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, F.L.; Li, T.; Gao, Q.Y.; Huang, Y.; Zhou, C.P.; Wang, W.; Qian, J.X. Association Between Vitamin D Supplementation and Fall Prevention. Front. Endocrinol. 2022, 13, 919839. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Male (n = 59) | Female (n = 17) | |
|---|---|---|
| Age mean (range) a | 37.22 (20–65) | 36.06 (22–49) |
| Underweight (%) | 0 (0%) | 0 (0%) |
| Normal weight (%) | 10 (16.949%) | 5 (29.41%) |
| Overweight (%) | 31 (52.54%) | 10 (58.82%) |
| Grade I obesity (%) | 18 (30.508%) | 2 (11.76%) |
| Vitamin D mild deficit (n = 8, 10.81%) a | 3 (5.08%) | 5 (29.41%) |
| All (n = 76) | Treatment group (n = 38) | Non-treatment group (n = 38) |
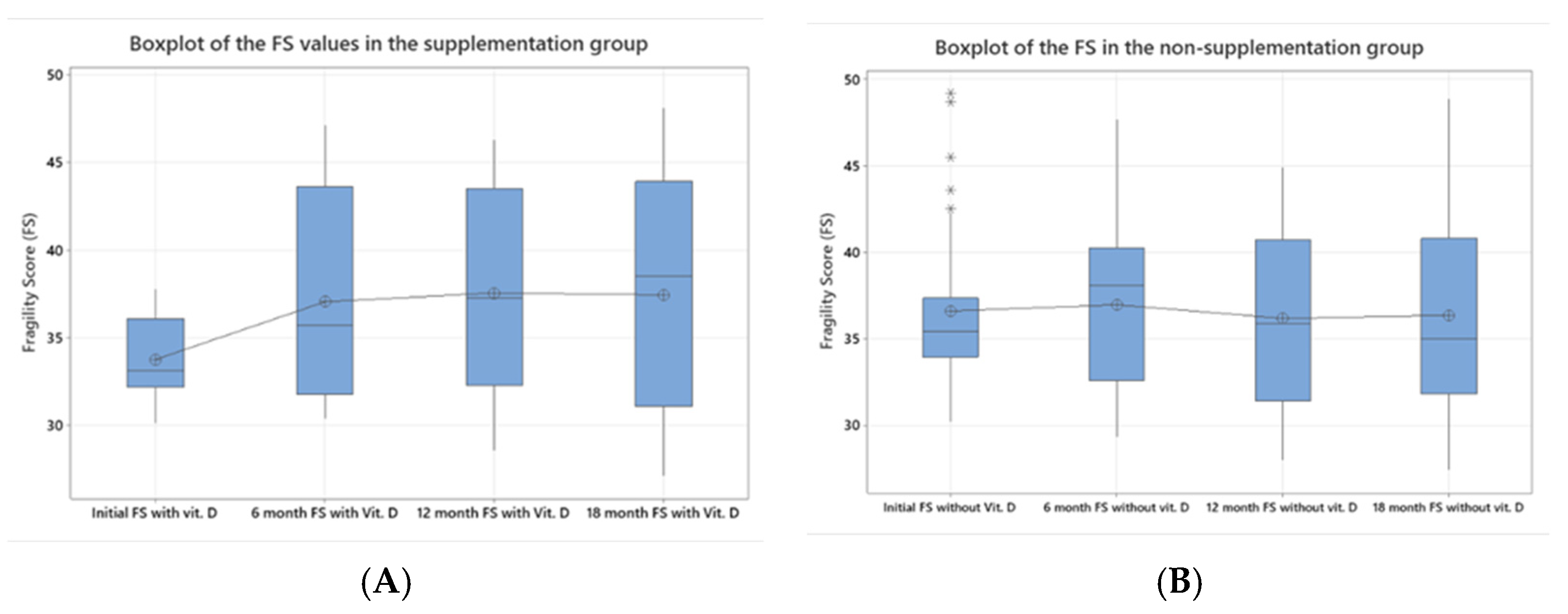
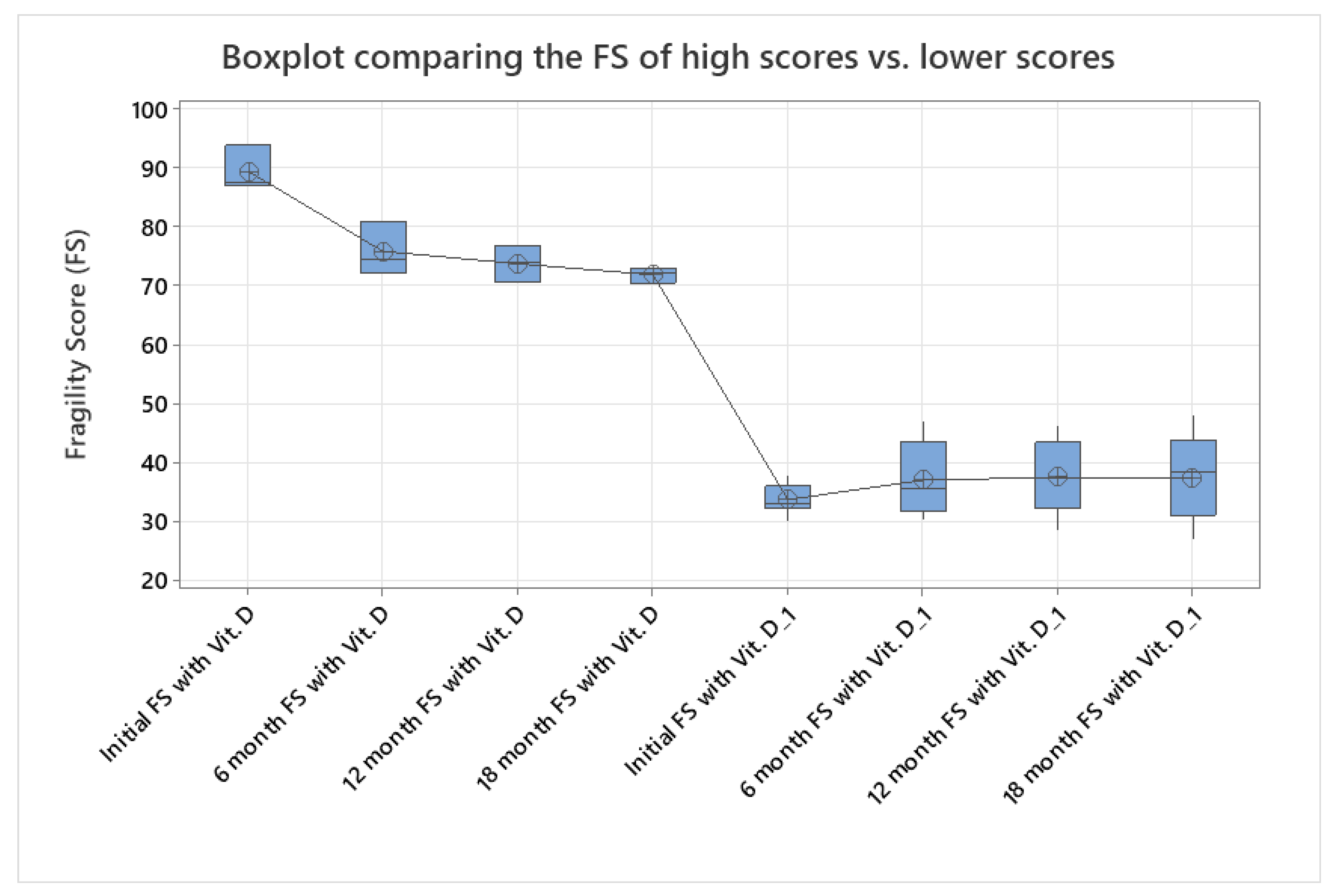
| Initial FS a | 38.15 (30.10–93.70) | 36.589 (30.20–49.20) |
| Initial muscle strength VAS a | 61.89 (10–100) | 52.57 (10–100) |
| Initial number of falls | 2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badea, I.-A.; Bojincă, M.; Bojincă, V.; Milicescu, M.; Ghițescu, G.; Casandra, N.; Ilina, A.-R.; Vulcan, M.-Ș.; Aramă, Ș.-S. Utility of the Fragility Score (FS) Determined Through Radiofrequency Ecographic Multi-Spectrometry (REMS) in the Follow-Up of Patients with Axial Spondyloarthritis (AxSpA). J. Clin. Med. 2025, 14, 2372. https://doi.org/10.3390/jcm14072372
Badea I-A, Bojincă M, Bojincă V, Milicescu M, Ghițescu G, Casandra N, Ilina A-R, Vulcan M-Ș, Aramă Ș-S. Utility of the Fragility Score (FS) Determined Through Radiofrequency Ecographic Multi-Spectrometry (REMS) in the Follow-Up of Patients with Axial Spondyloarthritis (AxSpA). Journal of Clinical Medicine. 2025; 14(7):2372. https://doi.org/10.3390/jcm14072372
Chicago/Turabian StyleBadea, Ionuț-Andrei, Mihai Bojincă, Violeta Bojincă, Mihaela Milicescu, Gabriel Ghițescu, Negoiță Casandra, Andreea-Ruxandra Ilina, Mădălina-Ștefania Vulcan, and Ștefan-Sorin Aramă. 2025. "Utility of the Fragility Score (FS) Determined Through Radiofrequency Ecographic Multi-Spectrometry (REMS) in the Follow-Up of Patients with Axial Spondyloarthritis (AxSpA)" Journal of Clinical Medicine 14, no. 7: 2372. https://doi.org/10.3390/jcm14072372
APA StyleBadea, I.-A., Bojincă, M., Bojincă, V., Milicescu, M., Ghițescu, G., Casandra, N., Ilina, A.-R., Vulcan, M.-Ș., & Aramă, Ș.-S. (2025). Utility of the Fragility Score (FS) Determined Through Radiofrequency Ecographic Multi-Spectrometry (REMS) in the Follow-Up of Patients with Axial Spondyloarthritis (AxSpA). Journal of Clinical Medicine, 14(7), 2372. https://doi.org/10.3390/jcm14072372






