Prognostic Impact of Chronic Kidney Disease After Percutaneous Coronary Intervention with Drug-Coated Balloons
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Assessment of Renal Function
2.3. Endpoints and Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Association Between CKD and Clinical Outcomes
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scheller, B.; Hehrlein, C.; Bocksch, W.; Rutsch, W.; Haghi, D.; Dietz, U.; Böhm, M.; Speck, U. Treatment of Coronary In-Stent Restenosis with a Paclitaxel-Coated Balloon Catheter. N. Engl. J. Med. 2006, 355, 2113–2124. [Google Scholar] [CrossRef] [PubMed]
- Jeger, R.V.; Farah, A.; Ohlow, M.-A.; Mangner, N.; Möbius-Winkler, S.; Leibundgut, G.; Weilenmann, D.; Wöhrle, J.; Richter, S.; Schreiber, M.; et al. Drug-Coated Balloons for Small Coronary Artery Disease (BASKET-SMALL 2): An Open-Label Randomised Non-Inferiority Trial. Lancet 2018, 392, 849–856. [Google Scholar] [CrossRef]
- Jeger, R.V.; Farah, A.; Ohlow, M.-A.; Mangner, N.; Möbius-Winkler, S.; Weilenmann, D.; Wöhrle, J.; Stachel, G.; Markovic, S.; Leibundgut, G.; et al. Long-Term Efficacy and Safety of Drug-Coated Balloons versus Drug-Eluting Stents for Small Coronary Artery Disease (BASKET-SMALL 2): 3-Year Follow-up of a Randomised, Non-Inferiority Trial. Lancet 2020, 396, 1504–1510. [Google Scholar] [CrossRef]
- Shin, E.S.; Jun, E.J.; Kim, S.; Kim, B.; Kim, T.H.; Sohn, C.B.; Her, A.Y.; Park, Y.; Cho, J.R.; Jeong, Y.H.; et al. Clinical Impact of Drug-Coated Balloon–Based Percutaneous Coronary Intervention in Patients with Multivessel Coronary Artery Disease. JACC Cardiovasc. Interv. 2023, 16, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Gitto, M.; Sticchi, A.; Chiarito, M.; Novelli, L.; Leone, P.P.; Mincione, G.; Oliva, A.; Condello, F.; Rossi, M.L.; Regazzoli, D.; et al. Drug-Coated Balloon Angioplasty for de Novo Lesions on the Left Anterior Descending Artery. Circ. Cardiovasc. Interv. 2023, 16, E013232. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.P.; Oliva, A.; Regazzoli, D.; Gitto, M.; Novelli, L.; Cozzi, O.; Stefanini, G.G.; Rossi, M.L.; Sticchi, A.; Tartaglia, F.; et al. Immediate and Follow-up Outcomes of Drug-Coated Balloon Angioplasty in de Novo Long Lesions on Large Coronary Arteries. EuroIntervention 2023, 19, e923–e925. [Google Scholar]
- Eckardt, K.-U.; Coresh, J.; Devuyst, O.; Johnson, R.J.; Köttgen, A.; Levey, A.S.; Levin, A. Evolving Importance of Kidney Disease: From Subspecialty to Global Health Burden. Lancet 2013, 382, 158–169. [Google Scholar] [CrossRef]
- Capodanno, D.; Angiolillo, D.J. Antithrombotic Therapy in Patients with Chronic Kidney Disease. Circulation 2012, 125, 2649–2661. [Google Scholar] [CrossRef]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic Kidney Disease: Global Dimension and Perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Coskun, U.; Orta Kilickesmez, K.; Abaci, O.; Kocas, C.; Bostan, C.; Yildiz, A.; Baskurt, M.; Arat, A.; Ersanli, M.; Gurmen, T. The Relationship between Chronic Kidney Disease and SYNTAX Score. Angiology 2011, 62, 504–508. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.-Y. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Lee, J.M.; Kang, J.; Lee, E.; Hwang, D.; Rhee, T.-M.; Park, J.; Kim, H.-L.; Lee, S.E.; Han, J.-K.; Yang, H.-M.; et al. Chronic Kidney Disease in the Second-Generation Drug-Eluting Stent Era: Pooled Analysis of the Korean Multicenter Drug-Eluting Stent Registry. JACC Cardiovasc. Interv. 2016, 9, 2097–2109. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e18–e114. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Kimura, T.; Ishihara, M.; Nakagawa, Y.; Nakao, K.; Miyauchi, K.; Sakamoto, T.; Tsujita, K.; Hagiwara, N.; Miyazaki, S.; et al. JCS 2018 Guideline on Diagnosis and Treatment of Acute Coronary Syndrome. Circ. J. 2019, 83, 1085–1196. [Google Scholar] [CrossRef]
- Nakamura, M.; Yaku, H.; Ako, J.; Arai, H.; Asai, T.; Chikamori, T.; Daida, H.; Doi, K.; Fukui, T.; Ito, T.; et al. JCS/JSCVS 2018 Guideline on Revascularization of Stable Coronary Artery Disease. Circ. J. 2022, 86, 477–588. [Google Scholar] [CrossRef]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Muramatsu, T.; Kozuma, K.; Tanabe, K.; Morino, Y.; Ako, J.; Nakamura, S.; Yamaji, K.; Kohsaka, S.; Amano, T.; Kobayashi, Y.; et al. Clinical Expert Consensus Document on Drug-Coated Balloon for Coronary Artery Disease from the Japanese Association of Cardiovascular Intervention and Therapeutics. Cardiovasc. Interv. Ther. 2023, 38, 166–176. [Google Scholar] [CrossRef]
- Jeger, R.V.; Eccleshall, S.; Wan Ahmad, W.A.; Ge, J.; Poerner, T.C.; Shin, E.-S.; Alfonso, F.; Latib, A.; Ong, P.J.; Rissanen, T.T.; et al. Drug-Coated Balloons for Coronary Artery Disease: Third Report of the International DCB Consensus Group. JACC Cardiovasc. Interv. 2020, 13, 1391–1402. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised Equations for Estimated GFR from Serum Creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- de Boer, I.H.; Caramori, L.M.; Chan, J.C.N.; Heerspink, H.J.L.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized Bleeding Definitions for Cardiovascular Clinical Trials: A Consensus Report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef]
- Stone, G.W.; Christiansen, E.H.; Ali, Z.A.; Andreasen, L.N.; Maehara, A.; Ahmad, Y.; Landmesser, U.; Holm, N.R. Intravascular Imaging-Guided Coronary Drug-Eluting Stent Implantation: An Updated Network Meta-Analysis. Lancet 2024, 403, 824–837. [Google Scholar] [CrossRef]
- Watanabe, H.; Domei, T.; Morimoto, T.; Natsuaki, M.; Shiomi, H.; Toyota, T.; Ohya, M.; Suwa, S.; Takagi, K.; Nanasato, M.; et al. Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI: The STOPDAPT-2 Randomized Clinical Trial. JAMA 2019, 321, 2414–2427. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, M.V.; Kirtane, A.J.; Redfors, B.; Généreux, P.; Ben-Yehuda, O.; Palmerini, T.; Benedetto, U.; Biondi-Zoccai, G.; Smits, P.C.; von Birgelen, C.; et al. Stent-Related Adverse Events >1 Year After Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2020, 75, 590–604. [Google Scholar] [CrossRef]
- Nakamura, D.; Attizzani, G.F.; Toma, C.; Sheth, T.; Wang, W.; Soud, M.; Aoun, R.; Tummala, R.; Leygerman, M.; Fares, A.; et al. Failure Mechanisms and Neoatherosclerosis Patterns in Very Late Drug-Eluting and Bare-Metal Stent Thrombosis. Circ. Cardiovasc. Interv. 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Gray, W.A.; Granada, J.F. Drug-Coated Balloons for the Prevention of Vascular Restenosis. Circulation 2010, 121, 2672–2680. [Google Scholar] [CrossRef]
- Hwang, D.; Kang, J.; Yang, H.-M.; Yang, S.; Park, J.; Han, J.-K.; Kang, H.-J.; Koo, B.-K.; Kim, H.-S. Better Prognosis After Complete Revascularization Using Contemporary Coronary Stents in Patients with Chronic Kidney Disease. Circ. Cardiovasc. Interv. 2019, 12, e007907. [Google Scholar] [CrossRef]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Trimarchi, G.; Pizzino, F.; Lilli, A.; De Caterina, A.R.; Esposito, A.; Dalmiani, S.; Mazzone, A.; Di Bella, G.; Berti, S.; Paradossi, U. Advanced Lung Cancer Inflammation Index as Predictor of All-Cause Mortality in ST-Elevation Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Intervention. J. Clin. Med. 2024, 13, 6059. [Google Scholar] [CrossRef] [PubMed]



| All Patients (n = 252) | No CKD (n = 130) | CKD (n = 122) | p Value | |
|---|---|---|---|---|
| Age, years | 68.8 ± 11.9 | 66.8 ± 12.3 | 70.8 ± 11.2 | 0.007 |
| Males, n (%) | 204 (81) | 115 (88) | 89 (73) | 0.003 |
| Body mass index, kg/m2 | 24.9 ± 4.0 | 25.4 ± 3.7 | 24.4 ± 4.2 | 0.059 |
| Hypertension, n (%) | 208 (83) | 97 (75) | 111 (91) | 0.001 |
| Dyslipidemia, n (%) | 142 (56) | 73 (56) | 69 (57) | 1.000 |
| Diabetes mellitus, n (%) | 116 (46) | 47 (36) | 69 (57) | 0.002 |
| Smoking, n (%) | 172 (68) | 97 (75) | 75 (62) | 0.035 |
| AF, n (%) | 39 (15) | 19 (15) | 20 (16) | 0.829 |
| Family history of CAD, n (%) | 27 (11) | 20 (15) | 7 (6) | 0.023 |
| Previous MI, n (%) | 58 (23) | 23 (18) | 35 (29) | 0.055 |
| Previous PCI, n (%) | 96 (38) | 41 (32) | 55 (45) | 0.037 |
| Previous CABG, n (%) | 10 (4) | 1 (1) | 9 (7) | 0.018 |
| Previous Stroke, n (%) | 32 (13) | 10 (8) | 22 (18) | 0.023 |
| Chronic heart failure, n (%) | 61 (24) | 12 (9) | 49 (40) | < 0.001 |
| PAD, n (%) | 20 (8) | 9 (7) | 11 (9) | 0.703 |
| AAA, n (%) | 14 (6) | 5 (4) | 9 (7) | 0.343 |
| COPD, n (%) | 18 (7) | 11 (9) | 7 (6) | 0.552 |
| Malignant tumor, n (%) | 36 (14) | 19 (15) | 17 (14) | 1.000 |
| Clinical presentation, n (%) | ||||
| STEMI | 30 (12) | 19 (15) | 11 (9) | 0.239 |
| NSTEMI | 54 (21) | 31 (24) | 23 (19) | 0.417 |
| Unstable angina | 14 (6) | 10 (8) | 4 (3) | 0.210 |
| CCS | 154 (61) | 70 (54) | 84 (69) | 0.021 |
| Complexity of disease, n (%) | ||||
| 1VD | 140 (56) | 80 (62) | 60 (49) | 0.065 |
| 2VD | 66 (26) | 32 (25) | 34 (28) | 0.657 |
| 3VD | 35 (14) | 12 (9) | 23 (19) | 0.043 |
| LMT | 11 (4) | 6 (5) | 5 (4) | 1.000 |
| CTO, n (%) | 37 (15) | 13 (10) | 24 (20) | 0.047 |
| DES-hybrid treatment, n (%) | 60 (24) | 26 (20) | 34 (28) | 0.188 |
| Echocardiographic data | ||||
| LVEF, % | 54.1 ± 13.5 | 57.7 ± 11.7 | 50.2 ± 14.3 | <0.001 |
| Biochemical data | ||||
| Sodium, mmol/L | 140.1 ± 3.0 | 140.2 ± 2.6 | 140.0 ± 3.3 | 0.536 |
| Potassium, mmol/L | 4.3 ± 0.5 | 4.2 ± 0.4 | 4.3 ± 0.5 | 0.022 |
| Chloride, mmol/L | 104.6 ± 3.1 | 104.5 ± 2.7 | 104.6 ± 3.5 | 0.864 |
| Medications, n (%) | ||||
| Aspirin, n (%) | 230 (91) | 118 (91) | 112 (92) | 0.946 |
| P2Y12 Inhibitors, n (%) | 235 (93) | 119 (92) | 116 (95) | 0.385 |
| Oral anticoagulant agents, n (%) | 43 (17) | 21 (16) | 22 (18) | 0.819 |
| Statins, n (%) | 215 (85) | 115 (89) | 100 (89) | 0.201 |
| RASi, n (%) | 182 (72) | 99 (76) | 83 (74) | 0.194 |
| MRA, n (%) | 34 (13) | 12 (9) | 22 (18) | 0.040 |
| β blockers, n (%) | 150 (60) | 66 (51) | 84 (69) | 0.005 |
| SGLT2 Inhibitors, n (%) | 44 (17) | 23 (18) | 21 (17) | 0.920 |
| No CKD (n = 130) | CKD (n = 122) | HR (95% CI) | p Value | Adjusted HR (95% CI) | p Value | |
|---|---|---|---|---|---|---|
| All-Cause Death | 3 (2.3%) | 16 (13.1%) | 5.689 (1.656–19.540) | 0.006 | 4.880 (1.337–17.804) | 0.016 |
| Cardiovascular Death | 0 (0%) | 3 (2.5%) | NA | NA | NA | NA |
| Any Myocardial Infarction | 3 (2.3%) | 9 (7.4%) | 3.282 (0.889–12.140) | 0.075 | 3.217 (0.770–13.450) | 0.109 |
| Target Vessel Revascularization | 17 (13.1%) | 17 (13.9%) | 1.081 (0.552–2.119) | 0.820 | 1.207 (0.576–2.531) | 0.618 |
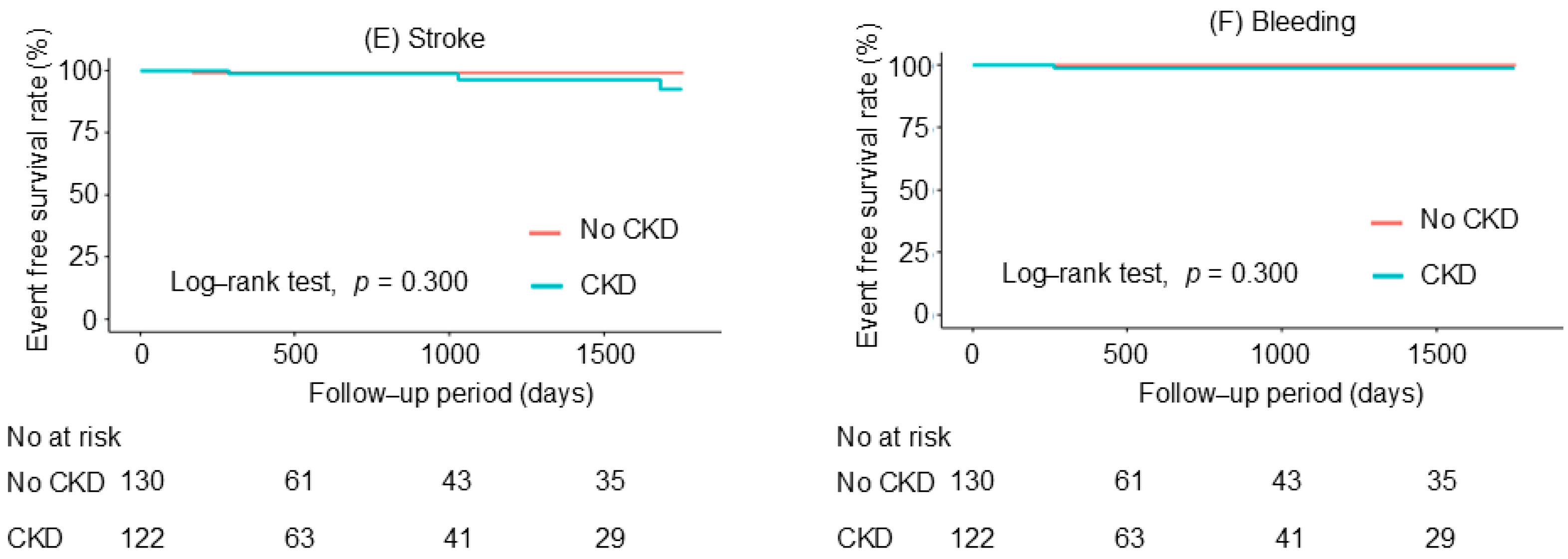
| Stroke | 1 (0.8%) | 3 (2.5%) | 3.390 (0.352–32.620) | 0.290 | 1.226 (0.102–14.694) | 0.872 |
| Bleeding | 0 (0%) | 1 (0.8%) | NA | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, T.; Watanabe, T.; Toyoshima, M.; Katawaki, W.; Toshima, T.; Kumagai, Y.; Yamanaka, T.; Watanabe, M. Prognostic Impact of Chronic Kidney Disease After Percutaneous Coronary Intervention with Drug-Coated Balloons. J. Clin. Med. 2025, 14, 2317. https://doi.org/10.3390/jcm14072317
Takahashi T, Watanabe T, Toyoshima M, Katawaki W, Toshima T, Kumagai Y, Yamanaka T, Watanabe M. Prognostic Impact of Chronic Kidney Disease After Percutaneous Coronary Intervention with Drug-Coated Balloons. Journal of Clinical Medicine. 2025; 14(7):2317. https://doi.org/10.3390/jcm14072317
Chicago/Turabian StyleTakahashi, Tetsuya, Tetsu Watanabe, Mashu Toyoshima, Wataru Katawaki, Taku Toshima, Yu Kumagai, Tamon Yamanaka, and Masafumi Watanabe. 2025. "Prognostic Impact of Chronic Kidney Disease After Percutaneous Coronary Intervention with Drug-Coated Balloons" Journal of Clinical Medicine 14, no. 7: 2317. https://doi.org/10.3390/jcm14072317
APA StyleTakahashi, T., Watanabe, T., Toyoshima, M., Katawaki, W., Toshima, T., Kumagai, Y., Yamanaka, T., & Watanabe, M. (2025). Prognostic Impact of Chronic Kidney Disease After Percutaneous Coronary Intervention with Drug-Coated Balloons. Journal of Clinical Medicine, 14(7), 2317. https://doi.org/10.3390/jcm14072317






