Additional Effects of Facilitatory Cerebellar Repetitive Transcranial Magnetic Stimulation on Inhibitory Repetitive Transcranial Magnetic Stimulation over the Unaffected Contralesional Primary Motor Cortex for Motor Recovery in Subacute Ischemic Stroke Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Determination of the MEP Response and Stimulation Location
2.4. rTMS Intervention
2.5. Baseline Characteristics and Outcome Measures
2.6. Comparative Analysis on Brain Lesions
2.7. Statistical Analysis
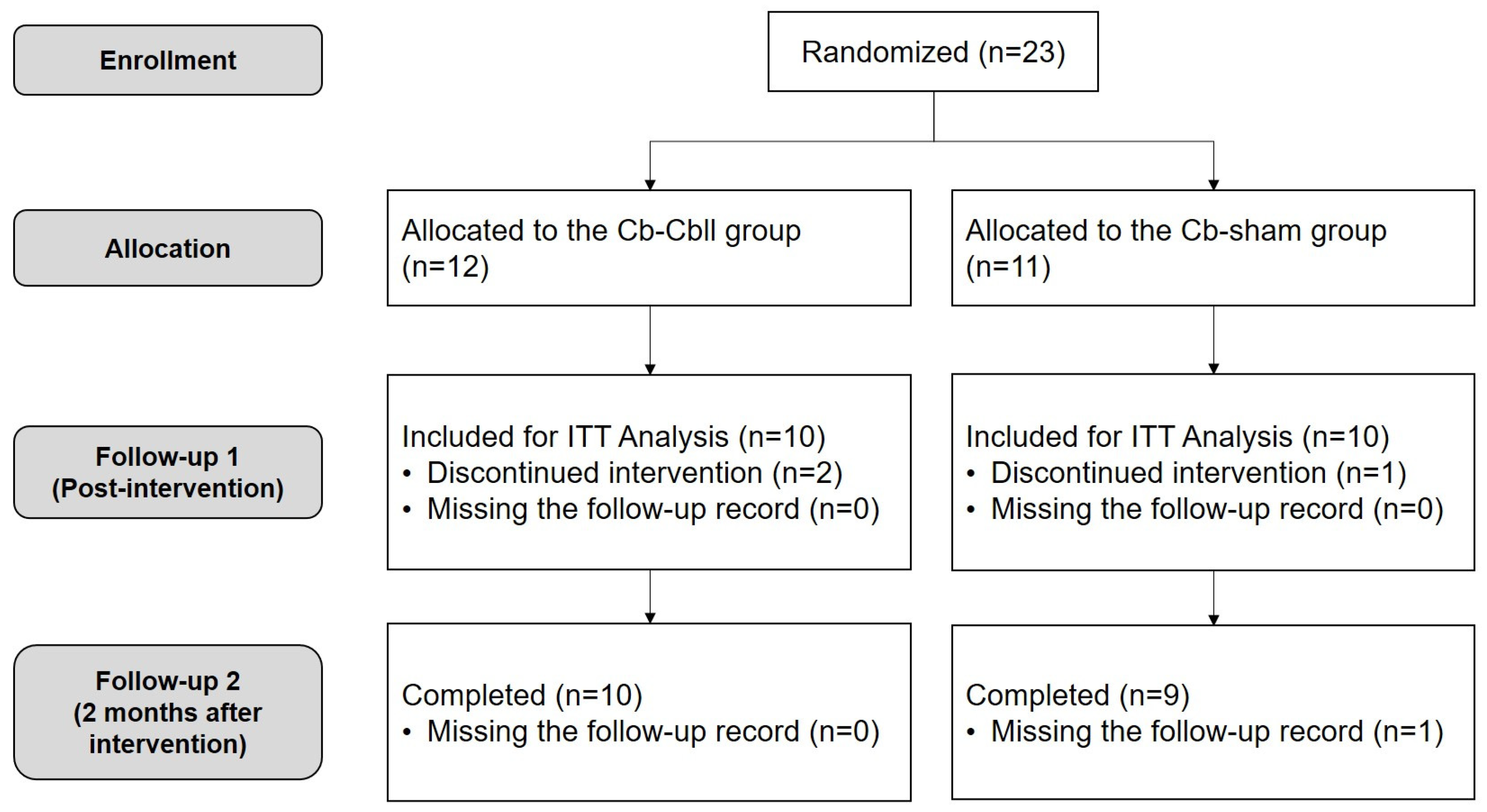
3. Results
3.1. Participants Characteristics
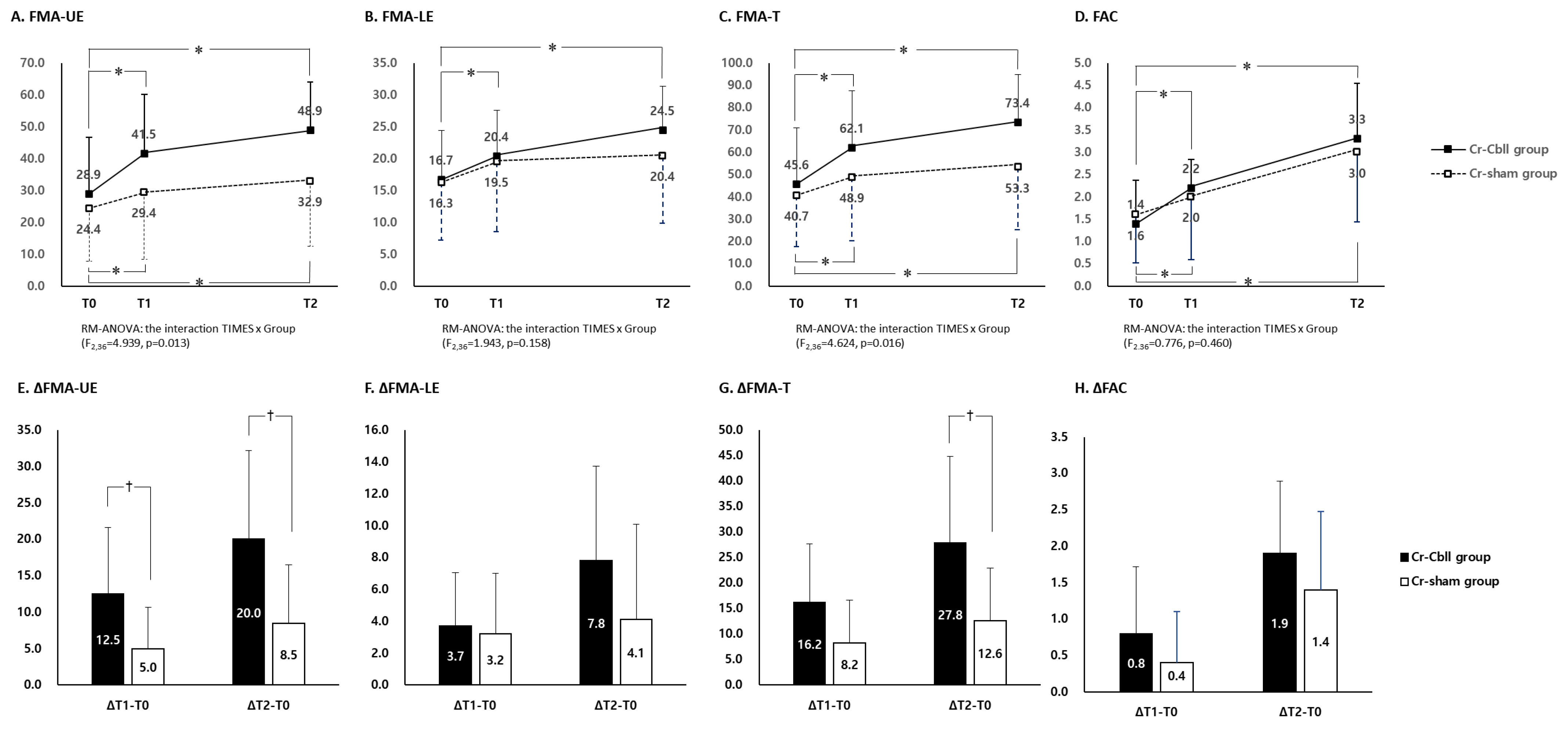
3.2. Changes in the Fugl-Meyer Assessment
3.3. Changes in Functional Ambulatory Category
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pascual-Leone, A.; Tormos, J.M.; Keenan, J.; Tarazona, F.; Canete, C.; Catala, M.D. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 1998, 15, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Murase, N.; Duque, J.; Mazzocchio, R.; Cohen, L.G. Influence of interhemispheric interactions on motor function in chronic stroke. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2004, 55, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar]
- Hofmeijer, J.; Ham, F.; Kwakkel, G. Evidence of rTMS for Motor or Cognitive Stroke Recovery: Hype or Hope? Stroke 2023, 54, 2500–2511. [Google Scholar] [CrossRef] [PubMed]
- Doyon, J.; Bellec, P.; Amsel, R.; Penhune, V.; Monchi, O.; Carrier, J.; Lehericy, S.; Benali, H. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav. Brain Res. 2009, 199, 61–75. [Google Scholar] [CrossRef]
- Doyon, J.; Penhune, V.; Ungerleider, L.G. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 2003, 41, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Doyon, J.; Song, A.W.; Karni, A.; Lalonde, F.; Adams, M.M.; Ungerleider, L.G. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc. Natl. Acad. Sci. USA 2002, 99, 1017–1022. [Google Scholar] [CrossRef]
- Hardwick, R.M.; Rottschy, C.; Miall, R.C.; Eickhoff, S.B. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage 2013, 67, 283–297. [Google Scholar] [CrossRef]
- Kim, W.S.; Jung, S.H.; Oh, M.K.; Min, Y.S.; Lim, J.Y.; Paik, N.J. Effect of repetitive transcranial magnetic stimulation over the cerebellum on patients with ataxia after posterior circulation stroke: A pilot study. J. Rehabil. Med. 2014, 46, 418–423. [Google Scholar] [CrossRef]
- Koch, G.; Bonni, S.; Casula, E.P.; Iosa, M.; Paolucci, S.; Pellicciari, M.C.; Cinnera, A.M.; Ponzo, V.; Maiella, M.; Picazio, S.; et al. Effect of Cerebellar Stimulation on Gait and Balance Recovery in Patients With Hemiparetic Stroke: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 170–178. [Google Scholar] [CrossRef]
- Qian, W.; Liao, X.; Ju, X.; Gao, Y.; Wu, M.; Xie, C.; Zhang, Y.; Long, X.; Qian, S.; Gong, Y. Effects of low frequency repetitive transcranial magnetic stimulation on motor recovery in subacute stroke patients with different motor evoked potential status: A randomized controlled trial. Front. Neurol. 2024, 15, 1460925. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Uhm, K.E.; Shin, Y.I.; Pascual-Leone, A.; Kim, Y.H. Factors influencing the response to high-frequency repetitive transcranial magnetic stimulation in patients with subacute stroke. Restor. Neurol. Neurosci. 2016, 34, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Fugl-Meyer, A.R. Post-stroke hemiplegia assessment of physical properties. Scand. J. Rehabil. Med. Suppl. 1980, 7, 85–93. [Google Scholar]
- Kim, W.S.; Paik, N.J. Safety Review for Clinical Application of Repetitive Transcranial Magnetic Stimulation. Brain Neurorehabil 2021, 14, e6. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Barker, A.T.; Berardelli, A.; Caramia, M.D.; Caruso, G.; Cracco, R.Q.; Dimitrijevic, M.R.; Hallett, M.; Katayama, Y.; Lucking, C.H.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 79–92. [Google Scholar]
- Wassermann, E.M.; Grafman, J.; Berry, C.; Hollnagel, C.; Wild, K.; Clark, K.; Hallett, M. Use and safety of a new repetitive transcranial magnetic stimulator. Electroencephalogr. Clin. Neurophysiol. 1996, 101, 412–417. [Google Scholar]
- Mennemeier, M.; Triggs, W.; Chelette, K.; Woods, A.; Kimbrell, T.; Dornhoffer, J. Sham Transcranial Magnetic Stimulation Using Electrical Stimulation of the Scalp. Brain Stimul. 2009, 2, 168–173. [Google Scholar] [CrossRef]
- Wessel, M.J.; Zimerman, M.; Timmermann, J.E.; Heise, K.F.; Gerloff, C.; Hummel, F.C. Enhancing Consolidation of a New Temporal Motor Skill by Cerebellar Noninvasive Stimulation. Cereb. Cortex 2016, 26, 1660–1667. [Google Scholar] [CrossRef]
- Temesi, J.; Gruet, M.; Rupp, T.; Verges, S.; Millet, G.Y. Resting and active motor thresholds versus stimulus-response curves to determine transcranial magnetic stimulation intensity in quadriceps femoris. J. Neuroeng. Rehabil. 2014, 11, 40. [Google Scholar] [CrossRef]
- Ah Sen, C.B.; Fassett, H.J.; El-Sayes, J.; Turco, C.V.; Hameer, M.M.; Nelson, A.J. Active and resting motor threshold are efficiently obtained with adaptive threshold hunting. PLoS ONE 2017, 12, e0186007. [Google Scholar] [CrossRef]
- Kim, T.L.; Hwang, S.H.; Lee, W.J.; Hwang, J.W.; Cho, I.; Kim, E.H.; Lee, J.A.; Choi, Y.; Park, J.H.; Shin, J.H. The Korean Version of the Fugl-Meyer Assessment: Reliability and Validity Evaluation. Ann. Rehabil. Med. 2021, 45, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.K.; Gill, K.M.; Magliozzi, M.R.; Nathan, J.; Piehl-Baker, L. Clinical gait assessment in the neurologically impaired. Reliability and meaningfulness. Phys. Ther. 1984, 64, 35–40. [Google Scholar] [CrossRef]
- Lehr, R. Sixteen S-squared over D-squared: A relation for crude sample size estimates. Stat. Med. 1992, 11, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Hiragami, S.; Inoue, Y.; Harada, K. Minimal clinically important difference for the Fugl-Meyer assessment of the upper extremity in convalescent stroke patients with moderate to severe hemiparesis. J. Phys. Ther. Sci. 2019, 31, 917–921. [Google Scholar] [CrossRef]
- Page, S.J.; Fulk, G.D.; Boyne, P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys. Ther. 2012, 92, 791–798. [Google Scholar] [CrossRef]
- Pandian, S.; Arya, K.N.; Kumar, D. Minimal clinically important difference of the lower-extremity fugl-meyer assessment in chronic-stroke. Top. Stroke Rehabil. 2016, 23, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Arya, K.N.; Verma, R.; Garg, R.K. Estimating the minimal clinically important difference of an upper extremity recovery measure in subacute stroke patients. Top. Stroke Rehabil. 2011, 18 (Suppl. 1), 599–610. [Google Scholar] [CrossRef]
- Dipietro, L.; Krebs, H.I.; Volpe, B.T.; Stein, J.; Bever, C.; Mernoff, S.T.; Fasoli, S.E.; Hogan, N. Learning, not adaptation, characterizes stroke motor recovery: Evidence from kinematic changes induced by robot-assisted therapy in trained and untrained task in the same workspace. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 48–57. [Google Scholar] [CrossRef]
- Krakauer, J.W. Motor learning: Its relevance to stroke recovery and neurorehabilitation. Curr. Opin. Neurol. 2006, 19, 84–90. [Google Scholar] [CrossRef]
- Kim, S.; Ogawa, K.; Lv, J.; Schweighofer, N.; Imamizu, H. Neural Substrates Related to Motor Memory with Multiple Timescales in Sensorimotor Adaptation. PLoS Biol. 2015, 13, e1002312. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, Y.H.; Jang, S.H.; Chang, W.H.; Park, C.H.; Kim, S.T. Dynamic changes in the cortico-subcortical network during early motor learning. NeuroRehabilitation 2010, 26, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.L.; Lindenberg, R.; Alexander, M.P.; Schlaug, G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke 2010, 41, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Wessel, M.J.; Hummel, F.C. Non-invasive Cerebellar Stimulation: A Promising Approach for Stroke Recovery? Cerebellum 2018, 17, 359–371. [Google Scholar] [CrossRef]
- Theoret, H.; Haque, J.; Pascual-Leone, A. Increased variability of paced finger tapping accuracy following repetitive magnetic stimulation of the cerebellum in humans. Neurosci. Lett. 2001, 306, 29–32. [Google Scholar] [CrossRef]
- Miall, R.C.; Christensen, L.O. The effect of rTMS over the cerebellum in normal human volunteers on peg-board movement performance. Neurosci. Lett. 2004, 371, 185–189. [Google Scholar] [CrossRef]
- Torriero, S.; Oliveri, M.; Koch, G.; Caltagirone, C.; Petrosini, L. Interference of left and right cerebellar rTMS with procedural learning. J. Cogn. Neurosci. 2004, 16, 1605–1611. [Google Scholar] [CrossRef]
- Manto, M.; Bower, J.M.; Conforto, A.B.; Delgado-Garcia, J.M.; da Guarda, S.N.; Gerwig, M.; Habas, C.; Hagura, N.; Ivry, R.B.; Marien, P.; et al. Consensus paper: Roles of the cerebellum in motor control--the diversity of ideas on cerebellar involvement in movement. Cerebellum 2012, 11, 457–487. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lisberger, S.G. Purkinje-cell plasticity and cerebellar motor learning are graded by complex-spike duration. Nature 2014, 510, 529–532. [Google Scholar] [CrossRef]
- Prevosto, V.; Graf, W.; Ugolini, G. Cerebellar inputs to intraparietal cortex areas LIP and MIP: Functional frameworks for adaptive control of eye movements, reaching, and arm/eye/head movement coordination. Cereb. Cortex 2010, 20, 214–228. [Google Scholar] [CrossRef]
- Doughty, C.; Wang, J.; Feng, W.; Hackney, D.; Pani, E.; Schlaug, G. Detection and Predictive Value of Fractional Anisotropy Changes of the Corticospinal Tract in the Acute Phase of a Stroke. Stroke 2016, 47, 1520–1526. [Google Scholar] [CrossRef]
- Groisser, B.N.; Copen, W.A.; Singhal, A.B.; Hirai, K.K.; Schaechter, J.D. Corticospinal tract diffusion abnormalities early after stroke predict motor outcome. Neurorehabil Neural Repair. 2014, 28, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, J.; Chhatbar, P.Y.; Doughty, C.; Landsittel, D.; Lioutas, V.A.; Kautz, S.A.; Schlaug, G. Corticospinal tract lesion load: An imaging biomarker for stroke motor outcomes. Ann. Neurol. 2015, 78, 860–870. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Cunningham, D.A.; Hogue, O.; Schroedel, M.; Campbell, B.A.; Plow, E.B.; Baker, K.B.; Machado, A.G. Cortico-Cerebellar Connectivity Underlying Motor Control in Chronic Poststroke Individuals. J. Neurosci. 2022, 42, 5186–5197. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Frey, B.M.; Koch, P.; Zimerman, M.; Bonstrup, M.; Feldheim, J.; Timmermann, J.E.; Schon, G.; Cheng, B.; Thomalla, G.; et al. Cortico-Cerebellar Structural Connectivity Is Related to Residual Motor Output in Chronic Stroke. Cereb. Cortex 2017, 27, 635–645. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Lim, S.H.; Kim, Y.; Kim, J.S.; Hong, B.Y.; Yoon, M.J.; Rim, H.; Park, G.Y. Structural Integrity of the Cerebellar Outflow Tract Predicts Long-Term Motor Function After Middle Cerebral Artery Ischemic Stroke. Neurorehabil Neural Repair. 2023, 37, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Antonioni, A.; Raho, E.M.; Straudi, S.; Granieri, E.; Koch, G.; Fadiga, L. The cerebellum and the Mirror Neuron System: A matter of inhibition? From neurophysiological evidence to neuromodulatory implications. A narrative review. Neurosci. Biobehav. Rev. 2024, 164, 105830. [Google Scholar] [CrossRef]
- Errante, A.; Fogassi, L. Activation of cerebellum and basal ganglia during the observation and execution of manipulative actions. Sci. Rep. 2020, 10, 12008. [Google Scholar] [CrossRef]
- Bonni, S.; Ponzo, V.; Caltagirone, C.; Koch, G. Cerebellar theta burst stimulation in stroke patients with ataxia. Funct. Neurol. 2014, 29, 41–45. [Google Scholar] [CrossRef]
- Chang, W.H.; Kim, M.S.; Cho, J.W.; Youn, J.; Kim, Y.K.; Kim, S.W.; Lee, A.; Kim, Y.H. Effect of cumulative repetitive transcranial magnetic stimulation on freezing of gait in patients with atypical Parkinsonism: A pilot study. J. Rehabil. Med. 2016, 48, 824–828. [Google Scholar] [CrossRef]
- Hatem, S.M.; Saussez, G.; Della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef]
- Antonioni, A.; Galluccio, M.; Toselli, R.; Baroni, A.; Fregna, G.; Schincaglia, N.; Milani, G.; Cosma, M.; Ferraresi, G.; Morelli, M.; et al. A Multimodal Analysis to Explore Upper Limb Motor Recovery at 4 Weeks After Stroke: Insights From EEG and Kinematics Measures. Clin. EEG Neurosci. 2024, 55, 465–476. [Google Scholar] [CrossRef] [PubMed]

| Cr-Cbll Group (n = 10) | Cr-Sham Group (n = 10) | p-Value | |
|---|---|---|---|
| Age (years) | 68.2 ± 5.2 | 67.2 ± 11.7 | 0.809 |
| Sex (male/female) | 8:2 | 6:4 | 0.628 |
| Stroke type (first-ever/recurrent) | 10:0 | 8:2 | 0.474 |
| Stroke side (right/left) | 6:4 | 4:6 | 0.638 |
| Stroke lesion (supratentorial/infratentorial) | 7:3 | 7:3 | 1.000 |
| Stroke duration (days) | 12.1 ± 4.7 | 15.1 ± 6.5 | 0.251 |
| rMT of affected M1 | - | - | - |
| rMT of unaffected M1 | 55.4 ± 12.0 | 46.6 ± 12.9 | 0.132 |
| Fugl-Meyer Assessment | |||
| FMA-UE | 28.9 ± 17.7 | 24.4 ± 16.5 | 0.564 |
| FMA-LE | 16.7 ± 7.7 | 16.3 ± 7.1 | 0.906 |
| FMA-T | 45.6 ± 25.2 | 40.7 ± 22.9 | 0.655 |
| T0 | T1 | T2 | ΔT1-T0 | ΔT2-T0 | ||
|---|---|---|---|---|---|---|
| FMA-UE-A | Cr-Cbll group (n = 10) | 17.2 ± 9.2 | 23.9 ± 9.4 * | 27.7 ± 7.1 * | 6.5 ± 5.2 † | 10.4 ± 5.7 † |
| Cr-sham group (n = 10) | 15.7 ± 9.1 | 17.9 ± 11.0 | 19.6 ± 10.6 * | 2.2 ± 2.7 | 3.9 ± 3.7 | |
| FMA-UE-B | Cr-Cbll group (n = 10) | 3.8 ± 3.1 | 5.5 ± 3.1 * | 7.2 ± 2.7 * | 1.7 ± 2.1 | 3.4 ± 2.8 |
| Cr-sham group (n = 10) | 2.4 ± 2.6 | 3.0 ± 2.6 | 3.8 ± 2.8 * | 0.6 ± 1.7 | 1.4 ± 2.5 | |
| FMA-UE-C | Cr-Cbll group (n = 10) | 5.7 ± 4.2 | 8.7 ± 4.6 * | 10.7 ± 4.3 * | 3.0 ± 2.4 | 5.0 ± 3.9 |
| Cr-sham group (n = 10) | 4.4 ± 3.4 | 6.2 ± 5.8 | 7.6 ± 5.7 * | 1.8 ± 2.9 | 3.2 ± 2.7 | |
| FMA-UE-D | Cr-Cbll group (n = 10) | 2.1 ± 1.9 | 3.4 ± 1.9 * | 3.3 ± 2.2 * | 1.3 ± 1.3 | 1.2 ± 1.5 † |
| Cr-sham group (n = 10) | 1.9 ± 2.0 | 2.3 ± 2.1 | 1.9 ± 2.0 * | 0.4 ± 1.0 | 0.0 ± 0.5 | |
| FMA-LE-E | Cr-Cbll group (n = 10) | 14.4 ± 5.9 | 17.2 ± 5.3 * | 20.6 ± 5.7 * | 2.8 ± 1.9 | 6.2 ± 4.6 |
| Cr-sham group (n = 10) | 13.3 ± 5.2 | 15.9 ± 6.9 | 16.6 ± 7.7 | 2.6 ± 3.1 | 3.3 ± 4.8 | |
| FMA-LE-F | Cr-Cbll group (n = 10) | 2.3 ± 2.1 | 3.2 ± 2.3 | 3.9 ± 1.4 | 0.8 ± 2.0 | 1.6 ± 2.0 |
| Cr-sham group (n = 10) | 3.0 ± 2.1 | 3.6 ± 2.0 | 3.8 ± 2.0 | 0.6 ± 1.6 | 0.8 ± 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Lee, H.S.; Kim, H.; Kim, D.H.; Chang, W.H. Additional Effects of Facilitatory Cerebellar Repetitive Transcranial Magnetic Stimulation on Inhibitory Repetitive Transcranial Magnetic Stimulation over the Unaffected Contralesional Primary Motor Cortex for Motor Recovery in Subacute Ischemic Stroke Patients. J. Clin. Med. 2025, 14, 2315. https://doi.org/10.3390/jcm14072315
Kim S, Lee HS, Kim H, Kim DH, Chang WH. Additional Effects of Facilitatory Cerebellar Repetitive Transcranial Magnetic Stimulation on Inhibitory Repetitive Transcranial Magnetic Stimulation over the Unaffected Contralesional Primary Motor Cortex for Motor Recovery in Subacute Ischemic Stroke Patients. Journal of Clinical Medicine. 2025; 14(7):2315. https://doi.org/10.3390/jcm14072315
Chicago/Turabian StyleKim, Sungwon, Ho Seok Lee, Heegoo Kim, Dae Hyun Kim, and Won Hyuk Chang. 2025. "Additional Effects of Facilitatory Cerebellar Repetitive Transcranial Magnetic Stimulation on Inhibitory Repetitive Transcranial Magnetic Stimulation over the Unaffected Contralesional Primary Motor Cortex for Motor Recovery in Subacute Ischemic Stroke Patients" Journal of Clinical Medicine 14, no. 7: 2315. https://doi.org/10.3390/jcm14072315
APA StyleKim, S., Lee, H. S., Kim, H., Kim, D. H., & Chang, W. H. (2025). Additional Effects of Facilitatory Cerebellar Repetitive Transcranial Magnetic Stimulation on Inhibitory Repetitive Transcranial Magnetic Stimulation over the Unaffected Contralesional Primary Motor Cortex for Motor Recovery in Subacute Ischemic Stroke Patients. Journal of Clinical Medicine, 14(7), 2315. https://doi.org/10.3390/jcm14072315






