The Effects of Functional Electrical Stimulation of Hip Abductor and Tibialis Anterior Muscles on Standing and Gait Characteristics in Patients with Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Setting and Ethical Approval
2.3. Patients, Sampling Method, Recruitment, and Data Collection
2.4. Sample Size Calculation
3. Instruments
3.1. Modified Ashworth Scale (MAS)
3.2. Five Times Sit-to-Stand Test (FTSST)
3.3. 10-Meter Walk Test (10 MWT)
3.4. C-Mill Analysis System
4. Functional Electrical Stimulation Management Programs
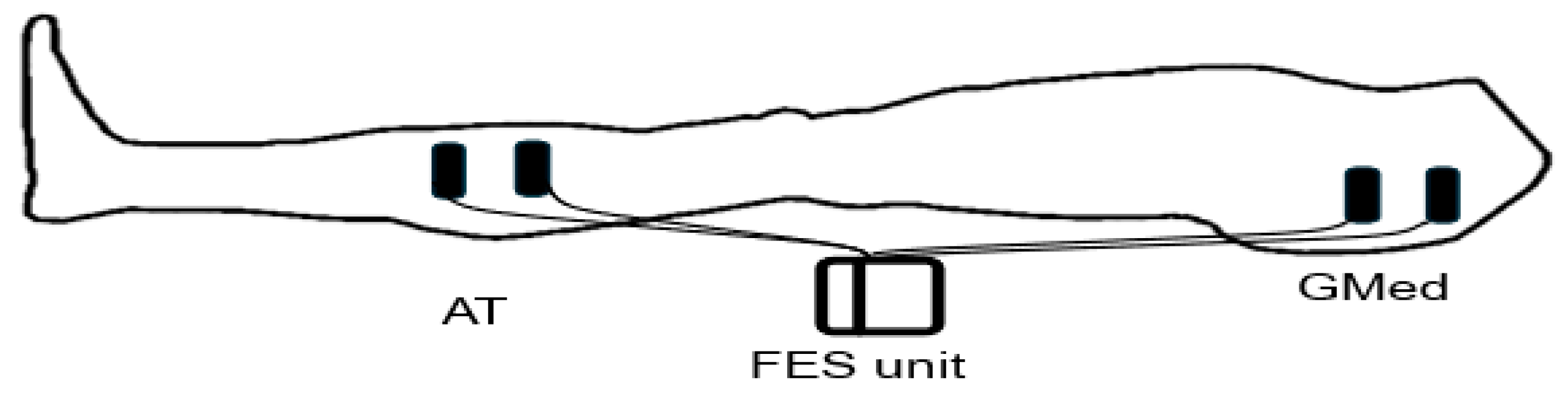
4.1. Functional Electrical Stimulation (FES) Unit
4.2. FES Management Programs
5. Assessment Procedure
Baseline Assessment Procedure
6. Patient Preparation for FES Management Programs
7. Short-Term FES Management Program and Assessment Procedure
8. Long-Term FES Management Program and Assessment Procedure
9. Statistical Analysis
10. Results
10.1. Baseline Data
10.2. Short-and-Long-Term Management Programs
11. Discussion
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Health Organization Definition of Palliative Care. World Health Organization Website. Available online: https://www.who.int/news-room/fact-sheets/detail/palliative-care (accessed on 25 March 2025).
- Memon, I.; Abu-Shaheen, A.; Heena, H.; Al-Tannir, M. Point prevalence study for stroke in Saudi Arabia: A cross-sectional survey. Saudi J. Health Sci. 2019, 8, 93–97. [Google Scholar]
- Basnawi, A.M.; Algrafi, M.B.; Alhamdi, M.S.G.; Alwakeel, A.A.A.; AlQahtani, R.F.; Abdellatif, R.A.; Alshehri, A.D.A.; Albalawi, A.N.A. Evaluation of knowledge, attitude and risk factor of Stroke in Tabuk Region, Saudi Arabia. Saudi Med. Horiz. J. 2024, 4, 1–13. [Google Scholar]
- Hawati, S.M.; Binobaid, F.; Melybari, R.Z.; Alabdali, S.; Alhazmi, G.; Namankani, A.; Abdrabuh, H.A. Awareness of Acute Stroke Among the General Population in the Western Region of Saudi Arabia. Cureus 2024, 16, e51979. [Google Scholar]
- Egan, M.; Kessler, D.; Gurgel-Juarez, N.; Chopra, A.; Linkewich, E.; Sikora, L.; Montgomery, P.; Duong, P. Stroke rehabilitation adaptive approaches: A theory-focused scoping review. Scand. J. Occup. Ther. 2024, 31, 1–13. [Google Scholar]
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar]
- Wang, W.; Li, K.; Yue, S.; Yin, C.; Wei, N. Associations between lower-limb muscle activation and knee flexion in post-stroke individuals: A study on the stance-to-swing phases of gait. PLoS ONE 2017, 12, e0183865. [Google Scholar]
- Hsiao, H.; Gray, V.L.; Creath, R.A.; Binder-Macleod, S.A.; Rogers, M.W. Control of lateral weight transfer is associated with walking speed in individuals post-stroke. J. Biomech. 2017, 60, 72–78. [Google Scholar] [CrossRef]
- Roelker, S.A.; Bowden, M.G.; Kautz, S.A.; Neptune, R.R. Paretic propulsion as a measure of walking performance and functional motor recovery post-stroke: A review. Gait Posture 2019, 68, 6–14. [Google Scholar] [CrossRef]
- Hsiao, H.; Knarr, B.A.; Higginson, J.S.; Binder-Macleod, S.A. Mechanisms to increase propulsive force for individuals poststroke. J. Neuroeng. Rehabil. 2015, 12, 40. [Google Scholar] [CrossRef]
- Shin, S.Y.; Lee, R.K.; Spicer, P.; Sulzer, J. Does kinematic gait quality improve with functional gait recovery? A longitudinal pilot study on early post-stroke individuals. J. Biomech. 2020, 105, 109761. [Google Scholar]
- Bowden, M.G.; Balasubramanian, C.K.; Neptune, R.R.; Kautz, S.A. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke 2006, 37, 872–876. [Google Scholar] [PubMed]
- Chemerinski, E.; Robinson, R.G.; Kosier, J.T. Improved recovery in activities of daily living associated with remission of poststroke depression. Stroke 2001, 32, 113–117. [Google Scholar] [PubMed]
- Forrester, L.W.; Roy, A.; Krebs, H.I.; Macko, R.F. Ankle training with a robotic device improves hemiparetic gait after a stroke. Neurorehabilit. Neural Repair 2011, 25, 369–377. [Google Scholar]
- Salbach, N.M.; Mayo, N.E.; Wood-Dauphinee, S.; Hanley, J.A.; Richards, C.L.; Cote, R. A task-orientated intervention enhances walking distance and speed in the first year post stroke: A randomized controlled trial. Clin. Rehabil. 2004, 18, 509–519. [Google Scholar]
- Hong, Z.; Sui, M.; Zhuang, Z.; Liu, H.; Zheng, X.; Cai, C.; Jin, D. Effectiveness of neuromuscular electrical stimulation on lower limbs of patients with hemiplegia after chronic stroke: A systematic review. Arch. Phys. Med. Rehabil. 2018, 99, 1011–1022.e1. [Google Scholar]
- Taylor, P.; Humphreys, L.; Swain, I. The long-term cost-effectiveness of the use of Functional Electrical Stimulation for the correction of dropped foot due to upper motor neuron lesion. J. Rehabil. Med. 2013, 45, 154–160. [Google Scholar]
- Shariat, A.; Nakhostin Ansari, N.; Honarpishe, R.; Moradi, V.; Hakakzadeh, A.; Cleland, J.A.; Kordi, R. Effect of cycling and functional electrical stimulation with linear and interval patterns of timing on gait parameters in patients after stroke: A randomized clinical trial. Disabil. Rehabil. 2021, 43, 1890–1896. [Google Scholar]
- Araki, S.; Kawada, M.; Miyazaki, T.; Nakai, Y.; Takeshita, Y.; Matsuzawa, Y.; Yamaguchi, Y.; Ohwatashi, A.; Tojo, R.; Nakamura, T. Effect of functional electrical stimulation of the gluteus medius during gait in patients following a stroke. BioMed Res. Int. 2020, 2020, 8659845. [Google Scholar]
- Oh, D.-G.; Yoo, K.-T. Effects of Functional Electrical Stimulation (FES) on the Temporal-spatial Gait Parameters and Activities of Daily Living in Hemiplegic Stroke Patients. J. Korean Soc. Phys. Med. 2021, 16, 37–44. [Google Scholar]
- Kumagai, M.; Shiba, N.; Higuchi, F.; Nishimura, H.; Inoue, A. Functional evaluation of hip abductor muscles with use of magnetic resonance imaging. J. Orthop. Res. 1997, 15, 888–893. [Google Scholar]
- Warshaw, M.E.; Baltz, M.J.; Hollman, J.H. Gait synchronized neuromuscular electrical stimulation to the gluteus medius on a patient with right hemiparesis: A case report. Physiother. Theory Pract. 2022, 38, 3180–3186. [Google Scholar] [PubMed]
- de Haart, M.; Geurts, A.C.; Huidekoper, S.C.; Fasotti, L.; van Limbeek, J. Recovery of standing balance in postacute stroke patients: A rehabilitation cohort study. Arch. Phys. Med. Rehabil. 2004, 85, 886–895. [Google Scholar] [PubMed]
- Vidmar, T.; Kregar, N.G.; Puh, U. Reliability of the Modified Ashworth Scale after stroke for 13 muscle groups. Arch. Phys. Med. Rehabil. 2023, 104, 1606–1611. [Google Scholar] [PubMed]
- Whitney, S.L.; Wrisley, D.M.; Marchetti, G.F.; Gee, M.A.; Redfern, M.S.; Furman, J.M. Clinical measurement of sit-to-stand performance in people with balance disorders: Validity of data for the Five-Times-Sit-to-Stand Test. Phys. Ther. 2005, 85, 1034–1045. [Google Scholar] [CrossRef]
- Dalgas, U.; Severinsen, K.; Overgaard, K. Relations between 6 minute walking distance and 10 meter walking speed in patients with multiple sclerosis and stroke. Arch. Phys. Med. Rehabil. 2012, 93, 1167–1172. [Google Scholar] [CrossRef]
- Cleland, B.T.; Arshad, H.; Madhavan, S. Concurrent validity of the GAITRite electronic walkway and the 10-m walk test for measurement of walking speed after stroke. Gait Posture 2019, 68, 458–460. [Google Scholar]
- Flansbjer, U.-B.; Holmbäck, A.M.; Downham, D.; Patten, C.; Lexell, J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J. Rehabil. Med. 2005, 37, 75–82. [Google Scholar]
- Van Der Veen, S.M.; Hammerbeck, U.; Baker, R.J.; Hollands, K.L. Validation of gait event detection by centre of pressure during target stepping in healthy and paretic gait. J. Biomech. 2018, 79, 218–222. [Google Scholar]
- Heeren, A.; Ooijen, M.; Geurts, A.C.; Day, B.L.; Janssen, T.; Beek, P.J.; Roerdink, M.; Weerdesteyn, V. Step by step: A proof of concept study of C-Mill gait adaptability training in the chronic phase after stroke. J. Rehabil. Med. 2013, 45, 616–622. [Google Scholar] [CrossRef]
- Hara, Y. Neurorehabilitation with new functional electrical stimulation for hemiparetic upper extremity in stroke patients. J. Nippon Med. Sch. 2008, 75, 4–14. [Google Scholar] [CrossRef]
- De Luca, C.J. Control properties of motor units. J. Exp. Biol. 1985, 115, 125–136. [Google Scholar] [PubMed]
- Azzollini, V.; Dalise, S.; Chisari, C. How does stroke affect skeletal muscle? State of the art and rehabilitation perspective. Front. Neurol. 2021, 12, 797559. [Google Scholar]
- Cho, M.-K.; Kim, J.-H.; Chung, Y.; Hwang, S. Treadmill gait training combined with functional electrical stimulation on hip abductor and ankle dorsiflexor muscles for chronic hemiparesis. Gait Posture 2015, 42, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Dorsch, S.; Ada, L.; Canning, C.G. Lower limb strength is significantly impaired in all muscle groups in ambulatory people with chronic stroke: A cross-sectional study. Arch. Phys. Med. Rehabil. 2016, 97, 522–527. [Google Scholar] [PubMed]
- Crone, C.; Nielsen, J. Central control of disynaptic reciprocal inhibition in humans. Acta Physiol. Scand. 1994, 152, 351–363. [Google Scholar]
- Boorman, G.; Lee, R.; Becker, W.; Windhorst, U. Impaired “natural reciprocal inhibition” in patients with spasticity due to incomplete spinal cord injury. Electroencephalogr. Clin. Neurophysiol./Electromyogr. Mot. Control 1996, 101, 84–92. [Google Scholar]
- Sommerfeld, D.K.; Eek, E.U.-B.; Svensson, A.-K.; Holmqvist, L.W.; Von Arbin, M.H. Spasticity after stroke: Its occurrence and association with motor impairments and activity limitations. Stroke 2004, 35, 134–139. [Google Scholar] [PubMed]
- Hultborn, H. Spinal reflexes, mechanisms and concepts: From Eccles to Lundberg and beyond. Prog. Neurobiol. 2006, 78, 215–232. [Google Scholar] [CrossRef]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M.; Siegelbaum, S.A.; Hudspeth, A.J. Principles of Neural Science, 5th ed.; McGraw Hill: New York, NY, USA, 2012. [Google Scholar]
- Burke, D.; Gandevia, S.C.; McKeon, B. Monosynaptic and oli-gosynaptic contributions to human ankle jerk and H-reflex. J. Physiol. 1977, 272, 545–565. [Google Scholar] [CrossRef]
- Enoka, R.M. Neuromechanics of Human Movement, 5th ed.; Human Kinetics: Champaign, IL, USA, 2015. [Google Scholar]
- Yan, T.; Hui-Chan, C.W.; Li, L.S. Functional electrical stimulation improves motor recovery of the lower extremity and walking ability of subjects with first acute stroke: A randomized placebo-controlled trial. Stroke 2005, 36, 80–85. [Google Scholar]
- Chung, Y.; Kim, J.H.; Cha, Y.; Hwang, S. Therapeutic effect of functional electrical stimulation-triggered gait training corresponding gait cycle for stroke. Gait Posture 2014, 40, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Sabut, S.K.; Sikdar, C.; Mondal, R.; Kumar, R.; Mahadevappa, M. Restoration of gait and motor recovery by functional electrical stimulation therapy in persons with stroke. Disabil. Rehabil. 2010, 32, 1594–1603. [Google Scholar] [PubMed]

| Measured Variables | Baseline (Mean ± SD) | p-Value | |
|---|---|---|---|
| Day 1 | Day 2 | ||
| MAS for ankle plantar flexor | 2.78 ± 0.80 | 2.78 ± 0.80 | 1.000 |
| MAS for hip adductors | 2.00 ± 0.55 | 2.00 ± 0.55 | 1.000 |
| FTSST (sec) | 21.29 ± 2.39 | 19.77 ± 2.00 | 0.124 |
| 10 MWT (sec) | 31.00 ± 11.42 | 30.78 ± 11.11 | 0.957 |
| Step width (cm) | 18.35 ± 3.61 | 18.33 ± 3.66 | 0.987 |
| Stride (cm) | 37.65 ± 18.77 | 40.52 ± 17.81 | 0.687 |
| Step length (cm) | 24.95 ± 8.81 | 25.03 ± 8.80 | 0.980 |
| Assessed Parameters | Short-Term Management Assessments | p-Value | Long-Term Management Assessments | |||
|---|---|---|---|---|---|---|
| Without FES | p-Value | With FES | p-Value | |||
| MAS for ankle plantar flexor | 1.71 ± 0.72 | 0.001 | 1.92 ± 0.91 | 0.006 | 0.85 ± 0.77 | 0.001 |
| MAS for hip adductors | 1.00 ± 0.55 | 0.001 | 1.00 ± 0.55 | 0.001 | 0.35 ± 0.63 | 0.001 |
| FTSST (sec) | 15.63 ± 2.69 | 0.001 | 17.59 ± 3.33 | 0.001 | 13.41 ± 2.20 | 0.001 |
| 10 MWT (sec) | 22.48 ± 0.21 | 0.039 | 21.49 ± 10.06 | 0.021 | 20.58 ± 9.97 | 0.012 |
| Step width (cm) | 21.85 ± 3.41 | 0.012 | 22.22 ± 3.57 | 0.006 | 23.73 ± 3.73 | 0.001 |
| Stride (cm) | 52.20 ± 9.07 | 0.044 | 53.35 ± 19.16 | 0.030 | 55.31 ± 19.00 | 0.028 |
| Step length (cm) | 32.67 ± 9.16 | 0.027 | 33.67 ± 9.16 | 0.013 | 35.45 ± 9.08 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlAbdulwahab, S.S.; Aldhaferi, A.S.; Alsubiheen, A.M.; Alharbi, S.H.; Alotaibi, F.H.; Alghamdi, M.A.; Basonbul, A.; El Sousai, A.; Al-Harbi, M.M.; Almurdi, M.M. The Effects of Functional Electrical Stimulation of Hip Abductor and Tibialis Anterior Muscles on Standing and Gait Characteristics in Patients with Stroke. J. Clin. Med. 2025, 14, 2309. https://doi.org/10.3390/jcm14072309
AlAbdulwahab SS, Aldhaferi AS, Alsubiheen AM, Alharbi SH, Alotaibi FH, Alghamdi MA, Basonbul A, El Sousai A, Al-Harbi MM, Almurdi MM. The Effects of Functional Electrical Stimulation of Hip Abductor and Tibialis Anterior Muscles on Standing and Gait Characteristics in Patients with Stroke. Journal of Clinical Medicine. 2025; 14(7):2309. https://doi.org/10.3390/jcm14072309
Chicago/Turabian StyleAlAbdulwahab, Sami S., Abdulaziz S. Aldhaferi, Abdulrahman M. Alsubiheen, Sultan H. Alharbi, Fahad H. Alotaibi, Mohammed A. Alghamdi, Abdulrahman Basonbul, Atta El Sousai, Mohammed M. Al-Harbi, and Muneera M. Almurdi. 2025. "The Effects of Functional Electrical Stimulation of Hip Abductor and Tibialis Anterior Muscles on Standing and Gait Characteristics in Patients with Stroke" Journal of Clinical Medicine 14, no. 7: 2309. https://doi.org/10.3390/jcm14072309
APA StyleAlAbdulwahab, S. S., Aldhaferi, A. S., Alsubiheen, A. M., Alharbi, S. H., Alotaibi, F. H., Alghamdi, M. A., Basonbul, A., El Sousai, A., Al-Harbi, M. M., & Almurdi, M. M. (2025). The Effects of Functional Electrical Stimulation of Hip Abductor and Tibialis Anterior Muscles on Standing and Gait Characteristics in Patients with Stroke. Journal of Clinical Medicine, 14(7), 2309. https://doi.org/10.3390/jcm14072309





