The PD1 Molecule May Contribute to Lower Treatment-Free Remission Rates in Patients with Chronic Myeloid Leukemia with the e13a2 Transcript
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Flow Cytometry for Detection of Immune Populations
- T cells, B cells, and regulatory T cells (Treg): anti-CD3-BV510 (catalog No: 564713), anti-CD4-PerCP (catalog No: 345770), anti-CD8-FITC (catalog No: 555634), anti-CD19-APC-Cy7 (catalog No: 557791), anti-CD25-PE-Cy7 (catalog No: 557741), and anti-CD127-BV421 (catalog No: 562436);
- Natural killer (NK) cells and natural killer T (NKT) cells: anti-CD45-BV510 (catalog No: 563204), anti-CD14-APC-H7 (catalog No: 560180), anti-CD3-PerCP (catalog No: 552851), anti-CD56-PE-Cy7 (catalog No: 557747), anti-CD16-BV421 (catalog No: 562874), anti-iNKT-FITC (catalog No: 558371), and anti-CD161-PE (catalog No: 556081);
- Conventional dendritic cells (cDC) and plasmacytoid dendritic cells (pDC): lineage cocktail FITC (anti-CD3, CD14, CD19, CD20, CD56; catalog No: 643397), anti-HLA-DR-BV510 (catalog No: 563083), anti-CD123-PE-Cy7 (catalog No: 560826), anti-BDCA-1-PE (catalog No: 564900), and anti-BDCA-2-BV421 (catalog No: 566427).
2.3. Real-Time Quantitative Reverse Transcriptase Polymerase Chain Reaction (RQ-PCR) and Droplet Digital Polymerase Chain Reaction (ddPCR) for the Detection of BCR::ABL1
2.4. Monte Carlo Feature Selection and Interdependency Discovery (MCFS-ID)
2.5. Principal Component Analysis (PCA)
2.6. Statistical Analysis
3. Results
3.1. Characterization Summary of Enrolled Patients
3.2. BCR::ABL1 Transcript Levels in CML Patients with the e13a2 or e14a2 Type in TFR
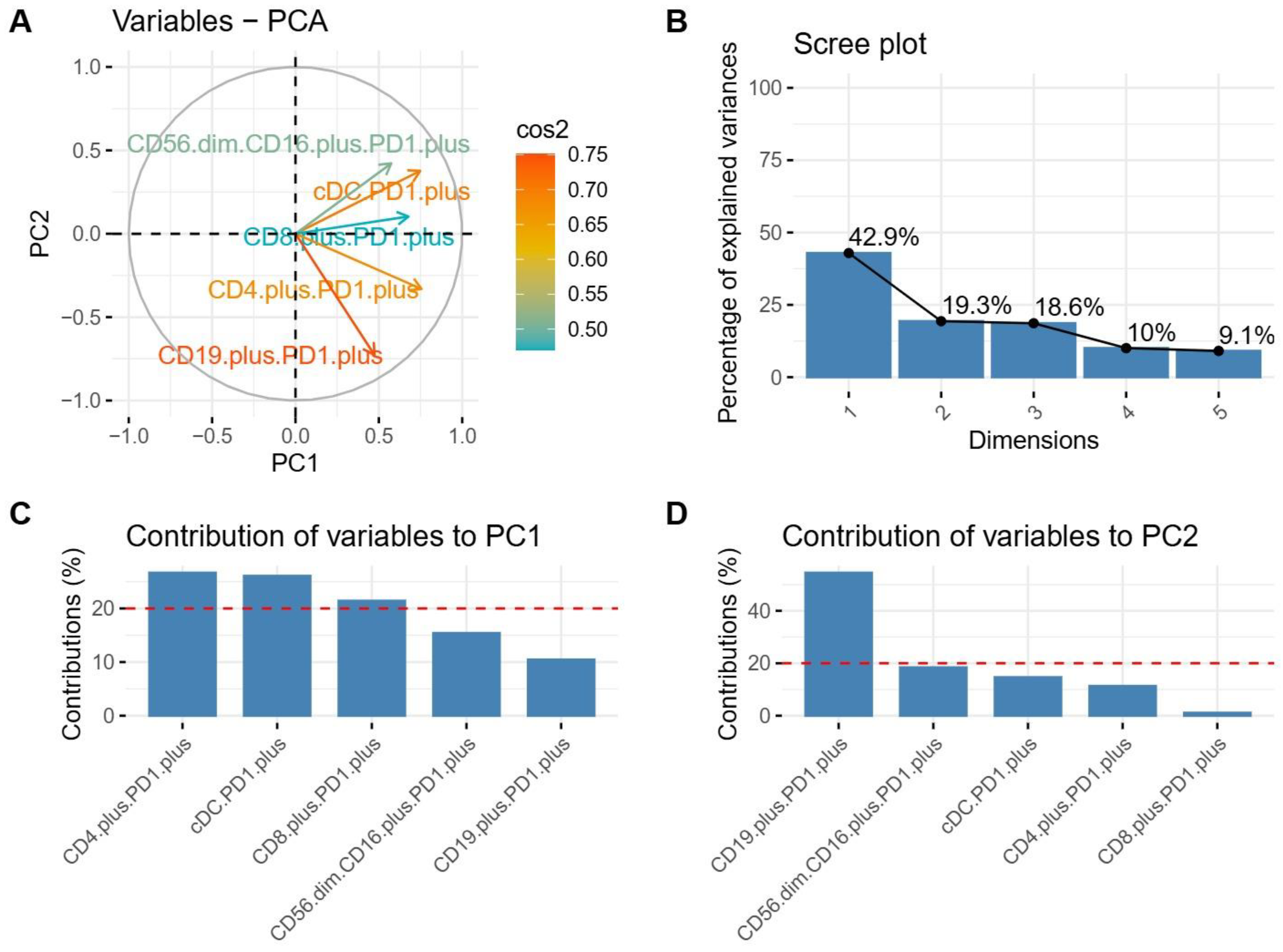
3.3. Selecting the Key Features That Distinguish Patients with e13a2 or e14a2 Transcripts
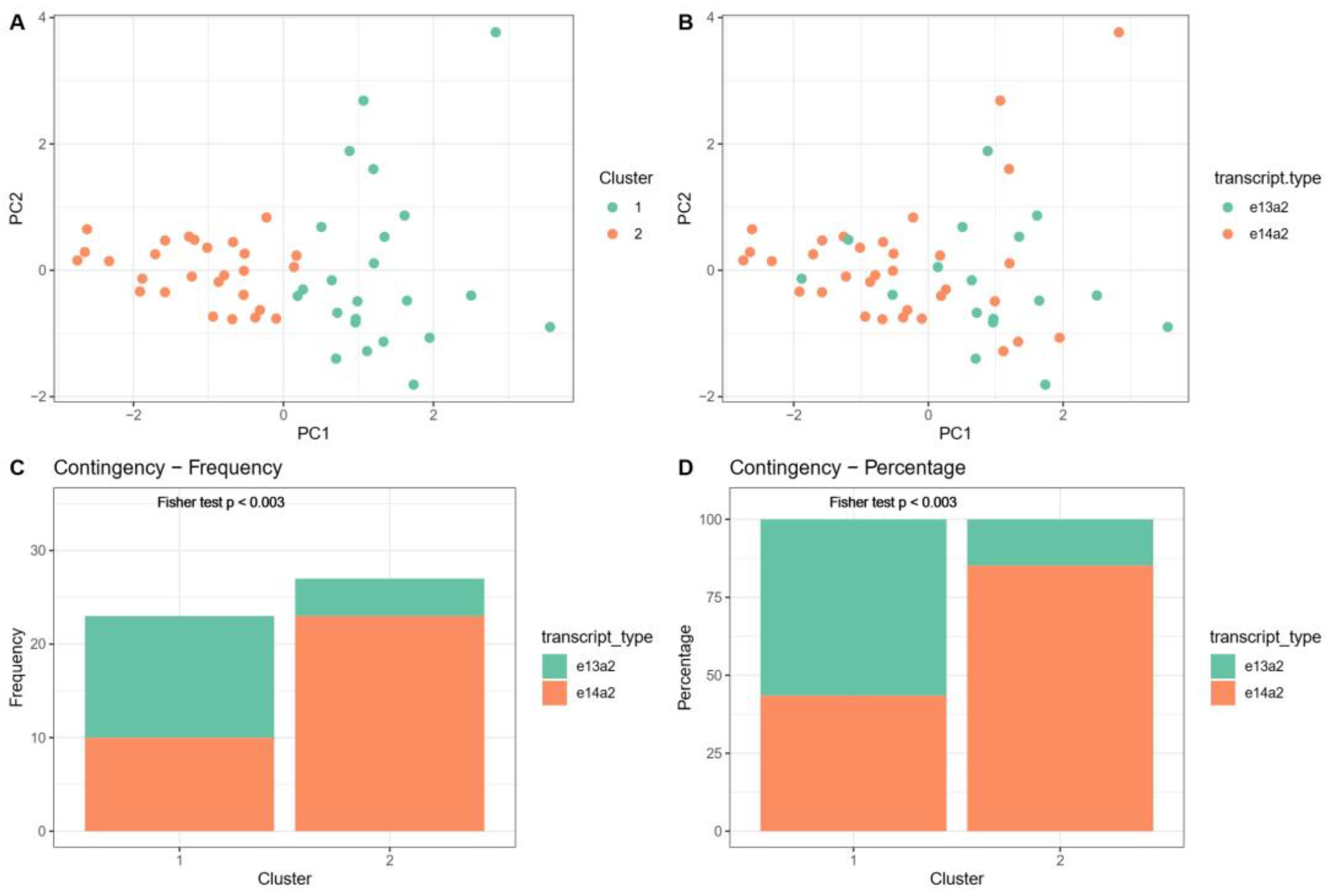
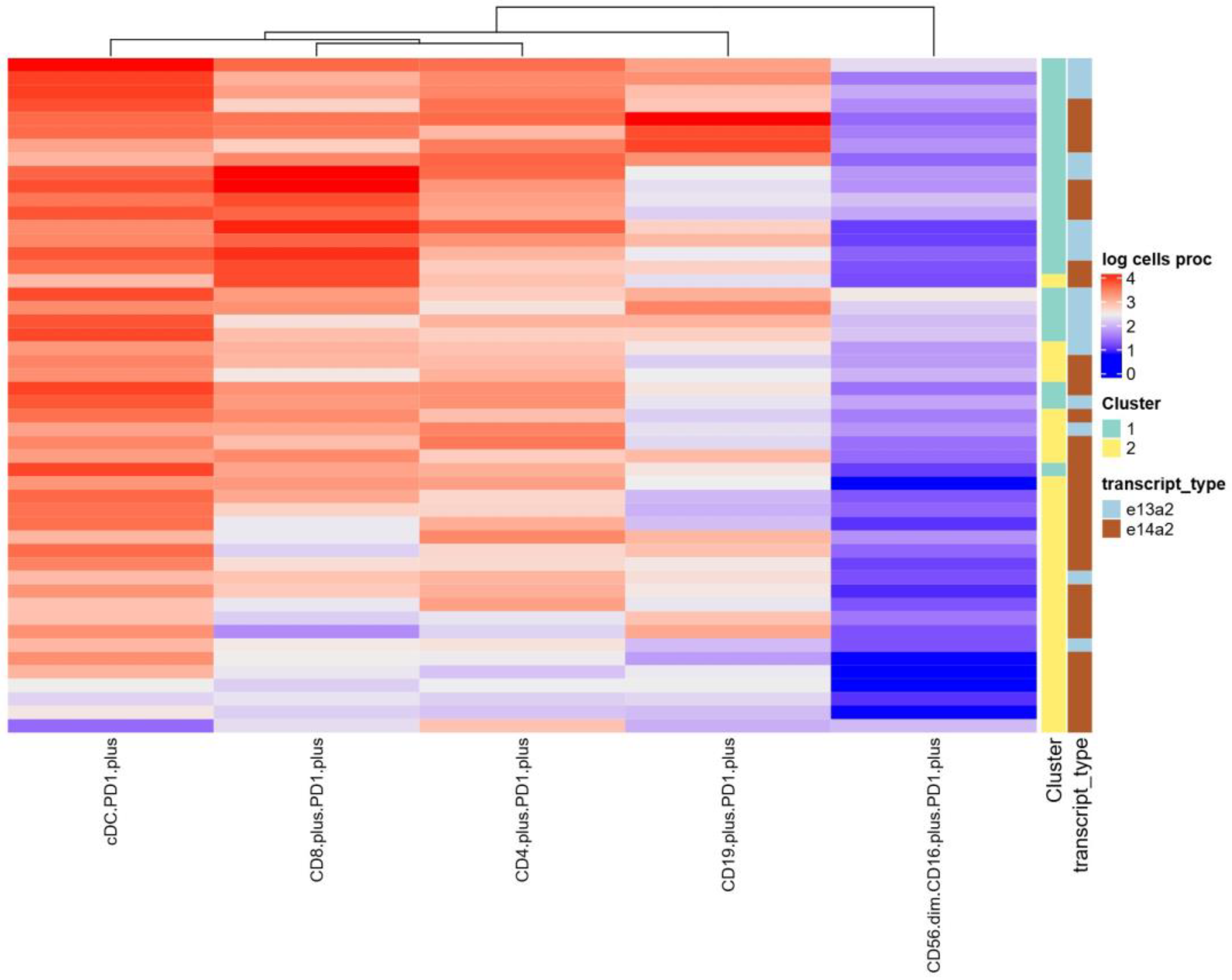
3.4. Distinguishing Groups of Patients with e14a2 or e13a2 Transcripts Based on Their Principal Component Analysis Results
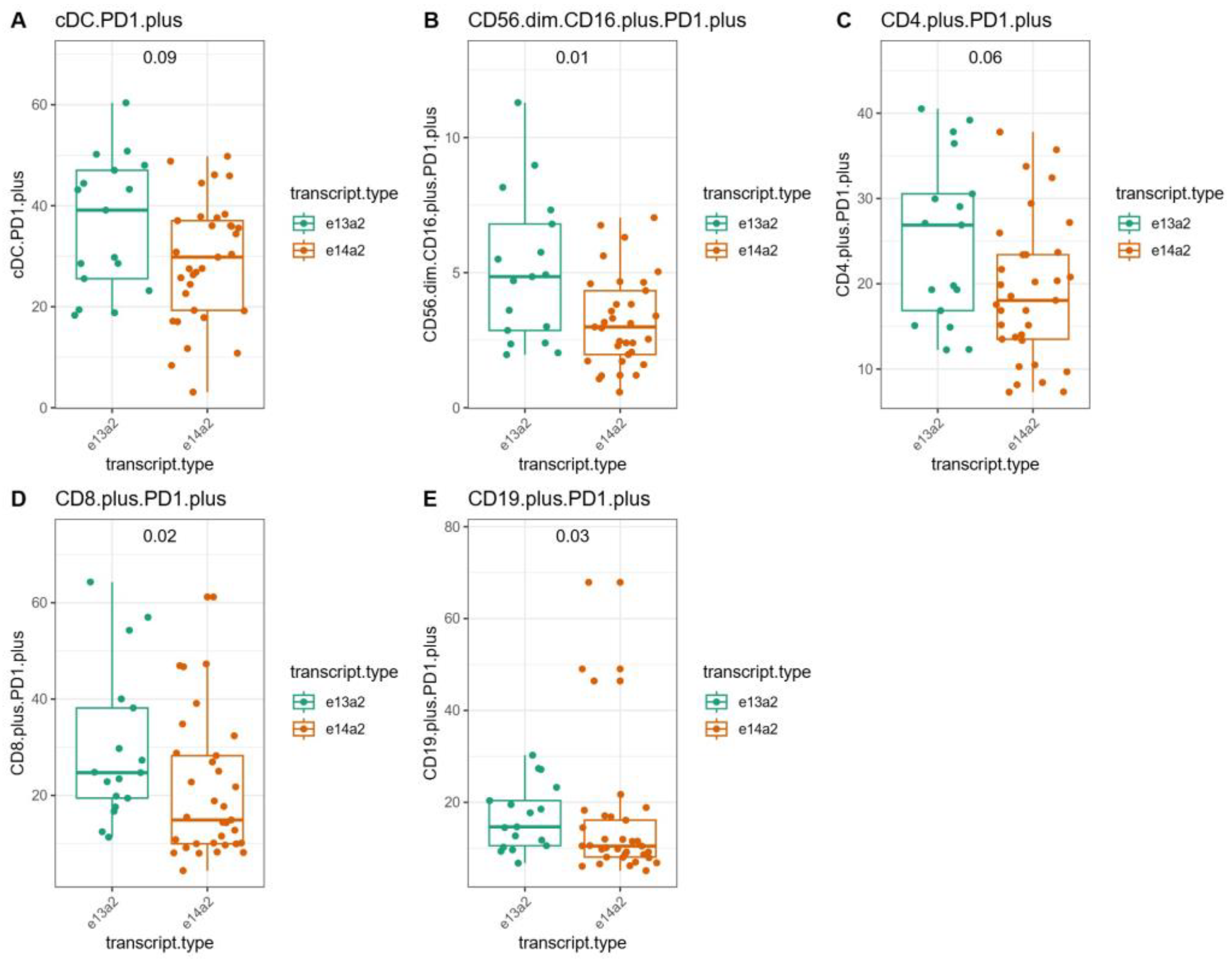
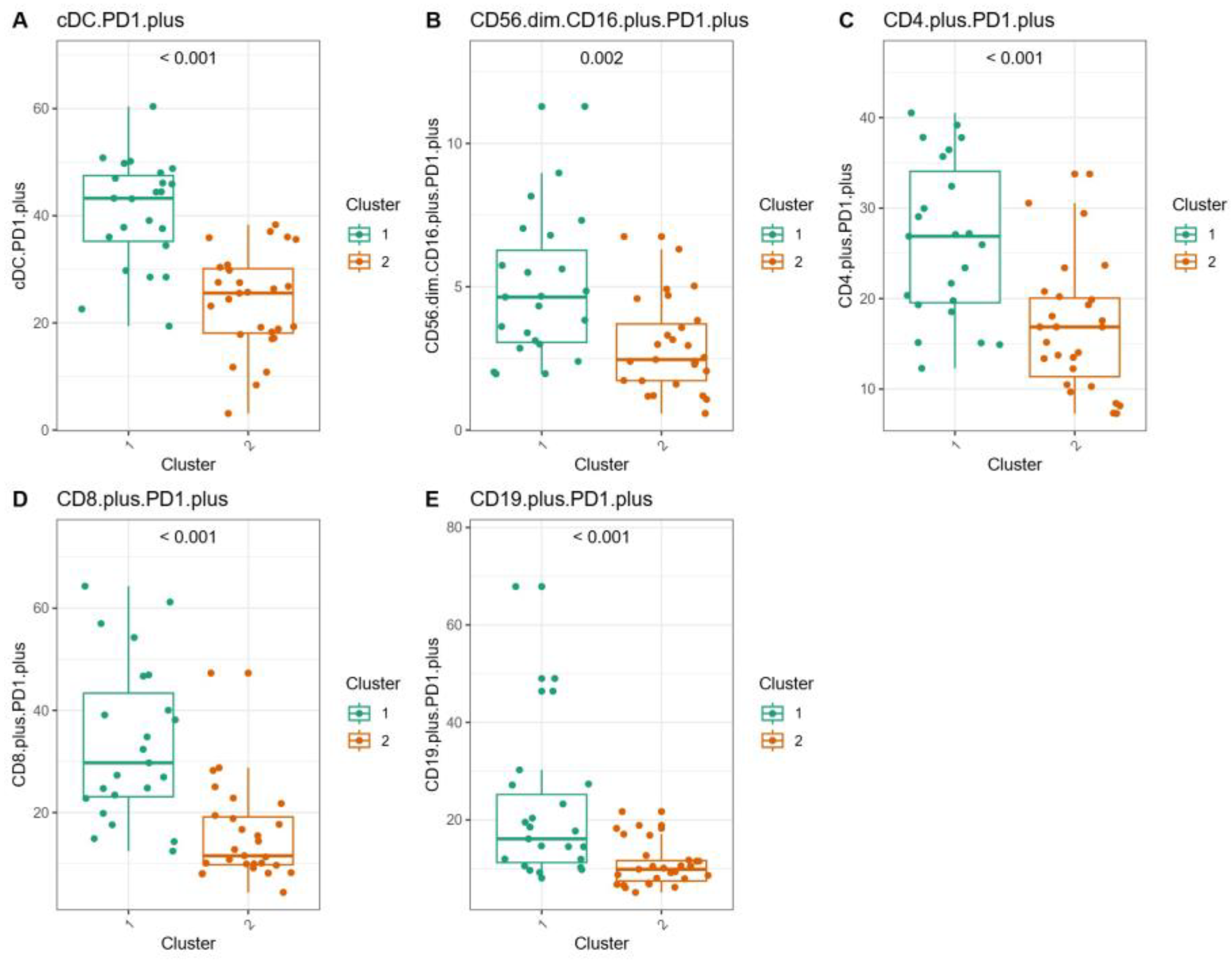
3.5. Immunological Characteristics of CML Patients with the e13a2 or e14a2 Transcripts After Imatinib Discontinuation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CML | Chronic Myeloid Leukemia |
| DC | Dendritic Cells |
| DMR | Deep Molecular Response |
| MCFS-ID | Monte Carlo Feature Selection and Interdependency Discovery |
| MMR | Major Molecular Response |
| NK | Natural Killers |
| NKT | Natural Killer T cells |
| PCA | Principal Component Analysis |
| PBMC | Peripheral Blood Mononuclear Cells |
| TFR | Treatment-Free Remission |
| TKI | Tyrosine Kinase Inhibitor |
| TREG | Regulatory T Lymphocytes |
References
- Jabbour, E.; Kantarjian, H. Chronic Myeloid Leukemia: 2025 Update on Diagnosis, Therapy, and Monitoring. Am. J. Hematol. 2024, 99, 2191–2212. [Google Scholar] [CrossRef]
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Deininger, M.W.; Guilhot, F.; et al. European LeukemiaNet 2020 Recommendations for Treating Chronic Myeloid Leukemia. Leukemia 2020, 34, 966–984. [Google Scholar]
- Saussele, S.; Richter, J.; Guilhot, J.; Gruber, F.X.; Hjorth-Hansen, H.; Almeida, A.; Janssen, J.J.W.M.; Mayer, J.; Koskenvesa, P.; Panayiotidis, P.; et al. Discontinuation of Tyrosine Kinase Inhibitor Therapy in Chronic Myeloid Leukaemia (EURO-SKI): A Prespecified Interim Analysis of a Prospective, Multicentre, Non-Randomised, Trial. Lancet Oncol. 2018, 19, 747–757. [Google Scholar] [CrossRef]
- Mahon, F.X.; Réa, D.; Guilhot, J.; Guilhot, F.; Huguet, F.; Nicolini, F.; Legros, L.; Charbonnier, A.; Guerci, A.; Varet, B.; et al. Discontinuation of Imatinib in Patients with Chronic Myeloid Leukaemia Who Have Maintained Complete Molecular Remission for at Least 2 Years: The Prospective, Multicentre Stop Imatinib (STIM) Trial. Lancet Oncol. 2010, 11, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.M.; Branford, S.; Seymour, J.F.; Schwarer, A.P.; Arthur, C.; Yeung, D.T.; Dang, P.; Goyne, J.M.; Slader, C.; Filshie, R.J.; et al. Safety and Efficacy of Imatinib Cessation for CML Patients with Stable Undetectable Minimal Residual Disease: Results from the TWISTER Study. Blood 2013, 122, 515–522. [Google Scholar] [CrossRef]
- Rousselot, P.; Charbonnier, A.; Cony-Makhoul, P.; Agape, P.; Nicolini, F.E.; Varet, B.; Gardembas, M.; Etienne, G.; Reá, D.; Roy, L.; et al. Loss of Major Molecular Response as a Trigger for Restarting Tyrosine Kinase Inhibitor Therapy in Patients with Chronic-Phase Chronic Myelogenous Leukemia Who Have Stopped Imatinib after Durable Undetectable Disease. J. Clin. Oncol. 2014, 32, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.G.; Sasaki, K.; Issa, G.C.; Garcia-Manero, G.; Ravandi, F.; Kadia, T.; Cortes, J.; Konopleva, M.; Pemmaraju, N.; Alvarado, Y.; et al. Treatment-Free Remission in Patients with Chronic Myeloid Leukemia Following the Discontinuation of Tyrosine Kinase Inhibitors. Am. J. Hematol. 2022, 97, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.C.; Kirschner, K.; Copland, M. Improving Outcomes in Chronic Myeloid Leukemia through Harnessing the Immunological Landscape. Leukemia 2021, 35, 1229–1242. [Google Scholar] [CrossRef]
- Ureshino, H. Treatment-Free Remission and Immunity in Chronic Myeloid Leukemia. Int. J. Hematol. 2021, 113, 642–647. [Google Scholar] [CrossRef]
- Ilander, M.; Olsson-Strömberg, U.; Schlums, H.; Guilhot, J.; Brück, O.; Lähteenmäki, H.; Kasanen, T.; Koskenvesa, P.; Söderlund, S.; Höglund, M.; et al. Increased Proportion of Mature NK Cells Is Associated with Successful Imatinib Discontinuation in Chronic Myeloid Leukemia. Leukemia 2017, 31, 1108–1116. [Google Scholar] [CrossRef]
- Schütz, C.; Inselmann, S.; Sausslele, S.; Dietz, C.T.; Müller, M.C.; Eigendorff, E.; Brendel, C.A.; Metzelder, S.K.; Brümmendorf, T.H.; Waller, C.; et al. Expression of the CTLA-4 Ligand CD86 on Plasmacytoid Dendritic Cells (PDC) Predicts Risk of Disease Recurrence after Treatment Discontinuation in CML. Leukemia 2017, 31, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Rea, D.; Henry, G.; Khaznadar, Z.; Etienne, G.; Guilhot, F.; Nicolini, F.; Guilhot, J.; Rousselot, P.; Huguet, F.; Legros, L.; et al. Natural Killer-Cell Counts Are Associated with Molecular Relapse-Free Survival after Imatinib Discontinuation in Chronic Myeloid Leukemia: The IMMUNOSTIM Study. Haematologica 2017, 102, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Kwaśnik, P.; Zaleska, J.; Link-Lenczowska, D.; Zawada, M.; Wysogląd, H.; Ochrem, B.; Bober, G.; Wasilewska, E.; Hus, I.; Szarejko, M.; et al. High Level of CD8+PD-1+ Cells in Patients with Chronic Myeloid Leukemia Who Experienced Loss of MMR after Imatinib Discontinuation. Cells 2024, 13, 723. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, J.; Cheng, F.; Li, W. Treatment-Free Remission in Chronic Myeloid Leukemia and New Approaches by Targeting Leukemia Stem Cells. Front. Oncol. 2021, 11, 769730. [Google Scholar] [CrossRef]
- Huuhtanen, J.; Adnan-Awad, S.; Theodoropoulos, J.; Forstén, S.; Warfvinge, R.; Dufva, O.; Bouhlal, J.; Dhapola, P.; Duàn, H.; Laajala, E.; et al. Single-Cell Analysis of Immune Recognition in Chronic Myeloid Leukemia Patients Following Tyrosine Kinase Inhibitor Discontinuation. Leukemia 2024, 38, 109–125. [Google Scholar] [CrossRef]
- Hughes, A.; Yong, A.S.M. Immune Effector Recovery in Chronic Myeloid Leukemia and Treatment-Free Remission. Front. Immunol. 2017, 8, 469. [Google Scholar]
- Costa, A.; Breccia, M. How to Improve Treatment-Free Remission Eligibility in Chronic Myeloid Leukaemia? Br. J. Haematol. 2024, 204, 434–448. [Google Scholar] [CrossRef]
- Claudiani, S.; Apperley, J.F.; Gale, R.P.; Clark, R.; Szydlo, R.; Deplano, S.; Palanicawandar, R.; Khorashad, J.; Foroni, L.; Milojkovic, D. E14a2 BCR-ABL1 Transcript Is Associated with a Higher Rate of Treatment-Free Remission in Individuals with Chronic Myeloid Leukemia after Stopping Tyrosine Kinase Inhibitor Therapy. Haematologica 2017, 102, e297–e299. [Google Scholar] [CrossRef]
- Chen, K.; Ruan, Y.; Tian, K.; Xiong, P.; Xia, N.; Li, J.; Huang, W.; Cao, F.; Chen, Q. Impact of BCR-ABL1 Transcript Type on Outcome in Chronic Myeloid Leukemia Patients Treated with Tyrosine Kinase Inhibitors: A Pairwise and Bayesian Network Meta-Analysis. Front. Oncol. 2022, 12, 841546. [Google Scholar] [CrossRef]
- Castagnetti, F.; Gugliotta, G.; Breccia, M.; Iurlo, A.; Levato, L.; Albano, F.; Vigneri, P.; Abruzzese, E.; Rossi, G.; Rupoli, S.; et al. The BCR-ABL1 Transcript Type Influences Response and Outcome in Philadelphia Chromosome-Positive Chronic Myeloid Leukemia Patients Treated Frontline with Imatinib. Am. J. Hematol. 2017, 92, 797–805. [Google Scholar] [CrossRef]
- Jain, P.; Kantarjian, H.; Patel, K.P.; Gonzalez, G.N.; Luthra, R.; Shamanna, R.K.; Sasaki, K.; Jabbour, E.; Romo, C.G.; Kadia, T.M.; et al. Impact of BCR-ABL Transcript Type on Outcome in Patients with Chronic-Phase CML Treated with Tyrosine Kinase Inhibitors. Blood 2016, 127, 1269. [Google Scholar] [CrossRef]
- D’Adda, M.; Farina, M.; Schieppati, F.; Borlenghi, E.; Bottelli, C.; Cerqui, E.; Ferrari, S.; Gramegna, D.; Pagani, C.; Passi, A.; et al. The E13a2 BCR-ABL Transcript Negatively Affects Sustained Deep Molecular Response and the Achievement of Treatment-Free Remission in Patients with Chronic Myeloid Leukemia Who Receive Tyrosine Kinase Inhibitors. Cancer 2019, 125, 1674–1682. [Google Scholar] [CrossRef]
- Pagnano, K.B.B.; Miranda, E.C.; Delamain, M.T.; Duarte, G.O.; de Paula, E.V.; Lorand-Metze, I.; de Souza, C.A. Influence of BCR-ABL Transcript Type on Outcome in Patients with Chronic-Phase Chronic Myeloid Leukemia Treated With Imatinib. Clin. Lymphoma. Myeloma Leuk. 2017, 17, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Sacha, T.; Zawada, M.; Czekalska, S.; Florek, I.; Mueller, M.; Gniot, M.; Jaźwiec, B.; Kyrcz-Krzemień, S.; Leszczyńska, A.; Lewandowski, K.; et al. Standaryzacja Ilościowej Oceny Ekspresji Genu BCR-ABL Metoda RQ-PCR u Chorych Na Przewlekła Białaczke Szpikowa We Współpracy z European Leukemia Net. Przegla̧d Lek. 2010, 67, 454–459. [Google Scholar]
- Kjaer, L.; Skov, V.; Andersen, M.T.; Aggerholm, A.; Clair, P.; Gniot, M.; Soeby, K.; Udby, L.; Dorff, M.H.; Hasselbalch, H.; et al. Variant-Specific Discrepancy When Quantitating BCR-ABL1 E13a2 and E14a2 Transcripts Using the Europe Against Cancer QPCR Assay. Eur. J. Haematol. 2019, 103, 26–34. [Google Scholar] [CrossRef]
- Draminski, M.; Koronacki, J. Rmcfs: An R Package for Monte Carlo Feature Selection and Interdependency Discovery. J. Stat. Softw. 2018, 85, 1–28. [Google Scholar] [CrossRef]
- Suttorp, M.; Thiede, C.; Tauer, J.T.; Range, U.; Schlegelberger, B.; von Neuhoff, N. Impact of the Type of the BCR-ABL Fusion Transcript on the Molecular Response in Pediatric Patients with Chronic Myeloid Leukemia. Haematologica 2010, 95, 852–853. [Google Scholar] [CrossRef]
- Lucas, C.M.; Harris, R.J.; Giannoudis, A.; Davies, A.; Knight, K.; Watmough, S.J.; Wang, L.; Clark, R.E. Chronic Myeloid Leukemia Patients with the E13a2 BCR-ABL Fusion Transcript Have Inferior Responses to Imatinib Compared to Patients with the E14a2 Transcript. Haematologica 2009, 94, 1362–1367. [Google Scholar] [CrossRef]
- Clark, R.E.; Anthony Dodi, I.; Hill, S.C.; Lill, J.R.; Aubert, G.; Macintyre, A.R.; Rojas, J.; Bourdon, A.; Bonner, P.L.R.; Wang, L.; et al. Direct Evidence That Leukemic Cells Present HLA-Associated Immunogenic Peptides Derived from the BCR-ABL B3a2 Fusion Protein. Blood 2001, 98, 2887–2893. [Google Scholar] [CrossRef]
- Hughes, A.; Clarson, J.; Tang, C.; Vidovic, L.; White, D.L.; Hughes, T.P.; Yong, A.S.M. CML Patients with Deep Molecular Responses to TKI Have Restored Immune Effectors and Decreased PD-1 and Immune Suppressors. Blood 2017, 129, 1166–1176. [Google Scholar] [CrossRef]
- Kwaśnik, P.; Giannopoulos, K. Treatment-Free Remission-A New Aim in the Treatment of Chronic Myeloid Leukemia. J. Pers. Med. 2021, 11, 697. [Google Scholar] [CrossRef] [PubMed]
- Breccia, M.; Molica, M.; Colafigli, G.; Massaro, F.; Quattrocchi, L.; Latagliata, R.; Mancini, M.; Diverio, D.; Guarini, A.; Alimena, G.; et al. Prognostic Factors Associated with a Stable MR4.5 Achievement in Chronic Myeloid Leukemia Patients Treated with Imatinib. Oncotarget 2017, 9, 7534–7540. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, M.; Scaffidi, L.; Binotto, G.; De Marchi, F.; Maino, E.; Calistri, E.; Bonalumi, A.; Frison, L.; Marin, L.; Medeot, M.; et al. Predictive Factors of Stable Deep Molecular Response in Chronic Myeloid Leukemia Patients Treated with Imatinib Standard Dose: A Study from the Gruppo Triveneto LMC. Blood 2015, 126, 597. [Google Scholar] [CrossRef]
- Hanfstein, B.; Lauseker, M.; Hehlmann, R.; Saussele, S.; Erben, P.; Dietz, C.; Fabarius, A.; Proetel, U.; Schnittger, S.; Haferlach, C.; et al. Distinct Characteristics of E13a2 versus E14a2 BCR-ABL1 Driven Chronic Myeloid Leukemia under First-Line Therapy with Imatinib. Haematologica 2014, 99, 1441. [Google Scholar] [CrossRef]
- Baccarani, M.; Rosti, G.; Soverini, S. Chronic Myeloid Leukemia: The Concepts of Resistance and Persistence and the Relationship with the BCR-ABL1 Transcript Type. Leukemia 2019, 33, 2358–2364. [Google Scholar] [CrossRef]
- Marcé, S.; Méndez, A.; Xicoy, B.; Estrada, N.; Cabezón, M.; Sturla, A.L.; García, M.R.; Angona, A.; Amat, P.; Escribano Serrat, S.; et al. E14a2 Transcript Favors Treatment-Free Remission in Chronic Myeloid Leukemia When Associated with Longer Treatment with Tyrosine Kinase Inhibitors and Sustained Deep Molecular Response. J. Clin. Med. 2024, 13, 779. [Google Scholar] [CrossRef]
- Eşkazan, A.E.; Kok, C.H.; Yeung, D.; Dalal, J.; Tiribelli, M. Editorial: Advances in the Treatment of Chronic Myeloid Leukemia. Front. Oncol. 2023, 13, 1166588. [Google Scholar] [CrossRef]
- Pagnano, K.B.; Miranda, E.C.; Seguro, F.S.; Magalhaes, G.H.; Clementino, N.D.; Moellmann-Coelho, A.; Da Silva, M.N.; Centrone, R.; Conchon, M.; Bortolini, J.; et al. E14a2 and e14a2+e13a2 BCR-ABL Transcripts Are Associated with Earlier Cytogenetic and Molecular Response Rates in CML-CP Treated with Imatinib. Blood 2017, 130, 1596. [Google Scholar]
- Garofalaki, M.; Tziotziou, E.; Nikolou, E.; Tsonis, I.; Dellatola, M.; Mellios, Z.; Psyllaki, M.; Grispou, E.; Delimpassi, S.; Baltadakis, I.; et al. E13A2, E14A2 Transcripts: Do They Have a Different Impact on Outcome In Patients with Chronic Myeloid Leukemia? EHA Library. 9 June 2021. p. 324194. Available online: https://library.ehaweb.org/eha/2021/eha2021-virtual-congress/324194/maria.garofalaki.e13a2.e14a2.transcripts.do.they.have.a.different.impact.on.html (accessed on 3 July 2024).
- Salmon, M.; White, H.E.; Zizkova, H.; Gottschalk, A.; Motlova, E.; Cerveira, N.; Colomer, D.; Coriu, D.; Franke, G.N.; Gottardi, E.; et al. Impact of BCR::ABL1 Transcript Type on RT-QPCR Amplification Performance and Molecular Response to Therapy. Leukemia 2022, 36, 1879–1886. [Google Scholar] [CrossRef]
- Dominy, K.M.; Claudiani, S.; O’Hare, M.; Szydlo, R.; Gerrard, G.; Foskett, P.; Foroni, L.; Milojkovic, D.; Apperley, J.F.; Khorashad, J. Assessment of Quantitative Polymerase Chain Reaction for BCR-ABL1 Transcripts in Chronic Myeloid Leukaemia: Are Improved Outcomes in Patients with E14a2 Transcripts an Artefact of Technology? Br. J. Haematol. 2022, 197, 52–62. [Google Scholar] [CrossRef]
- Pfirrmann, M.; Evtimova, D.; Saussele, S.; Castagnetti, F.; Cervantes, F.; Janssen, J.; Hoffmann, V.S.; Gugliotta, G.; Hehlmann, R.; Hochhaus, A.; et al. No Influence of BCR-ABL1 Transcript Types E13a2 and E14a2 on Long-Term Survival: Results in 1494 Patients with Chronic Myeloid Leukemia Treated with Imatinib. J. Cancer Res. Clin. Oncol. 2017, 143, 843–850. [Google Scholar] [CrossRef] [PubMed]


| e14a2 | e13a2 | p Value | |
|---|---|---|---|
| Number of patients, N (%) | 33 (64.7) | 18 (35.3) | |
| Gender, Male, N (%) | 16 (48.5) | 6 (33.3) | 0.39 |
| Age, years median (95% CI) | 65; 59–74 | 59.5; 46–76 | 0.74 |
| ELTS score, N (%) | 0.095 | ||
| low | 23 (69.7) | 9 (50.0) | |
| intermediate | 5 (15.2) | 2 (11.1) | |
| high | 0 (0.0) | 2 (11.1) | |
| EUTOS score, N (%) | 0.19 | ||
| low | 26 (78.8) | 13 (72.2) | |
| intermediate | 4 (12.1) | 0 (0.0) | |
| high | 3 (9.1) | 4 (22.2) | |
| Sokal score, N (%) | 0.69 | ||
| low | 26 (78.8) | 13 (72.2) | |
| intermediate | 4 (12.1) | 2 (11.1) | |
| high | 3 (9.1) | 3 (16.7) | |
| Hasford score, N (%) | 0.19 | ||
| low | 24 (72.7) | 10 (55.6) | |
| intermediate | 7 (21.2) | 3 (16.7) | |
| high | 2 (6.1) | 4 (22.2) | |
| Neutropenia, N (%) | 0.86 | ||
| 1/2 | 4 (12.1) | 2 (11.1) | |
| 2 | 0 (0.0) | 0 (0.0) | |
| 3 | 1(3.0) | 1 (5.6) | |
| 3/4 | 2 (6.1) | 2 (11.1) | |
| Anemia, N (%) | 0.47 | ||
| 1/2 | 14 (42.4) | 4 (22.2) | |
| 2 | 0 (0.0) | 1 (5.6) | |
| 3 | 0 (0.0) | 0 (0.0) | |
| 3/4 | 0 (0.0) | 0 (0.0) | |
| Thrombocytopenia, N (%) | 0.80 | ||
| 1/2 | 4 (12.1) | 2 (11.1) | |
| 2 | 0 (0.0) | 0 (0.0) | |
| 3 | 0 (0.0) | 1 (5.6) | |
| 3/4 | 1 (3.0) | 1 (5.6) | |
| Duration of DMR 1, months median (95% CI) | 83; 74–100 | 74.5; 51–91 | 0.22 |
| Duration of TKI treatment 1, months median (95% CI) | 116; 102–124 | 103.5; 61–128 | 0.16 |
| Lost MMR 2, N (%) | 8 (24.2) | 4 (22.2) | 0.68 |
| Lost DMR 2, N (%) | 11 (33.3) | 9 (50.0) | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwaśnik, P.; Kiełbus, M.; Zaleska, J.; Link-Lenczowska, D.; Zawada, M.; Wysogląd, H.; Sacha, T.; Giannopoulos, K. The PD1 Molecule May Contribute to Lower Treatment-Free Remission Rates in Patients with Chronic Myeloid Leukemia with the e13a2 Transcript. J. Clin. Med. 2025, 14, 2304. https://doi.org/10.3390/jcm14072304
Kwaśnik P, Kiełbus M, Zaleska J, Link-Lenczowska D, Zawada M, Wysogląd H, Sacha T, Giannopoulos K. The PD1 Molecule May Contribute to Lower Treatment-Free Remission Rates in Patients with Chronic Myeloid Leukemia with the e13a2 Transcript. Journal of Clinical Medicine. 2025; 14(7):2304. https://doi.org/10.3390/jcm14072304
Chicago/Turabian StyleKwaśnik, Paulina, Michał Kiełbus, Joanna Zaleska, Dorota Link-Lenczowska, Magdalena Zawada, Hubert Wysogląd, Tomasz Sacha, and Krzysztof Giannopoulos. 2025. "The PD1 Molecule May Contribute to Lower Treatment-Free Remission Rates in Patients with Chronic Myeloid Leukemia with the e13a2 Transcript" Journal of Clinical Medicine 14, no. 7: 2304. https://doi.org/10.3390/jcm14072304
APA StyleKwaśnik, P., Kiełbus, M., Zaleska, J., Link-Lenczowska, D., Zawada, M., Wysogląd, H., Sacha, T., & Giannopoulos, K. (2025). The PD1 Molecule May Contribute to Lower Treatment-Free Remission Rates in Patients with Chronic Myeloid Leukemia with the e13a2 Transcript. Journal of Clinical Medicine, 14(7), 2304. https://doi.org/10.3390/jcm14072304








