Management of SARS-CoV-2 Infection-Clinical Practice Guidelines of the Polish Association of Epidemiologists and Infectiologists, for 2025
Abstract
1. Introduction and Rationale for Developing the Guidelines
2. Characterization of SARS-CoV-2: Etiology, Molecular Evolution and Pathogenesis
3. Clinical Picture of the Disease
4. Laboratory Diagnostics
4.1. Antigen Tests
- CE certified;
- Clinical efficacy has been confirmed on samples taken from the nasal cavity, oropharyngeal cavity, or nasopharyngeal cavity;
- Sensitivity >80% in studies on symptomatic patients in the first seven days of symptom onset or in asymptomatic individuals whose SARS-CoV-2 infection was confirmed by molecular testing;
- Sensitivity >90% for individuals with SARS-CoV-2 genetic material detected with a detection threshold (cycle threshold, CT) <25, reflecting high viral load.
4.2. Molecular Tests
4.3. Serological Tests
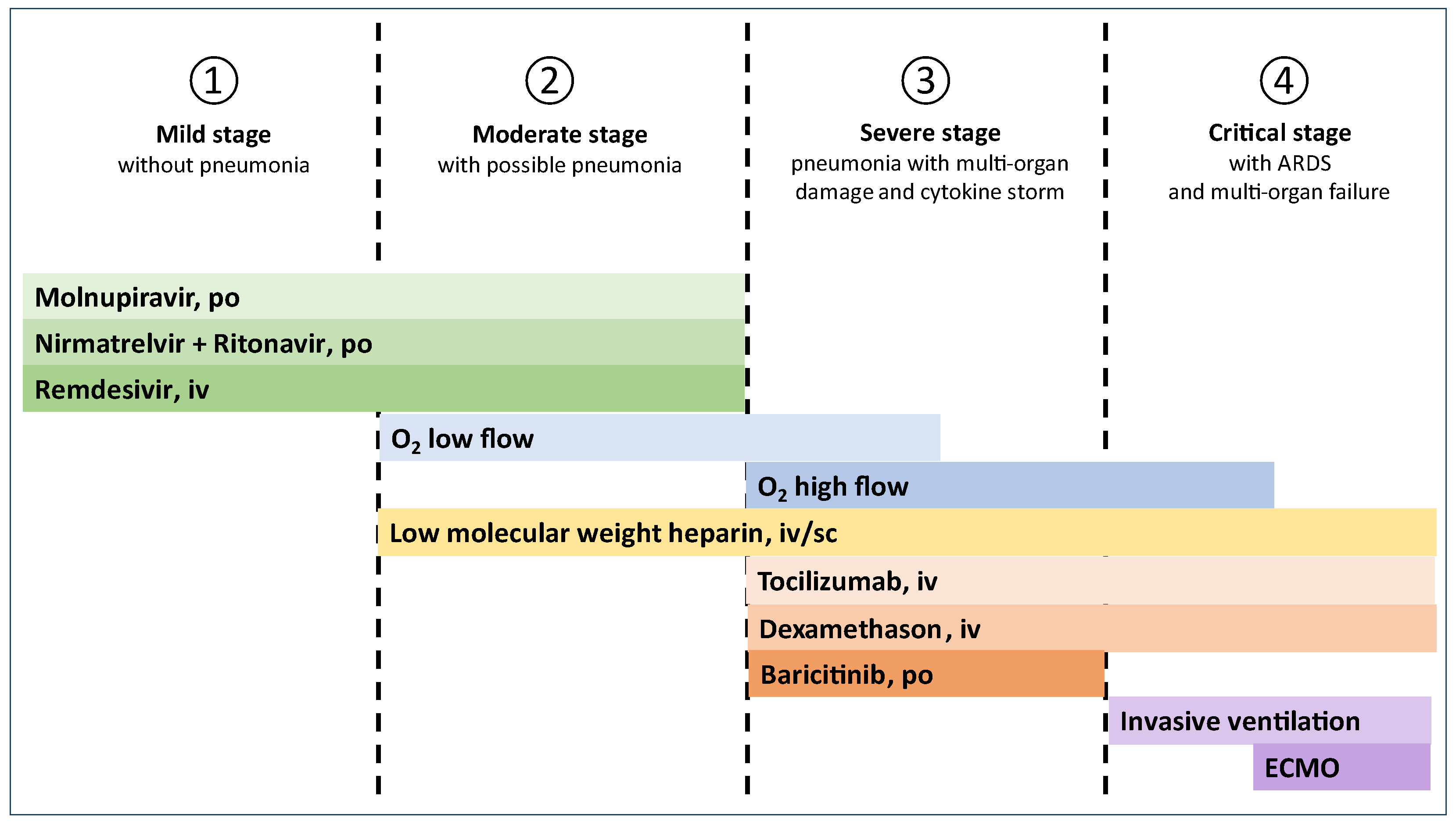
5. Treatment
5.1. Stage 1 (Mild, Without Pneumonia)
5.2. Stage 2 (Moderate, with Possible Pneumonia)
5.3. Stage 3 (Severe, Pneumonia with Multi-Organ Damage and Cytokine Storm)
| Stage 1 (mild, without pneumonia) | |
| |
| Basic treatment | Supportive treatment |
| Antiviral drugs (alphabetical order, does not indicate priority of selection). Initiation of antiviral therapy within 5 days of symptom onset and within 10 days in immunosuppressed patients. Recommended for patients at risk of severe COVID-19 * and to be considered in other patients.
|
|
| Notes: | |
| |
| Stage 2 (moderate, with possible pneumonia) | |
| |
| Basic treatment | Supportive treatment |
| Oxygen therapy: low-flow, up to 15 L/min. Antithrombotic drugs: Low-molecular-weight heparin in a prophylactic dose, which can be increased in justified cases. Antiviral drugs (alphabetical order, does not indicate priority of selection). Initiation of antiviral therapy within 5 days of the onset of symptoms and within 10 days in immunosuppressed patients.
|
|
| Notes: | |
| |
| Stage 3 (severe, pneumonia with multi-organ damage and cytokine storm) | |
| |
| Basic treatment | Supportive treatment |
| Oxygen therapy: high-flow, up to 60 L/min. Antithrombotic drugs: Low-molecular-weight heparin in a prophylactic dose, which can be increased in justified cases. Glucocorticosteroids: Dexamethasone administered intravenously in adults, 6 mg, in children, 0.1–0.15 mg/kg (maximum 6 mg) daily, for no longer than 10 days [78,79]. Immunomodulatory drugs:
|
|
| Notes: | |
| Antibiotic therapy only if there is a diagnosis or reasonable suspicion of an overlapping bacterial infection of the respiratory tract. | |
| Stage 4 (critical, with ARDS and multi-organ failure) | |
| |
| Basic treatment | Supportive treatment |
Oxygen therapy:
Glucocorticosteroids: Dexamethasone, adults, intravenous, daily dose of 12 mg, in children, 0.1–0.15 mg/kg (maximum 6 mg) daily, for no longer than 10 days. If dexamethasone is not available, other glucocorticoids in equivalent doses may be given. AND/OR Immunomodulatory drugs:
|
|
| Notes: | |
| Antibiotic therapy only if there is a diagnosis or reasonable suspicion of an overlapping bacterial infection of the respiratory tract. | |
5.4. Stage 4 (Critical, with Acute Respiratory Distress Syndrome (ARDS) and Multi-Organ Failure)
6. Therapies with Unproven Effectiveness
6.1. Antiviral/Anti-Infective Drugs
- ■
- Favipiravir (the major supporting article was retracted).
- ■
- Oseltamivir and zanamivir [88].
- ■
- Amantadine and rimantadine [89].
- ■
- Antiretroviral drugs (used to treat HIV infection) [90].
- ■
- Ivermectin [91].
- ■
- Fluvoxamine [92].
- ■
- Interferons.
- ■
- Intravenous immunoglobulin and specific immunoglobulin against SARS-CoV-2 [93].
- ■
- Anti-SARS-CoV-2 monoclonal antibodies—bamlaniwimab/etesewimab, casirivimab/imdewimab, thixagevimab/cilgavimab—lack of effectiveness against the Omicron variant of SARS-CoV-2 [94];
- ■
- Convalescent plasma [95].
6.2. Anti-Inflammatory Drugs
- ■
- Nonsteroidal anti-inflammatory drugs (NSAIDs) can be used for symptomatic treatment in therapeutic doses and for short periods [96];
- ■
- Anakinra, although initially recommended for use in stage 3, ultimately failed to demonstrate efficacy in clinical trials [97];
- ■
- Glucocorticosteroids are contraindicated in stage 1 of the disease.
6.3. Other Medications
- ■
- Metformin [98];
- ■
- Dietary supplements, including vitamins C and D and zinc [99];
- ■
- Azithromycin and other antibiotics should only be used in the presence of a concomitant bacterial infection [100];
- ■
- Chloroquine and hydroxychloroquine [101].
7. Post-COVID Syndrome
8. Clinical and Therapeutic Differences in Children
9. Vaccinations
9.1. Available Vaccines
- -
- mRNA vaccines:
- Comirnaty (Pfizer-BioNTech);
- Spikevax (Moderna).
- -
- Subunit protein vaccines (not available in Poland):
- Nuvaxovid (Novavax)—containing recombinant S protein and Matrix-M adjuvant;
- Bimervax (Hipra)—containing recombinant S protein and SQBA adjuvant.
9.2. Target Groups for Vaccinations
- People aged ≥60 years;
- People with chronic diseases, including diabetes, lung diseases, kidney diseases, cardiovascular diseases, obesity (BMI ≥25), neurodevelopmental disorders, active neoplastic disease, or immunosuppression (resulting from disease or treatment);
- People staying in long-term care facilities;
- Pregnant women, due to the reduction in COVID-19 complications among newborns;
- People working in health care or long-term care facilities;
9.3. Vaccination Schedule
- For individuals with severe immunodeficiency, it is recommended to administer the vaccine every 6 months, with a minimum interval of 2 months between doses. If possible, optimally ≥2 weeks before starting/continuing immunosuppressive therapy.
- Revaccination is recommended for patients vaccinated before or during hematopoietic cell transplantation or CAR T cell therapy ≥ 3 months after the procedure. Revaccination should be considered in patients vaccinated against COVID-19 during treatment with B cell-depleting therapies—vaccination is recommended 6 months after therapy. In the case of planned B cell-depleting therapy, COVID-19 vaccination should be administered 4 weeks before its initiation or resumption [131].
- For children aged 6 months to 11 years: for those vaccinated for the first time, we recommend 2 doses 4 weeks apart, and for children with severe immunodeficiency—3 doses (the first two 4 weeks apart, the third 2 months after the second dose). If possible, optimally ≥2 weeks before starting/continuing immunosuppressive therapy. Children with immunosuppression should receive subsequent doses of the vaccine every 6 months.
- It is safe and recommended to administer the COVID-19 vaccine concurrently with an inactivated influenza vaccine or a pneumococcal vaccine (in one visit), as well as other routinely administered vaccines.
- COVID-19 vaccines can be administered at any interval from other vaccines (including those recommended during pregnancy), except for the monkeypox vaccine (MPox), in which case a minimum interval of 4 weeks should be maintained.
- Vaccines are administered intramuscularly; we recommend continuing vaccinations with a vaccine from the same manufacturer whenever possible. It is not recommended to use vaccines with an outdated composition.
9.4. Contraindications and Situations Requiring Caution
- Severe allergic reaction (e.g., anaphylaxis) after the previous dose of the vaccine or to any of its components.
- Acute febrile illness or exacerbation of a chronic disease—vaccination should be postponed until the symptoms have subsided.
- Myocarditis or pericarditis.
- Multisystem inflammatory syndrome (MIS-C in children or MIS-A in adults).
- We recommend monitoring all vaccinated individuals for at least 15 min after vaccination.
9.5. Vaccine Safety
- Pain, redness, or swelling at the injection site.
- Fatigue, headache, muscle or joint pain, fever.
9.6. Organization of Vaccinations
- Primary health care (family doctors);
- Public pharmacies;
- Hospitals.
10. Pre-Exposure Prophylaxis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 2 March 2025).
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Pawłowska, M.; Parczewski, M.; Piekarska, A.; Simon, K.; Tomasiewicz, K.; Zarębska-Michaluk, D. Management of SARS-CoV-2 infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of March 31, 2020. Pol. Arch. Intern. Med. 2020, 130, 352–357. [Google Scholar]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Pawłowska, M.; Parczewski, M.; Piekarska, A.; Simon, K.; Tomasiewicz, K.; Zarębska-Michaluk, D. Management of SARS-CoV-2 infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists. Annex no. 1 as of June 8, 2020. Pol. Arch. Intern. Med. 2020, 130, 557–558. [Google Scholar] [PubMed]
- Flisiak, R.; Parczewski, M.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Pawłowska, M.; Piekarska, A.; Simon, K.; Tomasiewicz, K.; Zarębska-Michaluk, D. Management of SARS-CoV-2 infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists. Annex no. 2 as of October 13, 2020. Pol. Arch. Intern. Med. 2020, 130, 915–918. [Google Scholar]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Mastalerz-Migas, A.; Owczuk, R.; Parczewski, M.; Pawłowska, M.; Piekarska, A.; Simon, K.; et al. Management of SARS-CoV-2 infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of April 26, 2021. Pol. Arch. Intern. Med. 2021, 131, 487–496. [Google Scholar] [PubMed]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Mastalerz-Migas, A.; Owczuk, R.; Parczewski, M.; Pawłowska, M.; Piekarska, A.; Simon, K.; et al. Diagnosis and therapy of SARS-CoV-2 infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of November 12, 2021. Annex no. 1 to the Recommendations of April 26, 2021. Pol. Arch. Intern. Med. 2021, 131, 16140. [Google Scholar] [PubMed]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Mastalerz-Migas, A.; Owczuk, R.; Parczewski, M.; Pawłowska, M.; Piekarska, A.; Simon, K.; et al. Management of SARS-CoV-2 infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of February 23, 2022. Pol. Arch. Intern. Med. 2022, 132, 16230. [Google Scholar]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The Proximal Origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar]
- Boni, M.F.; Lemey, P.; Jiang, X.; Lam, T.T.-Y.; Perry, B.W.; Castoe, T.A.; Rambaut, A.; Robertson, D.L. Evolutionary Origins of the SARS-CoV-2 Sarbecovirus Lineage Responsible for the COVID-19 Pandemic. Nat. Microbiol. 2020, 5, 1408–1417. [Google Scholar]
- Li, L.-L.; Wang, J.-L.; Ma, X.-H.; Sun, X.-M.; Li, J.-S.; Yang, X.-F.; Shi, W.-F.; Duan, Z.-J. A Novel SARS-CoV-2 Related Coronavirus with Complex Recombination Isolated from Bats in Yunnan Province, China. Emerg. Microbes Infect. 2021, 10, 1683–1690. [Google Scholar]
- The Lancet Microbe. Searching for SARS-CoV-2 Origins: Confidence versus Evidence. Lancet Microbe 2023, 4, e200. [Google Scholar] [CrossRef] [PubMed]
- Statement on the Update of WHO’s Working Definitions and Tracking System for SARS-CoV-2 Variants of Concern and Variants of Interest. Available online: https://www.who.int/news/item/16-03-2023-statement-on-the-update-of-who-s-working-definitions-and-tracking-system-for-sars-cov-2-variants-of-concern-and-variants-of-interest (accessed on 2 March 2025).
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 Facilitates SARS-CoV-2 Cell Entry and Infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef]
- Gu, Y.; Cao, J.; Zhang, X.; Gao, H.; Wang, Y.; Wang, J.; He, J.; Jiang, X.; Zhang, J.; Shen, G.; et al. Receptome Profiling Identifies KREMEN1 and ASGR1 as Alternative Functional Receptors of SARS-CoV-2. Cell Res. 2022, 32, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Liu, X.; Ou, H.; Li, X.; Liu, R.; Lv, X.; Xiao, S.; Hu, M.; Liang, T.; Chen, T.; et al. The Histamine Receptor H1 Acts as an Alternative Receptor for SARS-CoV-2. MBio 2024, 15, e0108824. [Google Scholar] [CrossRef]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 Pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef]
- Zaidi, A.K.; Singh, R.B.; A A Rizvi, S.; Dehgani-Mobaraki, P.; Palladino, N. COVID-19 Pathogenesis. Prog. Mol. Biol. Transl. Sci. 2024, 202, 67–112. [Google Scholar] [PubMed]
- Willett, B.J.; Grove, J.; MacLean, O.A.; Wilkie, C.; De Lorenzo, G.; Furnon, W.; Cantoni, D.; Scott, S.; Logan, N.; Ashraf, S.; et al. SARS-CoV-2 Omicron Is an Immune Escape Variant with an Altered Cell Entry Pathway. Nat. Microbiol. 2022, 7, 1161–1179. [Google Scholar]
- Meng, B.; Abdullahi, A.; Ferreira, I.A.T.M.; Goonawardane, N.; Saito, A.; Kimura, I.; Yamasoba, D.; Gerber, P.P.; Fatihi, S.; Rathore, S.; et al. Altered TMPRSS2 Usage by SARS-CoV-2 Omicron Impacts Infectivity and Fusogenicity. Nature 2022, 603, 706–714. [Google Scholar] [CrossRef]
- Hui, K.P.Y.; Ho, J.C.W.; Cheung, M.-C.; Ng, K.-C.; Ching, R.H.H.; Lai, K.-L.; Kam, T.T.; Gu, H.; Sit, K.-Y.; Hsin, M.K.Y.; et al. SARS-CoV-2 Omicron Variant Replication in Human Bronchus and Lung Ex Vivo. Nature 2022, 603, 715–720. [Google Scholar] [CrossRef]
- Flisiak, R.; Zarębska-Michaluk, D.; Dobrowolska, K.; Rorat, M.; Rogalska, M.; Kryńska, J.A.; Moniuszko-Malinowska, A.; Czupryna, P.; Kozielewicz, D.; Jaroszewicz, J.; et al. Change in the Clinical Picture of Hospitalized Patients with COVID-19 between the Early and Late Period of Dominance of the Omicron SARS-CoV-2 Variant. J. Clin. Med. 2023, 12, 5572. [Google Scholar] [CrossRef] [PubMed]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Zhou, D.; Coomar, D.; Sheikh, J.; Lawson, H.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Kang, L.; Guo, Z.; Liu, J.; Liu, M.; Liang, W. Incubation Period of COVID-19 Caused by Unique SARS-CoV-2 Strains: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2228008. [Google Scholar]
- Rahmani, A.; Dini, G.; Leso, V.; Montecucco, A.; Kusznir Vitturi, B.; Iavicoli, I.; Durando, P. Duration of SARS-CoV-2 shedding and infectivity in the working age population: A systemic review and meta-analysis. Med. Lav. 2022, 113, e2022014. [Google Scholar] [PubMed]
- Shang, W.; Kang, L.; Cao, G.; Wang, Y.; Gao, P.; Liu, J.; Liu, M. Percentage of Asymptomatic Infections among SARS-CoV-2 Omicron Variant-Positive Individuals: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1049. [Google Scholar] [CrossRef]
- WHO. Clinical Management of COVID-19: Living Guideline. 18 August 2023. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2023.2 (accessed on 2 March 2025).
- Clinical Characteristics of COVID-19. European Center for Disease Prevention and Control. Updated: 30 May 2023. Available online: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/clinical (accessed on 2 March 2025).
- Coelho, D.H.; Reiter, E.R.; French, E.; Costanzo, R.M. Decreasing Incidence of Chemosensory Changes by COVID-19 Variant. Otolaryngol. Head Neck Surg. 2022, 168, 704–706. [Google Scholar]
- Tavares, J.; Figueiredo, D.; Passos, L.; Sobrinho, L.; Souza, E.; Pedreira, L. Atypical Presentation of COVID-19 in Older Adults: A Scoping Review. Port. J. Public Health. 2023, 41, 198–217. [Google Scholar]
- de Freitas, R.F.; Torres, S.C.; Martín-Sánchez, F.J.; Carbó, A.V.; Lauria, G. Nunes JPL. Syncope and COVID-19 disease—A systematic review. Auton. Neurosci. 2021, 235, 102872. [Google Scholar] [CrossRef]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef]
- Kemerley, A.; Gupta, A.; Thirunavukkarasu, M.; Maloney, M.; Burgwardt, S.; Maulik, N. COVID-19 Associated Cardiovascular Disease-Risks, Prevention and Management: Heart at Risk Due to COVID-19. Curr. Issues Mol. Biol. 2024, 46, 1904–1920. [Google Scholar] [CrossRef]
- Buckley, B.J.R.; Harrison, S.L.; Fazio-Eynullayeva, E.; Underhill, P.; Lane, D.A.; Lip, G.Y.H. Prevalence and clinical outcomes of myocarditis and pericarditis in 718,365 COVID-19 patients. Eur. J. Clin. Investig. 2021, 51, e13679. [Google Scholar] [CrossRef] [PubMed]
- Bavishi, C.; Bonow, R.O.; Trivedi, V.; Abbott, J.D.; Messerli, F.H.; Bhatt, D.L. Special Article—Acute myocardial injury in patients hospitalized with COVID-19 infection: A review. Prog. Cardiovasc. Dis. 2020, 63, 682–689. [Google Scholar] [CrossRef]
- Shiwani, H.; Artico, J.; Moon, J.C.; Gorecka, M.; McCann, G.P.; Roditi, G.; Morrow, A.; Mangion, K.; Lukaschuk, E.; Shanmuganathan, M.; et al. Clinical Significance of Myocardial Injury in Patients Hospitalized for COVID-19: A Prospective, Multicenter, Cohort Study. JACC Cardiovasc. Imaging. 2024, 17, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.; Walker, V.; Cooper, S.J.A.; Bolton, T.; Keene, S.; Denholm, R.; Akbari, A.; Abbasizanjani, H.; Torabi, F.; Omigie, E.; et al. Association of COVID-19 with major arterial and venous thrombotic diseases: A population-wide cohort study of 48 million adults in England and Wales. Circulation 2022, 146, 892–906. [Google Scholar] [PubMed]
- Liu, S.; Yu, C.; Tu, Q.; Zhang, Q.; Fu, Z.; Huang, Y.; He, C.; Yao, L. Bacterial co-infection in COVID-19: A call to stay vigilant. PeerJ 2024, 12, e18041. [Google Scholar]
- Frediani, J.K.; Parsons, R.; McLendon, K.B.; Westbrook, A.L.; Lam, W.; Martin, G.; Pollock, N.R. The New Normal: Delayed Peak SARS-CoV-2 Viral Loads Relative to Symptom Onset and Implications for COVID-19 Testing Programs. Clin. Infect. Dis. 2024, 78, 301–307. [Google Scholar]
- Eyre, D.W.; Futschik, M.; Tunkel, S.; Wei, J.; Cole-Hamilton, J.; Saquib, R.; Germanacos, N.; Dodgson, A.R.; Klapper, P.E.; Sudhanva, M.; et al. Performance of antigen lateral flow devices in the UK during the alpha, delta, and omicron waves of the SARS-CoV-2 pandemic: A diagnostic and observational study. Lancet Infect. Dis. 2023, 23, 922–932. [Google Scholar]
- EU Common List of COVID-19 Antigen tests. Final Bis Update: 26 July 2023. Available online: https://health.ec.europa.eu/system/files/2023-12/covid-19_eu-common-list-antigen-tests_en.pdf (accessed on 2 March 2025).
- Murphy, C.; Mak, L.; Cheng, S.M.S.; Gigi, Y.Z.L.; Leung, K.K.Y.; Sum, N.Y.W.; Poukka, E.; Peiris, J.S.M.; Cowling, B.J. Diagnostic performance of multiplex lateral flow tests in ambulatory patients with acute respiratory illness. Diagn. Microbiol. Infect. Dis. 2024, 110, 116421. [Google Scholar]
- De Arcos-Jiménez, J.C.; Quintero-Salgado, E.; Martínez-Ayala, P.; Rosales-Chávez, G.; Damian-Negrete, R.M.; Fernández-Diaz, O.F.; Ruiz-Briseño, M.d.R.; López-Romo, R.; Vargas-Becerra, P.N.; Rodríguez-Montaño, R.; et al. Population-Level SARS-CoV-2 RT-PCR Cycle Threshold Values and Their Relationships with COVID-19 Transmission and Outcome Metrics: A Time Series Analysis Across Pandemic Years. Viruses 2025, 17, 103. [Google Scholar] [CrossRef]
- Sepulcri, C.; Dentone, C.; Mikulska, M.; Bruzzone, B.; Lai, A.; Fenoglio, D.; Bozzano, F.; Bergna, A.; Parodi, A.; Altosole, T.; et al. The longest persistence of viable SARS-CoV-2 with recurrence of viremia and relapsing symptomatic COVID-19 in an immunocompromised patient—A case study. Open Forum Infect. Dis. 2021, 8, ofab217. [Google Scholar] [CrossRef]
- Muecksch, F.; Wise, H.; Templeton, K.; Batchelor, B.; Squires, M.; McCance, K.; Jarvis, L.; Malloy, K.; Furrie, E.; Richardson, C.; et al. Longitudinal variation in SARS-CoV-2 antibody levels and emergence of viral variants: A serological analysis. Lancet Microbe 2022, 3, e493–e502. [Google Scholar] [PubMed]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [PubMed]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar]
- Flisiak, R.; Zarębska-Michaluk, D.; Berkan-Kawińska, A.; Tudrujek-Zdunek, M.; Rogalska, M.; Piekarska, A.; Kozielewicz, D.; Kłos, K.; Rorat, M.; Bolewska, B.; et al. Remdesivir-based therapy improved the recovery of patients with COVID-19 in the multicenter, real-world SARSTer study. Pol. Arch. Intern. Med. 2021, 131, 103–110. [Google Scholar]
- Dobrowolska, K.; Zarębska-Michaluk, D.; Brzdęk, M.; Rzymski, P.; Rogalska, M.; Moniuszko-Malinowska, A.; Kozielewicz, D.; Hawro, M.; Rorat, M.; Sikorska, K.; et al. Retrospective analysis of the effectiveness of remdesivir in COVID-19 treatment during periods dominated by Delta and Omicron SARS-CoV-2 variants in clinical settings. J. Clin. Med. 2023, 12, 2371. [Google Scholar] [CrossRef]
- Flisiak, R.; Zarębska-Michaluk, D.; Rogalska, M.; Kryńska, J.A.; Kowalska, J.; Dutkiewicz, E.; Dobrowolska, K.; Jaroszewicz, J.; Moniuszko-Malinowska, A.; Rorat, M.; et al. Real-world experience with molnupiravir during the period of SARS-CoV-2 Omicron variant dominance. Pharmacol. Rep. 2022, 74, 1279–1285. [Google Scholar] [CrossRef]
- Schilling, W.H.K.; Jittamala, P.; Watson, J.A.; Boyd, S.; Luvira, V.; Siripoon, T.; Ngamprasertchai, T.; Batty, E.M.; Cruz, C.; Callery, J.J.; et al. Antiviral efficacy of molnupiravir versus ritonavir-boosted nirmatrelvir in patients with early symptomatic COVID-19 (PLATCOV): An open-label, phase 2, randomized, controlled, adaptive trial. Lancet Infect. Dis. 2024, 24, 36–45. [Google Scholar]
- Xie, Y.; Bowe, B.; Al-Aly, Z. Nirmatrelvir and risk of hospital admission or death in adults with COVID-19: Emulation of a randomized target trial using electronic health records. BMJ 2023, 381, e073312. [Google Scholar]
- Schwartz, K.L.; Wang, J.; Tadrous, M.; Langford, B.J.; Daneman, N.; Leung, V.; Gomes, T.; Friedman, L.; Daley, P.; Brown, K.A. Population-based evaluation of the effectiveness of nirmatrelvir-ritonavir for reducing hospital admissions and mortality from COVID-19. CMAJ 2023, 195, E220–E226. [Google Scholar]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.L.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.D.; Lye, D.C.B.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.D.; Galli, M.; Ahn, M.Y.; Nahass, R.G.; et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N. Engl. J. Med. 2020, 383, 1827–1837. [Google Scholar] [CrossRef]
- Lagevrio—Summary of Product Characteristics, MHRA Last Updated 19/10/2022. Available online: https://www.gov.uk/government/publications/regulatory-approval-of-lagevrio-molnupiravir/summary-of-product-characteristics-for-lagevrio (accessed on 2 March 2025).
- Paxlovid—Summary of Product Characteristics, EMA Last Updated 12/11/2024. Available online: https://www.ema.europa.eu/en/documents/product-information/paxlovid-epar-product-information_en.pdf (accessed on 2 March 2025).
- Veklury—Summary of Product Characteristics, EMA Last Updated 18/12/2024. Available online: https://www.ema.europa.eu/en/documents/product-information/veklury-epar-product-information_en.pdf (accessed on 2 March 2025).
- Liverpool COVID-19 Interactions Checker. Available online: https://www.covid19-druginteractions.org/checker (accessed on 2 March 2025).
- Ramakrishnan, S.; Nicolau, D.V.; Langford, B.; Mahdi, M.; Jeffers, H.; Mwasuku, C.; Krassowska, K.; Fox, R.; Binnian, I.; Glover, V.; et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): A phase 2, open-label, randomized controlled trial. Lancet Respir. Med. 2021, 9, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, R.; Gbinigie, O.; Ogburn, E.; Yu, L.M.; van Hecke, O.; Dorward, J.; Butler, C.; Saville, B. Inhaled Budesonide for COVID-19 in People at Higher Risk of Complications in the Community The UK National Community Randomi. Ann. Fam. Med. 2023, 21, 3859. [Google Scholar] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef]
- Ayerbe, L.; Risco, C.; Ayis, S. The association between treatment with heparin and survival in patients with COVID-19. J. Thromb. Thrombolysis. 2020, 50, 298–301. [Google Scholar] [CrossRef]
- PRINCIPLE Trial Collaborative Group. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): A randomised, controlled, open-label, adaptive platform trial. Lancet 2021, 397, 1063–1074. [Google Scholar] [CrossRef]
- Matta, M.; Gantzer, L.; Chakvetadze, C.; Moussiegt, A.; De Pontfarcy, A.; Lekens, B.; Diamantis, S. Antibiotic prescription in ambulatory care for COVID-19 patients: A cohort analysis in four European countries. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 115–119. [Google Scholar] [CrossRef]
- Hekmat, H.; Rasooli, A.; Siami, Z.; Rutajengwa, K.A.; Vahabi, Z.; Mirzadeh, F.A. A Review of Antibiotic Efficacy in COVID-19 Control. J. Immunol. Res. 2023, 2023, 6687437. [Google Scholar] [CrossRef]
- Tomasiewicz, K.; Piekarska, A.; Stempkowska-Rejek, J.; Serafińska, S.; Gawkowska, A.; Parczewski, M.; Niścigorska-Olsen, J.; Łapiński, T.W.; Zarębska-Michaluk, D.; Kowalska, J.D.; et al. Tocilizumab for patients with severe COVID-19: A retrospective, multi-center study. Expert. Rev. Anti Infect. Ther. 2021, 19, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Jaroszewicz, J.; Rogalska, M.; Łapiński, T.; Berkan-Kawińska, A.; Bolewska, B.; Tudrujek-Zdunek, M.; Kozielewicz, D.; Rorat, M.; Leszczyński, P.; et al. Tocilizumab improves the prognosis of COVID-19 in patients with high IL-6. J. Clin. Med. 2021, 10, 1583. [Google Scholar] [CrossRef]
- Zarębska-Michaluk, D.; Jaroszewicz, J.; Rogalska, M.; Martonik, D.; Pabjan, P.; Katarzyna Berkan-Kawińska, A.; Bolewska, B.; Oczko-Grzesik, B.; Kozielewicz, D.; Tudrujek-Zdunek, M.; et al. Effectiveness of Tocilizumab with and without Dexamethasone in Patients with Severe COVID-19: A Retrospective Study. J. Inflamm. Res. 2021, 14, 3359–3366. [Google Scholar] [CrossRef]
- REMAP-CAP Investigators. Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar]
- Almskog, L.M.; Sjöström, A.; Sundén-Cullberg, J.; Taxiarchis, A.; Ågren, A.; Freyland, S.; Börjesson, M.; Wikman, A.; Wahlgren, C.M.; Wanecek, M.; et al. Tocilizumab reduces hypercoagulation in COVID-19—Perspectives from the coagulation and immunomodulation Covid assessment (Coag-ImmCovA) clinical trial. Thromb. Res. 2024, 243, 109135. [Google Scholar] [CrossRef] [PubMed]
- RoActemra. Summary of Product Characteristics. Available online: https://ec.europa.eu/health/documents/community-register/2011/20110801106350/anx_106350_pl.pdf (accessed on 2 March 2025).
- Naik, N.B.; Puri, G.D.; Kajal, K.; Mahajan, V. High-dose dexamethasone versus tocilizumab in moderate to severe COVID-19 pneumonia: A randomized controlled trial. Cureus 2021, 13, e20353. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Higher dose corticosteroids in patients admitted to hospital with COVID-19 who are hypoxic but not requiring ventilatory support (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2023, 401, 1499–1507. [Google Scholar]
- Wang, S.; Chen, Z.; Zhang, X.; Wu, X.; Wang, Y.; Zhang, Q.; Huang, L.; Cui, X.; Cai, Y.; Huang, X.; et al. Impact of corticosteroid doses on prognosis of severe and critical COVID-19 patients with Omicron variant infection: A propensity score matching study. Inflammopharmacology 2024, 32, 3347–3356. [Google Scholar] [CrossRef]
- Olumiant—Highlights of Prescribing Information, FDA Last Updated 16/01/2025. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s006lbl.pdf (accessed on 2 March 2025).
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with COVID-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef]
- Ely, E.W.; Ramanan, A.V.; Kartman, C.E.; de Bono, S.; Liao, R.; Piruzeli, M.L.B.; Goldman, J.D.; Saraiva, J.F.K.; Chakladar, S.; Marconi, V.C. Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: An exploratory, randomised, placebo-controlled trial. Lancet Respir. Med. 2022, 10, 327–336. [Google Scholar] [PubMed]
- Wolfe, C.R.; Tomashek, K.M.; Patterson, T.F.; Gomez, C.A.; Marconi, V.C.; Jain, M.K.; Yang, O.O.; Paules, C.I.; Palacios, G.M.R.; Grossberg, R.; et al. Baricitinib versus dexamethasone for adults hospitalised with COVID-19 (ACTT-4): A randomised, double-blind, double placebo-controlled trial. Lancet Respir. Med. 2022, 10, 888–899. [Google Scholar] [PubMed]
- Granholm, A.; Munch, M.W.; Myatra, S.N.; Vijayaraghavan, B.K.T.; Cronhjort, M.; Wahlin, R.R.; Jakob, S.M.; Cioccari, L.; Kjær, M.B.N.; Vesterlund, G.K.; et al. Dexamethasone 12 mg versus 6 mg for patients with COVID-19 and severe hypoxaemia: A pre-planned, secondary Bayesian analysis of the COVID STEROID 2 trial. Intensive Care Med. 2022, 48, 45–55. [Google Scholar] [PubMed]
- Grasselli, G.; Calfee, C.S.; Camporota, L.; Poole, D.; Amato, M.B.P.; Antonelli, M.; Arabi, Y.M.; Baroncelli, F.; Beitler, J.R.; Bellani, G.; et al. ESICM guidelines on acute respiratory distress syndrome: Definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023, 49, 727–759. [Google Scholar]
- Badulak, J.; Antonini, M.V.; Stead, C.M.; Shekerdemian, L.; Raman, L.; Paden, M.L.; Agerstrand, C.; Bartlett, R.H.; Barrett, N.; Combes, A.; et al. Extracorporeal Membrane Oxygenation for COVID-19: Updated 2021 Guidelines from the Extracorporeal Life Support Organization. ASAIO J. 2021, 67, 485–495. [Google Scholar]
- Goodfellow, L.T.; Miller, A.G.; Varekojis, S.M.; LaVita, C.J.; Glogowski, J.T.; Hess, D.R. AARC Clinical Practice Guideline: Patient-Ventilator Assessment. Respiratory Care 2024, 69, 1042–1054. [Google Scholar]
- Aliyu, B.; Raji, Y.E.; Chee, H.Y.; Wong, M.Y.; Sekawi, Z.B. Systematic review and meta-analysis of the efficacy and safety of oseltamivir (Tamiflu) in the treatment of Coronavirus Disease 2019 (COVID-19). PLoS ONE 2022, 17, e0277206. [Google Scholar]
- Weis, N.; Bollerup, S.; Sund, J.D.; Glamann, J.B.; Vinten, C.; Jensen, L.R.; Sejling, C.; Kledal, T.N.; Rosenkilde, M.M. Amantadine for COVID-19 treatment (ACT) study: A randomized, double-blinded, placebo-controlled clinical trial. Clin. Microbiol. Infect. 2023, 29, 1313–1319. [Google Scholar]
- OVERY Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): A randomized, controlled, open-label, platform trial. Lancet 2020, 10259, 1345–1352. [Google Scholar]
- TOGETHER Investigators. Effect of early treatment with ivermectin among patients with COVID-19. N. Engl. J. Med. 2022, 386, 1721–1731. [Google Scholar]
- McCarthy, M.W.; Naggie, S.; Boulware, D.R.; Lindsell, C.J.; Stewart, T.G.; Felker, G.M.; Jayaweera, D.; Sulkowski, M.; Gentile, N.; Bramante, C.; et al. Effect of fluvoxamine vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: A randomized clinical trial. JAMA 2023, 329, 296–305. [Google Scholar]
- ITAC Study Group. Hyperimmune immunoglobulin for hospitalised patients with COVID-19 (ITAC): A double-blind, placebo-controlled, Phase 3, randomised trial. Lancet 2022, 399, 530–540. [Google Scholar]
- Francica, J.R.; Cai, Y.; Diallo, S.; Rosenthal, K.; Ren, K.; Flores, D.J.; Dippel, A.; Wu, Y.; Chen, X.; Cantu, E.; et al. The SARS-CoV-2 Monoclonal Antibody AZD3152 Potently Neutralizes Historical and Emerging Variants and is Being Developed for the Prevention and Treatment of COVID-19 in High-risk Individuals. Open Forum Infect. Dis. 2023, 10 (Suppl. S2), ofad500.1192. [Google Scholar]
- RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): A randomised controlled, open-label, platform trial. Lancet 2021, 397, 2049–2059. [Google Scholar]
- Chen, J.S.; Alfajaro, M.M.; Chow, R.D.; Wei, J.; Filler, R.B.; Eisenbarth, S.C.; Wilen, C.B. Non-steroidal anti-inflammatory drugs dampen the cytokine and antibody response to SARS-CoV-2 infection. J. Virology 2021, 95, e00014-21. [Google Scholar] [PubMed]
- Fanlo, P.; Gracia-Tello, B.D.C.; Fonseca Aizpuru, E.; Álvarez-Troncoso, J.; Gonzalez, A.; Prieto-González, S.; Freire, M.; Argibay, A.B.; Pallarés, L.; Todolí, J.A.; et al. Efficacy and Safety of Anakinra Plus Standard of Care for Patients With Severe COVID-19: A Randomized Phase 2/3 Clinical Trial. JAMA Netw. Open 2023, 6, e237243. [Google Scholar]
- Bramante, C.T.; Buse, J.B.; Liebovitz, D.M.; Nicklas, J.M.; Puskarich, M.A.; Cohen, K.; Belani, H.K.; Anderson, B.J.; Huling, J.D.; Tignanelli, C.J.; et al. COVID-OUT Study Team. Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): A multicentre, randomised, quadruple-blind, parallel-group, Phase 3 trial. Lancet Infect. Dis. 2023, 23, 1119–1129. [Google Scholar]
- Thomas, S.; Patel, D.; Bittel, B.; Wolski, K.; Wang, Q.; Kumar, A.; Il’Giovine, Z.J.; Mehra, R.; McWilliams, C.; Nissen, S.E.; et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: The COVID A to Z randomized clinical trial. JAMA Netw. Open. 2021, 4, e210369. [Google Scholar]
- Antonazzo, I.C.; Fornari, C.; Rozza, D.; Conti, S.; di Pasquale, R.; Cortesi, P.; Kaleci, S.; Ferrara, P.; Zucchi, A.; Maifredi, G.; et al. Azithromycin use and outcomes in patients with COVID-19: An observational real-world study. Int. J. Infect. Dis. 2022, 124, 27–34. [Google Scholar]
- WHO Solidarity Trial Consortium. Repurposed antiviral drugs for COVID-19—Interim WHO Solidarity trial results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar]
- National Institute of Health. COVID-19 Treatment Guidelines. Clinical Spectrum of SARS-CoV-2 Infection. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570371/pdf/Bookshelf_NBK570371.pdf (accessed on 2 March 2025).
- World Health Organization. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 2 March 2025).
- Hoshijima, H.; Mihara, T.; Seki, H.; Hyuga, S.; Kuratani, N.; Shiga, T. Incidence of long-term post-acute sequelae of SARS-CoV-2 infection related to pain and other symptoms: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0250909. [Google Scholar]
- Okarska-Napierała, M.; Ludwikowska, K.; Jackowska, T.; Książyk, J.; Buda, P.; Mazur, A.; Szenborn, L.; Werner, B.; Wysocki, J.; Kuchar, E. Approach to a child with pediatric inflammatory multisystemic syndrome with COVID-19. Przegl. Pediatr. 2021, 50, 1–11. [Google Scholar]
- Niedziela, J.T.; Głowacki, J.; Ochman, M.; Pudlo, R.; Adamczyk-Sowa, M.; Nowowiejska-Wiewióra, A.; Kułaczkowska, Z.; Sobala-Szczygieł, B.; Myrda, K.; Wiewióra, M.; et al. Post-COVID-19 complications in hospitalized and nonhospitalized patients: The Silesian database of COVID-19 complications (SILCOV-19). Pol. Arch. Intern. Med. 2022, 132, 16233. [Google Scholar] [PubMed]
- Huang, L.W.; Li, H.M.; He, B.; Wang, X.B.; Zhang, Q.Z.; Peng, W.X. Prevalence of cardiovascular symptoms in post-acute COVID-19 syndrome: A meta-analysis. BMC Med. 2025, 23, 70. [Google Scholar]
- Shah, D.P.; Thaweethai, T.; Karlson, E.W.; Bonilla, H.; Horne, B.D.; Mullington, J.M.; Wisnivesky, J.P.; Hornig, M.; Shinnick, D.J.; Klein, J.D.; et al. RECOVER Consortium. Sex Differences in Long COVID. JAMA Netw. Open. 2025, 8, e2455430. [Google Scholar]
- Terry, P.; Heidel, R.E.; Wilson, A.Q.; Dhand, R. Risk of long covid in patients with pre-existing chronic respiratory diseases: A systematic review and meta-analysis. BMJ Open Respir. Res. 2025, 12, e002528. [Google Scholar] [PubMed]
- Peter, R.S.; Nieters, A.; Göpel, S.; Merle, U.; Steinacker, J.M.; Deibert, P.; Friedmann-Bette, B.; Nieß, A.; Müller, B.; Schilling, C.; et al. EPILOC Phase 2 Study Group. Persistent symptoms and clinical findings in adults with post-acute sequelae of COVID-19/post-COVID-19 syndrome in the second year after acute infection: A population-based, nested case-control study. PLoS Med. 2025, 22, e1004511. [Google Scholar]
- The Royal Australian College of General Practitioners. Caring for Patients with Post–COVID–19 Conditions. December 2021. Available online: https://www.racgp.org.au/clinical-resources/covid-19-resources/clinical-care/caring-for-patients-with-post-covid-19-conditions (accessed on 2 March 2025).
- Spyropoulos, A.C.; Anderson, F.A., Jr.; FitzGerald, G.; Decousus, H.; Pini, M.; Chong, B.H.; Zotz, R.B.; Bergmann, J.F.; Tapson, V.; Froehlich, J.B.; et al. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest 2011, 140, 706–714. [Google Scholar]
- Spyropoulos, A.C.; Levy, J.H.; Ageno, W.; Connors, J.M.; Hunt, B.J.; Iba, T.; Levi, M.; Samama, C.M.; Thachil, J.; Giannis, D.; et al. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1859–1865. [Google Scholar]
- Del Corral, T.; Fabero-Garrido, R.; Plaza-Manzano, G.; Izquierdo-García, J.; López-Sáez, M.; García-García, R.; López-de-Uralde-Villanueva, I. Effect of respiratory rehabilitation on quality of life in individuals with post-COVID-19 symptoms: A randomised controlled trial. Ann. Phys. Rehabil. Med. 2025, 68, 101920. [Google Scholar] [CrossRef]
- Combet, E.; Haag, L.; Richardson, J.; Haig, C.E.; Cunningham, Y.; Fraser, H.L.; Brosnahan, N.; Ibbotson, T.; Ormerod, J.; White, C.; et al. Remotely delivered weight management for people with long COVID and overweight: The randomized wait-list-controlled ReDIRECT trial. Nat. Med. 2025, 31, 258–266. [Google Scholar] [CrossRef]
- Chow, N.K.N.; Tsang, C.Y.W.; Chan, Y.H.; Telaga, S.A.; Ng, L.Y.A.; Chung, C.M.; Yip, Y.M.; Cheung, P.P. The effect of pre-COVID and post-COVID vaccination on long COVID: A systematic review and meta-analysis. J. Infect. 2024, 89, 106358. [Google Scholar] [PubMed]
- Sun, G.; Lin, K.; Ai, J.; Zhang, W. The efficacy of antivirals, corticosteroids, and monoclonal antibodies as acute COVID-19 treatments in reducing the incidence of long COVID: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2024, 30, 1505–1513. [Google Scholar]
- Oeser, C.; Whitaker, H.; Borrow, R.; Linley, E.; Tonge, S.; Rowe, C.; Otter, A.; Warrener, L.; Campbell, C.; Ladhani, S.; et al. Following the Omicron wave, the majority of children in Eng-land have evidence of previous COVID infection. J. Infect. 2023, 86, 256–308. [Google Scholar] [PubMed]
- Franczak, J.; Moppert, J.; Sobolewska-Pilarczyk, M.; Pawłowska, M. The Seroprevalence of SARS-CoV-2 IgG Antibodies in Children Hospitalized for Reasons Other Than COVID-19. J. Clin. Med. 2022, 11, 3819. [Google Scholar] [CrossRef]
- Luo, C.; Chen, W.; Cai, J.; He, Y. The mechanisms of milder clinical symptoms of COVID-19 in children compared to adults. Ital. J. Pediatr. 2024, 50, 28. [Google Scholar] [PubMed]
- Powell, A.A.; Dowell, A.C.; Moss, P.; Ladhani, S.N.; sKIDs Investigation Team. Current state of COVID-19 in children: 4 years on. J. Infect. 2024, 88, 106134. [Google Scholar]
- Pokorska-Śpiewak, M.; Talarek, E.; Mania, A.; Pawłowska, M.; Popielska, J.; Zawadka, K.; Figlerowicz, M.; Mazur-Melewska, K.; Faltin, K.; Ciechanowski, P.; et al. Clinical and Epidemiological Characteristics of 1283 Pediatric Patients with Coronavirus Disease 2019 during the First and Second Waves of the Pandemic-Results of the Pediatric Part of a Multicenter Polish Register SARSTer. J. Clin. Med. 2021, 10, 5098. [Google Scholar] [CrossRef]
- Pawłowska, M.; Pokorska-Śpiewak, M.; Talarek, E.; Mania, A.; Hasiec, B.; Żwirek-Pytka, E.; Stankiewicz, M.; Stani, M.; Frańczak-Chmura, P.; Szenborn, L.; et al. Clinical Course and Severity of COVID-19 in 940 Infants with and without Comorbidities Hospitalized in 2020 and 2021: The Results of the National Multicenter Database SARSTer-PED. J. Clin. Med. 2023, 12, 2479. [Google Scholar] [CrossRef]
- Cohen, J.M.; Carter, M.J.; Cheung, R.C.; Ladhani, S. Lower risk of multisystem inflammatory syndrome in children (MIS-C) with the Delta and Omicron variants of SARS-CoV-2. Clin. Infect. Dis. 2022, 76, e518-21. [Google Scholar]
- Aparicio, C.; Willis, Z.I.; Nakamura, M.M.; Wolf, J.; Little, C.; Maron, G.M.; Sue, P.K.; Anosike, B.I.; Miller, C.; Bio, L.L.; et al. Risk Factors for Pediatric Critical COVID-19: A Systematic Review and Meta-Analysis. J. Ped. Infect. Dis. Soc. 2024, 13, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Mańdziuk, J.; Kuchar, E.; Okarska-Napierała, M. How international guidelines recommend treating children who have severe COVID-19 or risk disease progression. Acta Paediatr. 2024, 113, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Camporesi, A.; Morello, R.; La Rocca, A.; Zampino, G.; Vezzulli, F.; Munblit, D.; Raffaelli, F.; Valentini, P.; Buonsenso, D. Characteristics and predictors of Long Covid in children: A 3-year prospective cohort study. eClinicalMedicine 2024, 76, 102815. [Google Scholar] [CrossRef]
- Orban, E.; Li, L.Y.; Gilbert, M.; Napp, A.K.; Kaman, A.; Topf, S.; Boecker, M.; Devine, J.; Reiß, F.; Wendel, F.; et al. Mental health and quality of life in children and adolescents during the COVID-19 pandemic: A systematic review of longitudinal studies. Front. Public Health 2024, 11, 1275917. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Catalano, A.; Ceramella, J.; Pellegrino, M.; Marra, M.; Scali, E.; Sinicropi, M.S.; Aquaro, S. The Ongoing Impact of COVID-19 on Pediatric Obesity. Pediatr. Rep. 2024, 16, 135–150. [Google Scholar] [CrossRef]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/news/etf-recommends-updating-covid-19-vaccines-target-new-jn1-variant (accessed on 2 March 2025).
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html (accessed on 2 March 2025).
- CDC Interim Clinical Considerations for Use of COVID-19 Vaccines in the United States. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html# (accessed on 2 March 2025).
- World Health Organization (WHO). Coronavirus Disease (COVID-19) Epidemiological Updates and Monthly Operational Updates. WHO: COVID-19 Epidemiological Updates. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 2 March 2025).
- Comirnaty. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf (accessed on 2 March 2025).
- Spikevax. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/spikevax-epar-product-information_en.pdf (accessed on 2 March 2025).
- Nuvaxovid. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/nuvaxovid-epar-product-information_en.pdf (accessed on 2 March 2025).
- Ministerstwo Zdrowia—Komunikat Ministra Zdrowia nr 36 z Dnia 22 Października 2024 r. Available online: https://www.gov.pl/attachment/ba7d5589-e7bf-484c-9552-9fe416b5c885 (accessed on 2 March 2025).
- Ministerstwo Zdrowia. Komunikat nr 37 Ministra Zdrowia z 26.11.2024 r. w Sprawie Realizacji Szczepień u Dzieci 6 Mies.–11 Lat Dostęp 12 Luty 2025. Available online: https://www.gov.pl/attachment/801f5af3-f7d1-42ff-857f-d2b868911ff0 (accessed on 2 March 2025).
- Program Szczepień Ochronnych na rok 2025. Available online: https://www.gov.pl/attachment/83bef042-106b-4228-a011-56bcb58470bb (accessed on 2 March 2025).
- Comirnaty. Periodic Safety Update Report Assessment 19 June 2023 to 18 December 2023. Available online: www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/comirnaty-periodic-safety-update-report-assessment-19-june-2023-18-december-2023_en.pdf (accessed on 2 March 2025).
- Spikevax. Periodic Safety Update Report Assessment 18 June 2023 to 17 December 2023. Available online: www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/spikevax-periodic-safety-update-report-assessment-18-june-2023-17-december-2023_en.pdf (accessed on 2 March 2025).
- WHO. Statement—Update on COVID-19: Omicron Wave Threatening to Overcome Health Workforce, 11-01-2022. Available online: https://www.who.int/poland/multi-media/details/statement---update-on-covid-19--omicron-wave-threatening-to-overcome-health-workforce--11-01-2022 (accessed on 2 March 2025).
- ETF Statement on the Loss of Activity of Anti-Spike Protein Monoclonal Antibodies Due to Emerging SARS-CoV-2 Variants. 9 December 2024. Available online: https://www.ema.europa.eu/en/documents/other/etf-statement-loss-activity-anti-spike-protein-monoclonal-antibodies-due-emerging-sars-cov-2-variants-december-2024-update_en.pdf (accessed on 2 March 2025).
- Wang, Q.; Guo, Y.; Ho, J.; Ho, D.D. Activity of research-grade pemivibart against recent SARS-CoV-2 JN.1 sublineages. N. Engl. J. Med. 2024, 391, 1863–1864. [Google Scholar]
- Emergency Use Authorization (EUA) for Pemgarda, Center for Drug Evaluation and Research (CDER). Review Memorandum. Available online: https://www.fda.gov/media/182220/download?attachment (accessed on 2 March 2025).
- Esmaeili, S.; Owens, K.; Wagoner, J.; Polyak, S.J.; White, J.M.; Schiffer, J.T. A unifying model to explain frequent SARS-CoV-2 rebound after nirmatrelvir treatment and limited prophylactic efficacy. Nat. Commun. 2024, 15, 5478. [Google Scholar] [CrossRef]
- Rahmati, M.; Shamsi, M.M.; Khoramipour, K.; Malakoutinia, F.; Woo, W.; Park, S.; Yon, D.K.; Lee, S.W.; Shin, J.I.; Smith, L. Baseline physical activity is associated with reduced mortality and disease outcomes in COVID-19: A systematic review and meta-analysis. Rev. Med. Virol. 2022, 32, e2349. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flisiak, R.; Jaroszewicz, J.; Kozielewicz, D.; Kuchar, E.; Parczewski, M.; Pawłowska, M.; Piekarska, A.; Rzymski, P.; Simon, K.; Tomasiewicz, K.; et al. Management of SARS-CoV-2 Infection-Clinical Practice Guidelines of the Polish Association of Epidemiologists and Infectiologists, for 2025. J. Clin. Med. 2025, 14, 2305. https://doi.org/10.3390/jcm14072305
Flisiak R, Jaroszewicz J, Kozielewicz D, Kuchar E, Parczewski M, Pawłowska M, Piekarska A, Rzymski P, Simon K, Tomasiewicz K, et al. Management of SARS-CoV-2 Infection-Clinical Practice Guidelines of the Polish Association of Epidemiologists and Infectiologists, for 2025. Journal of Clinical Medicine. 2025; 14(7):2305. https://doi.org/10.3390/jcm14072305
Chicago/Turabian StyleFlisiak, Robert, Jerzy Jaroszewicz, Dorota Kozielewicz, Ernest Kuchar, Miłosz Parczewski, Małgorzata Pawłowska, Anna Piekarska, Piotr Rzymski, Krzysztof Simon, Krzysztof Tomasiewicz, and et al. 2025. "Management of SARS-CoV-2 Infection-Clinical Practice Guidelines of the Polish Association of Epidemiologists and Infectiologists, for 2025" Journal of Clinical Medicine 14, no. 7: 2305. https://doi.org/10.3390/jcm14072305
APA StyleFlisiak, R., Jaroszewicz, J., Kozielewicz, D., Kuchar, E., Parczewski, M., Pawłowska, M., Piekarska, A., Rzymski, P., Simon, K., Tomasiewicz, K., & Zarębska-Michaluk, D. (2025). Management of SARS-CoV-2 Infection-Clinical Practice Guidelines of the Polish Association of Epidemiologists and Infectiologists, for 2025. Journal of Clinical Medicine, 14(7), 2305. https://doi.org/10.3390/jcm14072305










