Does Preliminary Chest Shape Assessment Improve the Prognostic Risk Stratification of Individuals with Mitral Annular Disjunction? A Case Report and Narrative Review
Abstract
1. Introduction
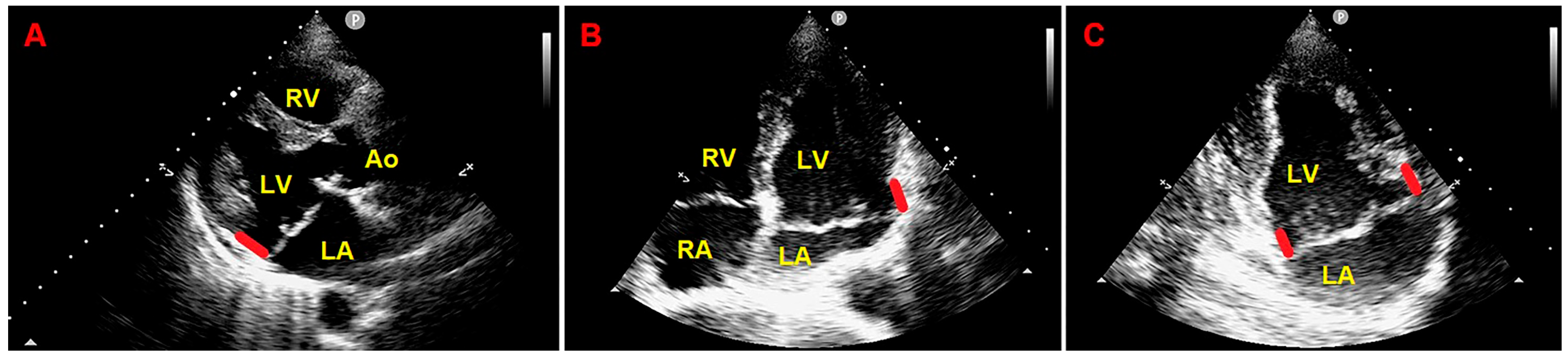
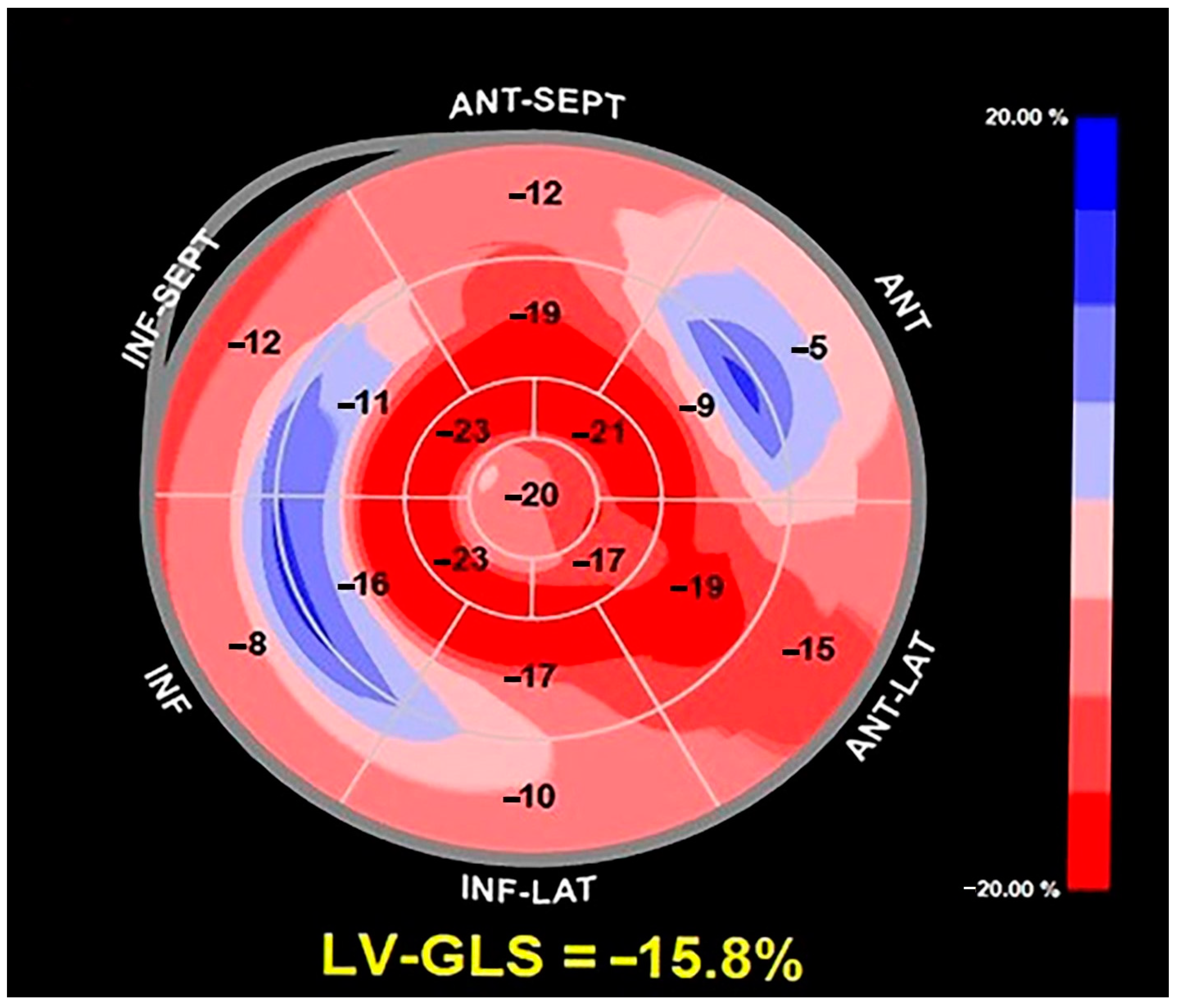
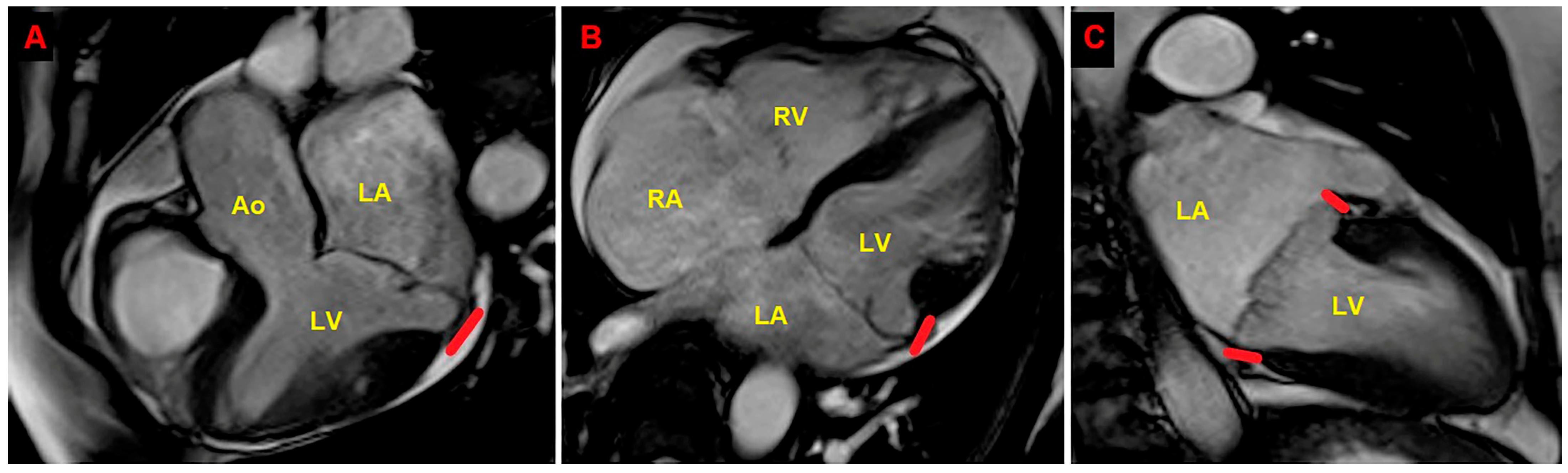
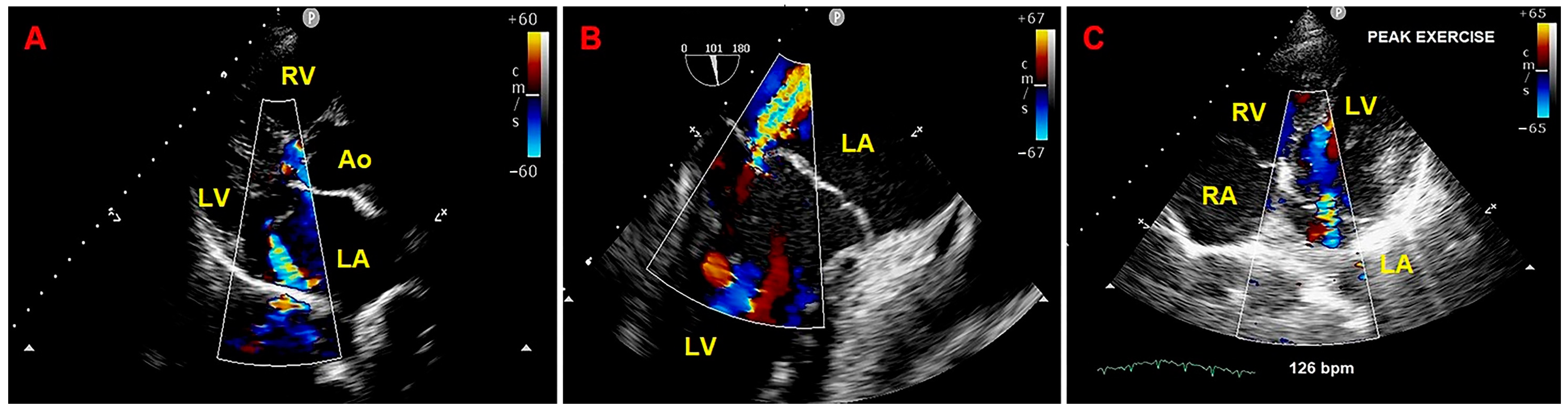
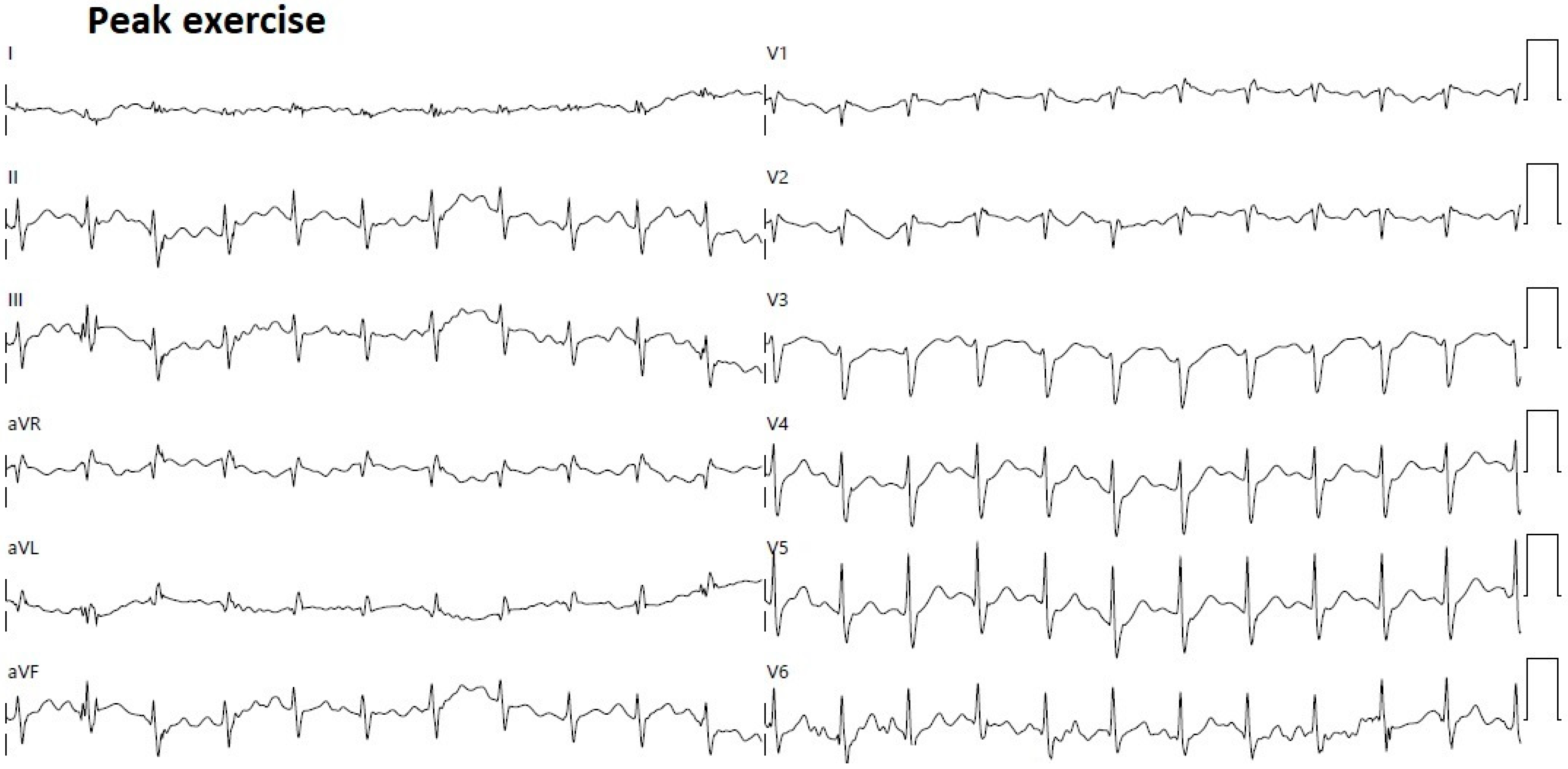
2. Clinical Case
3. Discussion
3.1. The Role of CMR in MAD Assessment
3.2. The Role of Echocardiographic Techniques in MAD Assessment
3.3. The Role of CT in MAD Assessment
3.4. The Role of Chest Shape Conformation in MVP Individuals with MAD
3.5. Implications for Clinical Practice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van der Bijl, P.; Stassen, J.; Haugaa, K.H.; Essayagh, B.; Basso, C.; Thiene, G.; Faletra, F.F.; Edvardsen, T.; Enriquez-Sarano, M.; Nihoyannopoulos, P.; et al. Mitral Annular Disjunction in the Context of Mitral Valve Prolapse: Identifying the At-Risk Patient. JACC Cardiovasc. Imaging 2024, 17, 1229–1245. [Google Scholar] [CrossRef] [PubMed]
- Cesmat, A.P.; Chaudry, A.M.; Gupta, S.; Sivaraj, K.; Weickert, T.T.; Simpson, R.J., Jr.; Syed, F.F. Prevalence and predictors of mitral annular disjunction and ventricular ectopy in mitral valve prolapse. Heart Rhythm 2024, 21, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Figliozzi, S.; Stankowski, K.; Tondi, L.; Catapano, F.; Gitto, M.; Lisi, C.; Bombace, S.; Olivieri, M.; Cannata, F.; Fazzari, F.; et al. Mitral Annulus Disjunction in consecutive patients undergoing Cardiac Magnetic Resonance: Where is the boundary between normality and disease? J. Cardiovasc. Magn. Reson. 2024, 26, 101056. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Muti-Schünemann, G.E.U.; Lombardo, M.; Muti, P. The Prevalence, Pathophysiological Role and Determinants of Mitral Annular Disjunction Among Patients with Mitral Valve Prolapse: A Systematic Review. J. Clin. Med. 2025, 14, 1423. [Google Scholar] [CrossRef]
- Lee, A.P.; Jin, C.N.; Fan, Y.; Wong, R.H.L.; Underwood, M.J.; Wan, S. Functional Implication of Mitral Annular Disjunction in Mitral Valve Prolapse: A Quantitative Dynamic 3D Echocardiographic Study. JACC Cardiovasc. Imaging 2017, 10, 1424–1433. [Google Scholar] [CrossRef]
- Dejgaard, L.A.; Skjølsvik, E.T.; Lie, Ø.H.; Ribe, M.; Stokke, M.K.; Hegbom, F.; Scheirlynck, E.S.; Gjertsen, E.; Andresen, K.; Helle-Valle, T.M.; et al. The Mitral Annulus Disjunction Arrhythmic Syndrome. J. Am. Coll. Cardiol. 2018, 72, 1600–1609. [Google Scholar] [CrossRef]
- Angelini, A.; Ho, S.Y.; Anderson, R.H.; Becker, A.E.; Davies, M.J. Disjunction of the mitral annulus in floppy mitral valve. N. Engl. J. Med. 1988, 318, 188–189. [Google Scholar] [CrossRef]
- Carmo, P.; Andrade, M.J.; Aguiar, C.; Rodrigues, R.; Gouveia, R.; Silva, J.A. Mitral annular disjunction in myxomatous mitral valve disease: A relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc. Ultrasound 2010, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Perazzolo Marra, M.; Basso, C.; De Lazzari, M.; Rizzo, S.; Cipriani, A.; Giorgi, B.; Lacognata, C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Morphofunctional Abnormalities of Mitral Annulus and Arrhythmic Mitral Valve Prolapse. Circ. Cardiovasc. Imaging 2016, 9, e005030. [Google Scholar] [CrossRef]
- Han, H.C.; Ha, F.J.; The, A.W.; Calafiore, P.; Jones, E.F.; Johns, J.; Koshy, A.N.; O’Donnell, D.; Hare, D.L.; Farouque, O.; et al. Mitral Valve Prolapse and Sudden Cardiac Death: A Systematic Review. J. Am. Heart Assoc. 2018, 7, e010584. [Google Scholar] [CrossRef]
- Essayagh, B.; Iacuzio, L.; Civaia, F.; Avierinos, J.F.; Tribouilloy, C.; Levy, F. Usefulness of 3-Tesla Cardiac Magnetic Resonance to Detect Mitral Annular Disjunction in Patients with Mitral Valve Prolapse. Am. J. Cardiol. 2019, 124, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Perazzolo Marra, M.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2015, 132, 556–566. [Google Scholar] [CrossRef]
- Eriksson, M.J.; Bitkover, C.Y.; Omran, A.S.; David, T.E.; Ivanov, J.; Ali, M.J.; Woo, A.; Siu, S.C.; Rakowski, H. Mitral annular disjunction in advanced myxomatous mitral valve disease: Echocardiographic detection and surgical correction. J. Am. Soc. Echocardiogr. 2005, 18, 1014–1022. [Google Scholar] [CrossRef]
- Mantegazza, V.; Tamborini, G.; Muratori, M.; Gripari, P.; Fusini, L.; Italiano, G.; Volpato, V.; Sassi, V.; Pepi, M. Mitral Annular Disjunction in a Large Cohort of Patients with Mitral Valve Prolapse and Significant Regurgitation. JACC Cardiovasc. Imaging 2019, 12, 2278–2280. [Google Scholar] [CrossRef]
- Mantegazza, V.; Volpato, V.; Gripari, P.; Ghulam Ali, S.; Fusini, L.; Italiano, G.; Muratori, M.; Pontone, G.; Tamborini, G.; Pepi, M. Multimodality imaging assessment of mitral annular disjunction in mitral valve prolapse. Heart 2021, 107, 25–32. [Google Scholar] [CrossRef]
- Essayagh, B.; Sabbag, A.; Antoine, C.; Benfari, G.; Batista, R.; Yang, L.T.; Maalouf, J.; Thapa, P.; Asirvatham, S.; Michelena, H.I.; et al. The Mitral Annular Disjunction of Mitral Valve Prolapse: Presentation and Outcome. JACC Cardiovasc. Imaging 2021, 14, 2073–2087. [Google Scholar] [CrossRef]
- Bennett, S.; Tafuro, J.; Brumpton, M.; Bardolia, C.; Heatlie, G.; Duckett, S.; Ridley, P.; Nanjaiah, P.; Kwok, C.S. Echocardiographic description and outcomes in a heterogeneous cohort of patients undergoing mitral valve surgery with and without mitral annular disjunction: A health service evaluation. Echo Res. Pract. 2022, 9, 4. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M. The influence of chest wall conformation on myocardial strain parameters in a cohort of mitral valve prolapse patients with and without mitral annular disjunction. Int. J. Cardiovasc. Imaging 2023, 39, 61–76. [Google Scholar] [CrossRef]
- Gray, R.; Indraratna, P.; Cranney, G.; Lam, H.; Yu, J.; Mathur, G. Mitral annular disjunction in surgical mitral valve prolapse: Prevalence; characteristics and outcomes. Echo Res. Pract. 2023, 10, 21. [Google Scholar] [CrossRef]
- Vaksmann, G.; Bouzguenda, I.; Guillaume, M.P.; Gras, P.; Silvestri, V.; Richard, A. Mitral annular disjunction and Pickelhaube sign in children with mitral valve prolapse: A prospective cohort study. Arch. Cardiovasc. Dis. 2023, 116, 514–522. [Google Scholar] [CrossRef]
- Shechter, A.; Vaturi, M.; Hong, G.J.; Kaewkes, D.; Patel, V.; Seok, M.; Nagasaka, T.; Koren, O.; Koseki, K.; Skaf, S.; et al. Implications of Mitral Annular Disjunction in Patients Undergoing Transcatheter Edge-to-Edge Repair for Degenerative Mitral Regurgitation. JACC Cardiovasc. Interv. 2023, 16, 2835–2849. [Google Scholar] [CrossRef] [PubMed]
- Özyıldırım, S.; Guven, B.; Yumuk, M.T.; Barman, H.A.; Dogan, O.; Topel, C.; Atici, A.; Donmez, A.; Kucukoglu, M.S.; Dogan, S.M. Evaluation of left ventricular function in patients with mitral annular disjunction using speckle tracking echocardiography. Echocardiography 2024, 41, e15813. [Google Scholar] [CrossRef] [PubMed]
- Meucci, M.C.; Mantegazza, V.; Wu, H.W.; van Wijngaarden, A.L.; Garlaschè, A.; Tamborini, G.; Pepi, M.; Bax, J.J.; Ajmone Marsan, N. Structural and functional abnormalities of left-sided cardiac chambers in Barlow’s disease without significant mitral regurgitation. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 1296–1305. [Google Scholar] [CrossRef]
- Biondi, R.; Ribeyrolles, S.; Diakov, C.; Amabile, N.; Ricciardi, G.; Khelil, N.; Berrebi, A.; Zannis, K. Mapping of the myxomatous mitral valve: The three-dimensional extension of mitral annular disjunction in surgically repaired mitral prolapse. Front. Cardiovasc. Med. 2022, 9, 1036400. [Google Scholar] [CrossRef]
- Figliozzi, S.; Georgiopoulos, G.; Lopes, P.M.; Bauer, K.B.; Moura-Ferreira, S.; Tondi, L.; Mushtaq, S.; Censi, S.; Pavon, A.G.; Bassi, I.; et al. Myocardial Fibrosis at Cardiac MRI Helps Predict Adverse Clinical Outcome in Patients with Mitral Valve Prolapse. Radiology 2023, 306, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Bhagia, G.; Doyle, M.; Rayarao, G.; Williams, R.B.; Biederman, R.W.W. Mitral annular disjunction; how accurate are we? A cardiovascular MRI study defining risk. Int. J. Cardiol. Heart Vasc. 2023, 49, 101298. [Google Scholar] [CrossRef]
- Perazzolo Marra, M.; Cecere, A.; Cipriani, A.; Migliore, F.; Zorzi, A.; De Lazzari, M.; Lorenzoni, G.; Cecchetto, A.; Brunetti, G.; Graziano, F.; et al. Determinants of Ventricular Arrhythmias in Mitral Valve Prolapse. JACC Clin. Electrophysiol. 2024, 10, 670–681. [Google Scholar] [CrossRef]
- Blondeel, M.; L’Hoyes, W.; Robyns, T.; Verbrugghe, P.; De Meester, P.; Dresselaers, T.; Masci, P.G.; Willems, R.; Bogaert, J.; Vandenberk, B. Serial Cardiac Magnetic Resonance Imaging in Patients with Mitral Valve Prolapse-A Single-Center Retrospective Registry. J. Clin. Med. 2024, 13, 2669. [Google Scholar] [CrossRef]
- Putnam, A.J.; Kebed, K.; Mor-Avi, V.; Rashedi, N.; Sun, D.; Patel, B.; Balkhy, H.; Lang, R.M.; Patel, A.R. Prevalence of mitral annular disjunction in patients with mitral valve prolapse and severe regurgitation. Int. J. Cardiovasc. Imaging 2020, 36, 1363–1370. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Baravelli, M.; Vincenti, A.; Trevisan, R.; Zompatori, M.; Nicolosi, G.L.; Lombardo, M.; Anzà, C. A New modified anthropometric haller index obtained without radiological exposure. Int. J. Cardiovasc. Imaging 2018, 34, 1505–1509. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Haugaa, K. Improving the imaging diagnosis of mitral annular disjunction. Heart 2021, 107, 4–5. [Google Scholar] [CrossRef]
- Vermes, E.; Altes, A.; Iacuzio, L.; Levy, F.; Bohbot, Y.; Renard, C.; Grigioni, F.; Maréchaux, S.; Tribouilloy, C. The evolving role of cardiovascular magnetic resonance in the assessment of mitral valve prolapse. Front. Cardiovasc. Med. 2023, 10, 1093060. [Google Scholar] [CrossRef]
- Faletra, F.F.; Leo, L.A.; Paiocchi, V.L.; Caretta, A.; Viani, G.M.; Schlossbauer, S.A.; Demertzis, S.; Ho, S.Y. Anatomy of mitral annulus insights from non-invasive imaging techniques. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Morningstar, J.E.; Gensemer, C.; Moore, R.; Fulmer, D.; Beck, T.C.; Wang, C.; Moore, K.; Guo, L.; Sieg, F.; Nagata, Y.; et al. Mitral Valve Prolapse Induces Regionalized Myocardial Fibrosis. J. Am. Heart Assoc. 2021, 10, e022332. [Google Scholar] [CrossRef]
- Han, Y.; Peters, D.C.; Salton, C.J.; Bzymek, D.; Nezafat, R.; Goddu, B.; Kissinger, K.V.; Zimetbaum, P.J.; Manning, W.J.; Yeon, S.B. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc. Imaging 2008, 1, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Romero Daza, A.; Chokshi, A.; Pardo, P.; Maneiro, N.; Guijarro Contreras, A.; Larrañaga-Moreira, J.M.; Ibañez, B.; Fuster, V.; Fernández Friera, L.; Solís, J.; et al. Mitral valve prolapse morphofunctional features by cardiovascular magnetic resonance: More than just a valvular disease. J. Cardiovasc. Magn. Reson. 2021, 23, 107. [Google Scholar] [CrossRef]
- Kitkungvan, D.; Nabi, F.; Kim, R.J.; Bonow, R.O.; Khan, M.A.; Xu, J.; Little, S.H.; Quinones, M.A.; Lawrie, G.M.; Zoghbi, W.A.; et al. Myocardial Fibrosis in Patients with Primary Mitral Regurgitation With and Without Prolapse. J. Am. Coll. Cardiol. 2018, 72, 823–834. [Google Scholar] [CrossRef]
- Constant Dit Beaufils, A.L.; Huttin, O.; Jobbe-Duval, A.; Senage, T.; Filippetti, L.; Piriou, N.; Cueff, C.; Venner, C.; Mandry, D.; Sellal, J.M.; et al. Replacement Myocardial Fibrosis in Patients with Mitral Valve Prolapse: Relation to Mitral Regurgitation, Ventricular Remodeling, and Arrhythmia. Circulation 2021, 143, 1763–1774. [Google Scholar] [CrossRef]
- de Meester de Ravenstein, C.; Bouzin, C.; Lazam, S.; Boulif, J.; Amzulescu, M.; Melchior, J.; Pasquet, A.; Vancraeynest, D.; Pouleur, A.C.; Vanoverschelde, J.L.; et al. Histological Validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from Modified Look-Locker imaging (MOLLI) T1 mapping at 3 T. J. Cardiovasc. Magn. Reson. 2015, 17, 48. [Google Scholar] [CrossRef]
- Fontana, M.; White, S.K.; Banypersad, S.M.; Sado, D.M.; Maestrini, V.; Flett, A.S.; Piechnik, S.K.; Neubauer, S.; Roberts, N.; Moon, J.C. Comparison of T1 mapping techniques for ECV quantification. Histological validation and reproducibility of ShMOLLI versus multibreath-hold T1 quantification equilibrium contrast CMR. J. Cardiovasc. Magn. Reson. 2012, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Pepi, M.; Tamborini, G.; Maltagliati, A.; Galli, C.A.; Sisillo, E.; Salvi, L.; Naliato, M.; Porqueddu, M.; Parolari, A.; Zanobini, M.; et al. Head-to-head comparison of two- and three-dimensional transthoracic and transesophageal echocardiography in the localization of mitral valve prolapse. J. Am. Coll. Cardiol. 2006, 48, 2524–2530. [Google Scholar] [CrossRef]
- Gripari, P.; Muratori, M.; Fusini, L.; Tamborini, G.; Pepi, M. Three-Dimensional Echocardiography: Advancements in Qualitative and Quantitative Analyses of Mitral Valve Morphology in Mitral Valve Prolapse. J. Cardiovasc. Echogr. 2014, 24, 1–9. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Picano, E.; Pibarot, P.; Lancellotti, P.; Monin, J.L.; Bonow, R.O. The emerging role of exercise testing and stress echocardiography in valvular heart disease. J. Am. Coll. Cardiol. 2009, 54, 2251–2260. [Google Scholar] [CrossRef]
- Bakkestrøm, R.; Banke, A.; Christensen, N.L.; Pecini, R.; Irmukhamedov, A.; Andersen, M.; Borlaug, B.A.; Møller, J.E. Hemodynamic Characteristics in Significant Symptomatic and Asymptomatic Primary Mitral Valve Regurgitation at Rest and During Exercise. Circ. Cardiovasc. Imaging 2018, 11, e007171. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Hidaka, T.; Susawa, H.; Izumi, K.; Harada, Y.; Kinoshita, M.; Itakura, K.; Masada, K.; Kihara, Y. Exercise-Stress Echocardiography and Effort Intolerance in Asymptomatic/Minimally Symptomatic Patients with Degenerative Mitral Regurgitation Combined Invasive-Noninvasive Hemodynamic Monitoring. Circ. Cardiovasc. Imaging 2018, 11, e007282. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Trevisan, R.; Lombardo, M.; Grasso, E.; Gensini, G.F.; Ambrosio, G. The influence of pectus excavatum on cardiac kinetics and function in otherwise healthy individuals: A systematic review. Int. J. Cardiol. 2023, 381, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M. Impact of Chest Wall Conformation on the Outcome of Primary Mitral Regurgitation due to Mitral Valve Prolapse. J. Cardiovasc. Echogr. 2022, 32, 29–37. [Google Scholar] [CrossRef]
- Blanke, P.; Dvir, D.; Cheung, A.; Ye, J.; Levine, R.A.; Precious, B.; Berger, A.; Stub, D.; Hague, C.; Murphy, D.; et al. A simplified D-shaped model of the mitral annulus to facilitate CT-based sizing before transcatheter mitral valve implantation. J. Cardiovasc. Comput. Tomogr. 2014, 8, 459–467. [Google Scholar] [CrossRef]
- Tsianaka, T.; Matziris, I.; Kobe, A.; Euler, A.; Kuzo, N.; Erhart, L.; Leschka, S.; Manka, R.; Kasel, A.M.; Tanner, F.C.; et al. Mitral annular disjunction in patients with severe aortic stenosis: Extent and reproducibility of measurements with computed tomography. Eur. J. Radiol. Open. 2021, 8, 100335. [Google Scholar] [CrossRef] [PubMed]
- Udoshi, M.B.; Shah, A.; Fisher, V.J.; Dolgin, M. Incidence of mitral valve prolapse in subjects with thoracic skeletal abnormalities--a prospective study. Am. Heart J. 1979, 97, 303–311. [Google Scholar] [CrossRef]
- Movahed, A.; Majdalany, D.; Gillinov, M.; Schiavone, W. Association Between Myxomatous Mitral Valve Disease and Skeletal Back Abnormalities. J. Heart Valve Dis. 2017, 26, 564–568. [Google Scholar] [PubMed]
- Nomura, K.; Ajiro, Y.; Nakano, S.; Matsushima, M.; Yamaguchi, Y.; Hatakeyama, N.; Ohata, M.; Sakuma, M.; Nonaka, T.; Harii, M.; et al. Characteristics of mitral valve leaflet length in patients with pectus excavatum: A single center cross-sectional study. PLoS ONE 2019, 14, e0212165. [Google Scholar] [CrossRef] [PubMed]
- Boudoulas, K.D.; Pitsis, A.A.; Mazzaferri, E.L.; Gumina, R.J.; Triposkiadis, F.; Boudoulas, H. Floppy mitral valve/mitral valve prolapse: A complex entity with multiple genotypes and phenotypes. Prog. Cardiovasc. Dis. 2020, 63, 308–326. [Google Scholar] [CrossRef]
- Bijnens, B.H.; Cikes, M.; Claus, P.; Sutherland, G.R. Velocity and deformation imaging for the assessment of myocardial dysfunction. Eur. J. Echocardiogr. 2009, 10, 216–226. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Rigamonti, E.; Nicolosi, G.L.; Lombardo, M. Prognostic Value of Modified Haller Index in Patients with Suspected Coronary Artery Disease Referred for Exercise Stress Echocardiography. J. Cardiovasc. Echogr. 2021, 31, 85–95. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonaglioni, A.; Nicolosi, G.L.; Muti-Schünemann, G.E.U.; Rispoli, G.A.; Lombardo, M.; Muti, P. Does Preliminary Chest Shape Assessment Improve the Prognostic Risk Stratification of Individuals with Mitral Annular Disjunction? A Case Report and Narrative Review. J. Clin. Med. 2025, 14, 2277. https://doi.org/10.3390/jcm14072277
Sonaglioni A, Nicolosi GL, Muti-Schünemann GEU, Rispoli GA, Lombardo M, Muti P. Does Preliminary Chest Shape Assessment Improve the Prognostic Risk Stratification of Individuals with Mitral Annular Disjunction? A Case Report and Narrative Review. Journal of Clinical Medicine. 2025; 14(7):2277. https://doi.org/10.3390/jcm14072277
Chicago/Turabian StyleSonaglioni, Andrea, Gian Luigi Nicolosi, Giovanna Elsa Ute Muti-Schünemann, Gaetana Anna Rispoli, Michele Lombardo, and Paola Muti. 2025. "Does Preliminary Chest Shape Assessment Improve the Prognostic Risk Stratification of Individuals with Mitral Annular Disjunction? A Case Report and Narrative Review" Journal of Clinical Medicine 14, no. 7: 2277. https://doi.org/10.3390/jcm14072277
APA StyleSonaglioni, A., Nicolosi, G. L., Muti-Schünemann, G. E. U., Rispoli, G. A., Lombardo, M., & Muti, P. (2025). Does Preliminary Chest Shape Assessment Improve the Prognostic Risk Stratification of Individuals with Mitral Annular Disjunction? A Case Report and Narrative Review. Journal of Clinical Medicine, 14(7), 2277. https://doi.org/10.3390/jcm14072277