Tricuspid Regurgitation in the Era of Transcatheter Interventions: The Pivotal Role of Multimodality Imaging
Abstract
1. Introduction
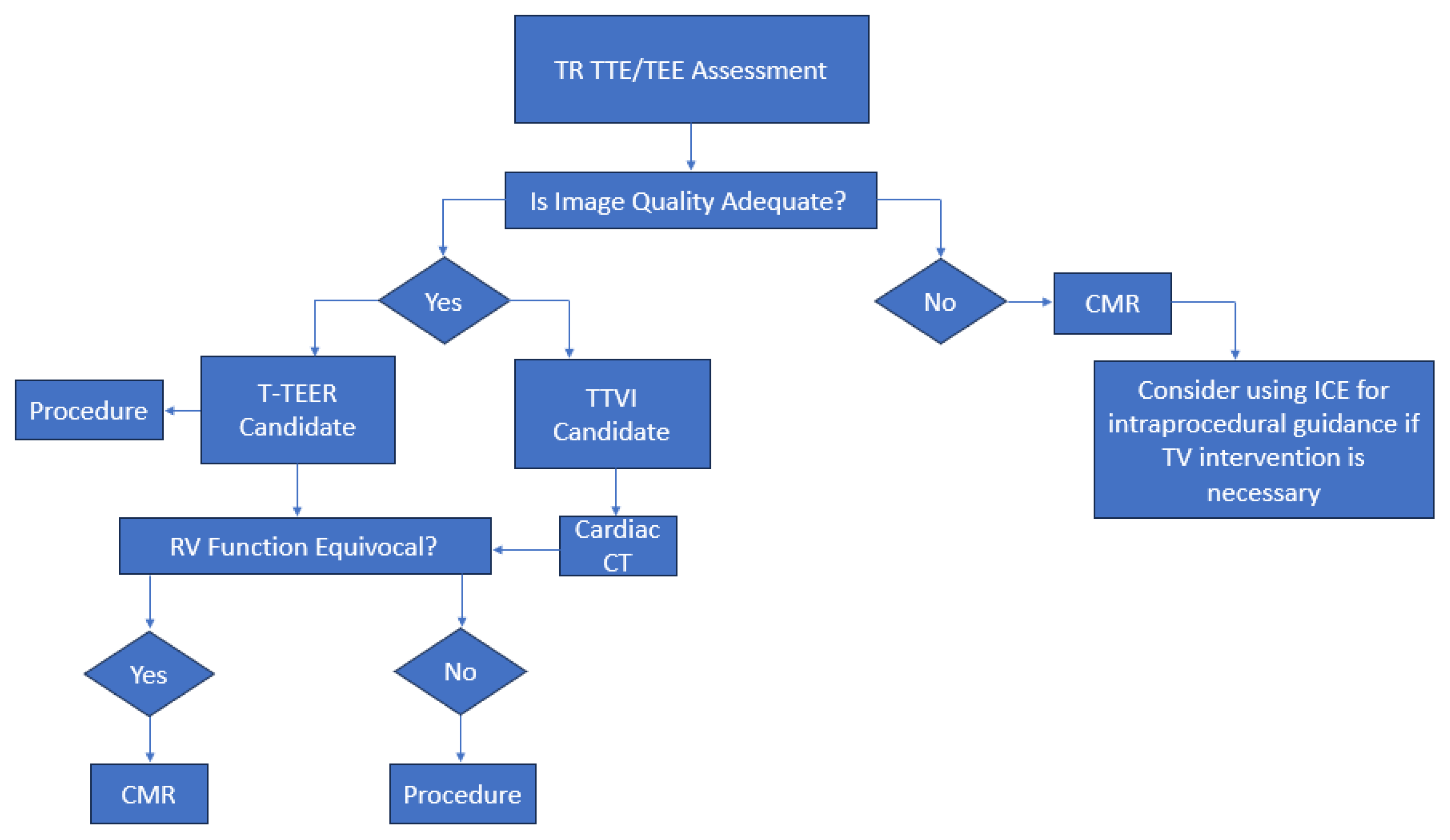
2. Role of Echocardiography in Initial Diagnosis
3. Multimodality Imaging for Procedural Planning
4. Imaging-Based Eligibility Criteria
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TTVI | Transcatheter Tricuspid Valve Intervention |
| TRJ | Tricuspid Regurgitation |
| CT | Computed Tomography |
| CMR | Cardiovascular Magnetic Resonance |
| KCCQ | Kansas City Cardiomyopathy Questionnaire |
| NHYA | New York Heart Association |
| TTE | Transthoracic Echocardiography |
| RV | Right Ventricle |
| CW | Continuous-Wave |
| PW | Pulsed-Wave |
| EROA | Effective Regurgitant Orifice Area |
| aFTR | Atrial Functional TR |
| vFTR | Ventricular Functional TR |
| CIED | Cardiac Implantable Electronic Device |
| FAC | Fractional Area Change |
| EF | Ejection Fraction |
| TAPSE | Tricuspid Annular Plane Systolic Excursion |
| PH | Pulmonary Hypertension |
| sPAP | Systolic Pulmonary Artery Pressure |
| HFH | Heart Failure Hospitalization |
| PA | Pulmonary Artery |
| XGB | Extreme Gradient Boosting |
| IVC | Inferior Vena Cava |
| 3D | Three-Dimensional |
| MPR | Multiplanar Reconstruction |
| TA | Tricuspid Annulus |
| Reg Vol | Regurgitant Volume |
| VC | Vena Contracta |
| VCA | Vena Contracta Area |
| T-TEER | Transcatheter Tricuspid Edge-to-Edge Repair |
| RCA | Right Coronary Artery |
| TEE | Trans Esophageal Echocardiography |
| LTR-A | Lead-Associated Tricuspid Regurgitation Type A |
| TTVR | Transcatheter Tricuspid Valve Replacement |
References
- Prihadi, E.A.; Van Der Bijl, P.; Gursoy, E.; Abou, R.; Mara Vollema, E.; Hahn, R.T.; Stone, G.W.; Leon, M.B.; Ajmone Marsan, N.; Delgado, V.; et al. Development of Significant Tricuspid Regurgitation over Time and Prognostic Implications: New Insights into Natural History. Eur. Heart J. 2018, 39, 3574–3581. [Google Scholar] [CrossRef]
- Hahn, R.T.; Chandrashekhar, Y. Tricuspid Regurgitation. JACC Cardiovasc. Imaging 2019, 12, 572–575. [Google Scholar] [CrossRef]
- Topilsky, Y.; Maltais, S.; Medina Inojosa, J.; Oguz, D.; Michelena, H.; Maalouf, J.; Mahoney, D.W.; Enriquez-Sarano, M. Burden of Tricuspid Regurgitation in Patients Diagnosed in the Community Setting. JACC Cardiovasc. Imaging 2019, 12, 433–442. [Google Scholar] [CrossRef]
- Rodés-Cabau, J.; Hahn, R.T.; Latib, A.; Laule, M.; Lauten, A.; Maisano, F.; Schofer, J.; Campelo-Parada, F.; Puri, R.; Vahanian, A. Transcatheter Therapies for Treating Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2016, 67, 1829–1845. [Google Scholar] [CrossRef]
- Dreyfus, G.D.; Essayagh, B. Transcatheter Treatment Options for Severe Tricuspid Regurgitation. JACC Cardiovasc. Interv. 2021, 14, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Von Bardeleben, R.S.; Lurz, P.; Sorajja, P.; Ruf, T.; Hausleiter, J.; Sitges, M.; Da Rocha E Silva, J.; Näbauer, M.; Weber, M.; Tang, G.H.L.; et al. Two-Year Outcomes for Tricuspid Repair with a Transcatheter Edge-to-Edge Valve Repair From the Transatlantic TRILUMINATE Trial. Circ. Cardiovasc. Interv. 2023, 16, e012888. [Google Scholar] [CrossRef] [PubMed]
- Kodali, S.; Hahn, R.T.; Makkar, R.; Makar, M.; Davidson, C.J.; Puthumana, J.J.; Zahr, F.; Chadderdon, S.; Fam, N.; Ong, G.; et al. Transfemoral Tricuspid Valve Replacement and One-Year Outcomes: The TRISCEND Study. Eur. Heart J. 2023, 44, 4862–4873. [Google Scholar] [CrossRef] [PubMed]
- Albertini, A.; Nerla, R.; Castriota, F.; Squeri, A. Right Ventricle Remodeling after Transcatheter Tricuspid Leaflet Repair in Patients with Functional Tricuspid Regurgitation: Lessons from the Surgical Experience. Front. Cardiovasc. Med. 2022, 9, 977142. [Google Scholar] [CrossRef]
- Beghini, A.; Sammartino, A.M.; Papp, Z.; Von Haehling, S.; Biegus, J.; Ponikowski, P.; Adamo, M.; Falco, L.; Lombardi, C.M.; Pagnesi, M.; et al. 2024 Update in Heart Failure. ESC Heart Fail. 2025, 12, 8–42. [Google Scholar] [CrossRef]
- Hahn, R.T.; Lawlor, M.K.; Davidson, C.J.; Badhwar, V.; Sannino, A.; Spitzer, E.; Lurz, P.; Lindman, B.R.; Topilsky, Y.; Baron, S.J.; et al. Tricuspid Valve Academic Research Consortium Definitions for Tricuspid Regurgitation and Trial Endpoints. J. Am. Coll. Cardiol. 2023, 82, 1711–1735. [Google Scholar] [CrossRef]
- Davidson, L.J.; Tang, G.H.L.; Ho, E.C.; Fudim, M.; Frisoli, T.; Camaj, A.; Bowers, M.T.; Masri, S.C.; Atluri, P.; Chikwe, J.; et al. The Tricuspid Valve: A Review of Pathology, Imaging, and Current Treatment Options: A Scientific Statement From the American Heart Association. Circulation 2024, 149, e1223–e1238. [Google Scholar] [CrossRef]
- Hahn, R.T. State-of-the-Art Review of Echocardiographic Imaging in the Evaluation and Treatment of Functional Tricuspid Regurgitation. Circ. Cardiovasc. Imaging 2016, 9, e005332. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Ro, R.; Tang, G.H.L.; Seetharam, K.; Khera, S.; Sharma, S.K.; Kini, A.S.; Lerakis, S. Echocardiographic Imaging for Transcatheter Tricuspid Edge-to-Edge Repair. J. Am. Heart Assoc. 2020, 9, e015682. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, M.; Penso, M.; Badano, L.P.; Clement, A.; Radu, N.; Heilbron, F.; Gavazzoni, M.; Hădăreanu, D.R.; Oliverio, G.; Fisicaro, S.; et al. Association with Outcomes of Correcting the Proximal Isovelocity Surface Area Method to Quantitate Secondary Tricuspid Regurgitation. J. Am. Soc. Echocardiogr. 2025, 38, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, M.; Penso, M.; Badano, L.P.; Clement, A.; Radu, N.; Heilbron, F.; Benzoni, G.; Hădăreanu, D.R.; Springhetti, P.; Giorgio, O.; et al. Right Ventricular Function and Outcomes Stratified by the Effective Regurgitant Orifice Area in Secondary Tricuspid Regurgitation. Can. J. Cardiol. 2025, 41, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Brener, M.I.; Lurz, P.; Hausleiter, J.; Rodés-Cabau, J.; Fam, N.; Kodali, S.K.; Rommel, K.-P.; Muntané-Carol, G.; Gavazzoni, M.; Nazif, T.M.; et al. Right Ventricular-Pulmonary Arterial Coupling and Afterload Reserve in Patients Undergoing Transcatheter Tricuspid Valve Repair. J. Am. Coll. Cardiol. 2022, 79, 448–461. [Google Scholar] [CrossRef]
- Sannino, A.; Ilardi, F.; Hahn, R.T.; Lancellotti, P.; Lurz, P.; Smith, R.L.; Esposito, G.; Grayburn, P.A. Clinical and Echocardiographic Outcomes of Transcatheter Tricuspid Valve Interventions: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 919395. [Google Scholar] [CrossRef]
- Surkova, E.; Cosyns, B.; Gerber, B.; Gimelli, A.; La Gerche, A.; Ajmone Marsan, N. The Dysfunctional Right Ventricle: The Importance of Multi-Modality Imaging. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 885–897. [Google Scholar] [CrossRef]
- Schlotter, F.; Miura, M.; Kresoja, K.-P.; Alushi, B.; Alessandrini, H.; Attinger-Toller, A.; Besler, C.; Biasco, L.; Braun, D.; Brochet, E.; et al. Outcomes of Transcatheter Tricuspid Valve Intervention by Right Ventricular Function: A Multicentre Propensity-Matched Analysis. EuroIntervention 2021, 17, e343–e352. [Google Scholar] [CrossRef] [PubMed]
- Lurz, P.; Orban, M.; Besler, C.; Braun, D.; Schlotter, F.; Noack, T.; Desch, S.; Karam, N.; Kresoja, K.-P.; Hagl, C.; et al. Clinical Characteristics, Diagnosis, and Risk Stratification of Pulmonary Hypertension in Severe Tricuspid Regurgitation and Implications for Transcatheter Tricuspid Valve Repair. Eur. Heart J. 2020, 41, 2785–2795. [Google Scholar] [CrossRef] [PubMed]
- Stolz, L.; Kresoja, K.-P.; Von Stein, J.; Fortmeier, V.; Koell, B.; Rottbauer, W.; Kassar, M.; Goebel, B.; Denti, P.; Achouh, P.; et al. Impact of Pulmonary Hypertension on Outcomes After Transcatheter Tricuspid Valve Edge-to-Edge Repair. JACC Cardiovasc. Interv. 2025, 18, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Fortmeier, V.; Lachmann, M.; Körber, M.I.; Unterhuber, M.; Von Scheidt, M.; Rippen, E.; Harmsen, G.; Gerçek, M.; Friedrichs, K.P.; Roder, F.; et al. Solving the Pulmonary Hypertension Paradox in Patients with Severe Tricuspid Regurgitation by Employing Artificial Intelligence. JACC Cardiovasc. Interv. 2022, 15, 381–394. [Google Scholar] [CrossRef]
- Fortmeier, V.; Lachmann, M.; Stolz, L.; Von Stein, J.; Unterhuber, M.; Kassar, M.; Gerçek, M.; Schöber, A.R.; Stocker, T.J.; Omran, H.; et al. Artificial Intelligence–Enabled Assessment of Right Ventricular to Pulmonary Artery Coupling in Patients Undergoing Transcatheter Tricuspid Valve Intervention. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 558–572. [Google Scholar] [CrossRef]
- Badano, L.P.; Tomaselli, M.; Muraru, D.; Galloo, X.; Li, C.H.P.; Ajmone Marsan, N. Advances in the Assessment of Patients with Tricuspid Regurgitation: A State-of-the-Art Review on the Echocardiographic Evaluation Before and After Tricuspid Valve Interventions. J. Am. Soc. Echocardiogr. 2024, 37, 1083–1102. [Google Scholar] [CrossRef]
- Kuwajima, K.; Ogawa, M.; Ruiz, I.; Yamane, T.; Hasegawa, H.; Yagi, N.; Rader, F.; Siegel, R.J.; Shiota, T. Comparison of Prognostic Value among Echocardiographic Surrogates of Right Ventricular–Pulmonary Arterial Coupling: A Three-dimensional Echocardiographic Study. Echocardiography 2024, 41, e15717. [Google Scholar] [CrossRef]
- Gavazzoni, M.; Badano, L.P.; Cascella, A.; Heilbron, F.; Tomaselli, M.; Caravita, S.; Baratto, C.; Perelli, F.; Radu, N.; Perger, E.; et al. Clinical Value of a Novel Three-Dimensional Echocardiography–Derived Index of Right Ventricle–Pulmonary Artery Coupling in Tricuspid Regurgitation. J. Am. Soc. Echocardiogr. 2023, 36, 1154–1166.e3. [Google Scholar] [CrossRef]
- Hungerford, S.L.; Rye, E.E.; Hansen, P.S.; Bhindi, R.; Choong, C. Key Echocardiographic Considerations for Tricuspid Valve Transcatheter Edge-to-Edge Repair. J. Am. Soc. Echocardiogr. 2023, 36, 366–380.e1. [Google Scholar] [CrossRef]
- Hahn, R.T.; Weckbach, L.T.; Noack, T.; Hamid, N.; Kitamura, M.; Bae, R.; Lurz, P.; Kodali, S.K.; Sorajja, P.; Hausleiter, J.; et al. Proposal for a Standard Echocardiographic Tricuspid Valve Nomenclature. JACC Cardiovasc. Imaging 2021, 14, 1299–1305. [Google Scholar] [CrossRef]
- Lebehn, M.; Nikolou, E.; Grapsa, J.; Hahn, R.T. Edge-to-Edge Tricuspid Valve Repair. JACC Case Rep. 2020, 2, 1093–1096. [Google Scholar] [CrossRef]
- Chouchani, M.; Michaelsen, J.; Langenbrink, L.; Piatkowski, M.; Altiok, E.; Hoffmann, R. Quantification of Tricuspid Regurgitation Area by 3-dimensional Color Doppler Echocardiography Considering Different Clinical Settings. Echocardiography 2020, 37, 1120–1129. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Tsang, W.; Adams, D.H.; Agricola, E.; Buck, T.; Faletra, F.F.; Franke, A.; Hung, J.; Pérez De Isla, L.; et al. EAE/ASE Recommendations for Image Acquisition and Display Using Three-Dimensional Echocardiography. J. Am. Soc. Echocardiogr. 2012, 25, 3–46. [Google Scholar] [CrossRef] [PubMed]
- Cammalleri, V.; Antonelli, G.; De Luca, V.M.; Piscione, M.; Carpenito, M.; Gaudio, D.; Nusca, A.; Cocco, N.; Mega, S.; Grigioni, F.; et al. 3D Transoesophageal Echocardiographic Assessment of Acute Reverse Remodelling of the Tricuspid Annulus after Transcatheter Edge-to-Edge Repair. Eur. Heart J. Cardiovasc. Imaging 2025, 26, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Mediratta, A.; Addetia, K.; Yamat, M.; Moss, J.D.; Nayak, H.M.; Burke, M.C.; Weinert, L.; Maffessanti, F.; Jeevanandam, V.; Mor-Avi, V.; et al. 3D Echocardiographic Location of Implantable Device Leads and Mechanism of Associated Tricuspid Regurgitation. JACC Cardiovasc. Imaging 2014, 7, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Agricola, E.; Asmarats, L.; Maisano, F.; Cavalcante, J.L.; Liu, S.; Milla, F.; Meduri, C.; Rodés-Cabau, J.; Vannan, M.; Pibarot, P. Imaging for Tricuspid Valve Repair and Replacement. JACC Cardiovasc. Imaging 2021, 14, 61–111. [Google Scholar] [CrossRef]
- Muraru, D.; Gavazzoni, M.; Heilbron, F.; Mihalcea, D.J.; Guta, A.C.; Radu, N.; Muscogiuri, G.; Tomaselli, M.; Sironi, S.; Parati, G.; et al. Reference Ranges of Tricuspid Annulus Geometry in Healthy Adults Using a Dedicated Three-Dimensional Echocardiography Software Package. Front. Cardiovasc. Med. 2022, 9, 1011931. [Google Scholar] [CrossRef]
- Cotella, J.I.; Blitz, A.; Clement, A.; Tomaselli, M.; Muraru, D.; Badano, L.P.; Sauber, N.; Font Calvarons, A.; Degel, M.; Rucki, A.; et al. Three-Dimensional Transthoracic Echocardiography for Semiautomated Analysis of the Tricuspid Annulus: Validation and Normal Values. J. Am. Soc. Echocardiogr. 2025, 38, 33–43.e3. [Google Scholar] [CrossRef]
- Dahou, A.; Levin, D.; Reisman, M.; Hahn, R.T. Anatomy and Physiology of the Tricuspid Valve. JACC Cardiovasc. Imaging 2019, 12, 458–468. [Google Scholar] [CrossRef]
- Schoenhagen, P.; Numburi, U.; Halliburton, S.S.; Aulbach, P.; Von Roden, M.; Desai, M.Y.; Rodriguez, L.L.; Kapadia, S.R.; Tuzcu, E.M.; Lytle, B.W. Three-Dimensional Imaging in the Context of Minimally Invasive and Transcatheter Cardiovascular Interventions Using Multi-Detector Computed Tomography: From Pre-Operative Planning to Intra-Operative Guidance. Eur. Heart J. 2010, 31, 2727–2740. [Google Scholar] [CrossRef]
- Hell, M.M.; Emrich, T.; Kreidel, F.; Kreitner, K.-F.; Schoepf, U.J.; Münzel, T.; Von Bardeleben, R.S. Computed Tomography Imaging Needs for Novel Transcatheter Tricuspid Valve Repair and Replacement Therapies. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 601–610. [Google Scholar] [CrossRef]
- Cammalleri, V.; Carpenito, M.; De Stefano, D.; Ussia, G.P.; Bono, M.C.; Mega, S.; Nusca, A.; Cocco, N.; Nobile, E.; De Filippis, A.; et al. Novel Computed Tomography Variables for Assessing Tricuspid Valve Morphology: Results from the TRIMA (Tricuspid Regurgitation IMAging) Study. J. Clin. Med. 2022, 11, 2825. [Google Scholar] [CrossRef]
- Van Rosendael, P.J.; Kamperidis, V.; Kong, W.K.F.; Van Rosendael, A.R.; Van Der Kley, F.; Ajmone Marsan, N.; Delgado, V.; Bax, J.J. Computed Tomography for Planning Transcatheter Tricuspid Valve Therapy. Eur. Heart J. 2017, 38, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Hinzpeter, R.; Eberhard, M.; Burghard, P.; Tanner, F.; Taramasso, M.; Manka, R.; Feuchtner, G.; Maisano, F.; Alkadhi, H. Computed Tomography in Patients with Tricuspid Regurgitation Prior to Transcatheter Valve Repair: Dynamic Analysis of the Annulus with an Individually Tailored Contrast Media Protocol. EuroIntervention 2017, 12, e1828–e1836. [Google Scholar] [CrossRef] [PubMed]
- Praz, F.; Khalique, O.K.; Dos Reis Macedo, L.G.; Pulerwitz, T.C.; Jantz, J.; Wu, I.Y.; Kantor, A.; Patel, A.; Vahl, T.; Bapat, V.; et al. Comparison between Three-Dimensional Echocardiography and Computed Tomography for Comprehensive Tricuspid Annulus and Valve Assessment in Severe Tricuspid Regurgitation: Implications for Tricuspid Regurgitation Grading and Transcatheter Therapies. J. Am. Soc. Echocardiogr. 2018, 31, 1190–1202.e3. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Lerakis, S.; Delgado, V.; Addetia, K.; Burkhoff, D.; Muraru, D.; Pinney, S.; Friedberg, M.K. Multimodality Imaging of Right Heart Function. J. Am. Coll. Cardiol. 2023, 81, 1954–1973. [Google Scholar] [CrossRef]
- Doldi, P.M.; Weckbach, L.T.; Fink, N.; Stolz, L.; Ennin, C.; Dinkel, J.; Lurz, P.; Thiele, H.; Hahn, R.T.; Cavalcante, J.L.; et al. 3D Echocardiographic and CMR Imaging for the Assessment of Right Ventricular Function and Tricuspid Regurgitation Severity. Circ Cardiovasc. Imaging 2025, 18, e017638. [Google Scholar] [CrossRef]
- Donal, E.; Unger, P.; Coisne, A.; Pibarot, P.; Magne, J.; Sitges, M.; Habib, G.; Clavel, M.-A.; Von Bardeleben, R.S.; Plein, S.; et al. The Role of Multi-Modality Imaging in Multiple Valvular Heart Diseases: A Clinical Consensus Statement of the European Association of Cardiovascular Imaging of the European Society of Cardiology. Eur. Heart J. Cardiovasc. Imaging 2025, 26, 593–608. [Google Scholar] [CrossRef]
- Tanaka, T.; Vogelhuber, J.; Öztürk, C.; Silaschi, M.; Bakhtiary, F.; Zimmer, S.; Nickenig, G.; Weber, M.; Sugiura, A. Eligibility for Transcatheter Tricuspid Valve Interventions in Patients with Tricuspid Regurgitation. JACC Cardiovasc. Interv. 2024, 17, 2732–2744. [Google Scholar] [CrossRef]
- Gerçek, M.; Narang, A.; Körber, M.I.; Friedrichs, K.P.; Puthumana, J.J.; Ivannikova, M.; Al-Kazaz, M.; Cremer, P.; Baldridge, A.S.; Meng, Z.; et al. GLIDE Score. JACC Cardiovasc. Imaging 2024, 17, 729–742. [Google Scholar] [CrossRef]
- Taramasso, M.; Alessandrini, H.; Latib, A.; Asami, M.; Attinger-Toller, A.; Biasco, L.; Braun, D.; Brochet, E.; Connelly, K.A.; Denti, P.; et al. Outcomes After Current Transcatheter Tricuspid Valve Intervention. JACC Cardiovasc. Interv. 2019, 12, 155–165. [Google Scholar] [CrossRef]
- Besler, C.; Orban, M.; Rommel, K.-P.; Braun, D.; Patel, M.; Hagl, C.; Borger, M.; Nabauer, M.; Massberg, S.; Thiele, H.; et al. Predictors of Procedural and Clinical Outcomes in Patients with Symptomatic Tricuspid Regurgitation Undergoing Transcatheter Edge-to-Edge Repair. JACC Cardiovasc. Interv. 2018, 11, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak, J.; Vivekanantham, H.; Kassar, M.; Dernektsi, C.; Agarwal, V.; Lebehn, M.; Windecker, S.; Brugger, N.; Hahn, R.T.; Praz, F. Computed Tomography Anatomic Predictors of Outcomes in Patients Undergoing Tricuspid Transcatheter Edge-to-Edge Repair. J. Cardiovasc. Comput. Tomogr. 2024, 18, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.J.; Hussein, N.; Peel, B.; Coles, J.; Arsdell, G.S.V.; Honjo, O.; Haller, C.; Lam, C.Z.; Seed, M.; Barron, D. 3D Modeling and Printing in Congenital Heart Surgery: Entering the Stage of Maturation. Front. Pediatr. 2021, 9, 621672. [Google Scholar] [CrossRef] [PubMed]
- Quader, N.; Kaneko, T.; Williford, N.; Sintek, M.; Kachroo, P.; Brescia, A.A.; Roberts, H.G., Jr.; Zajarias, A. Transesophageal Echocardiography and Intracardiac Echocardiography to Guide EVOQUE. JACC Case Rep. 2025, 30, 103628. [Google Scholar] [CrossRef] [PubMed]
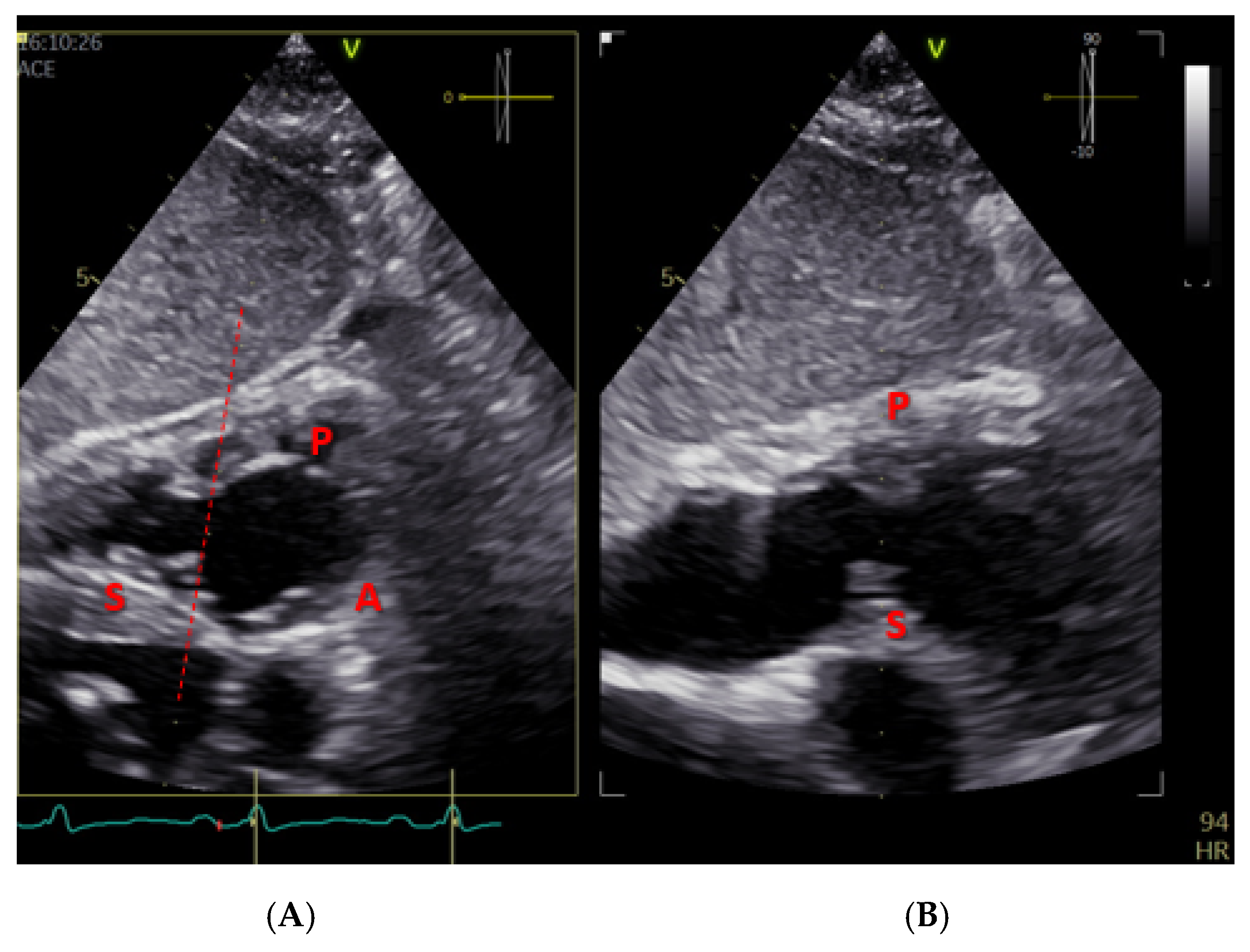
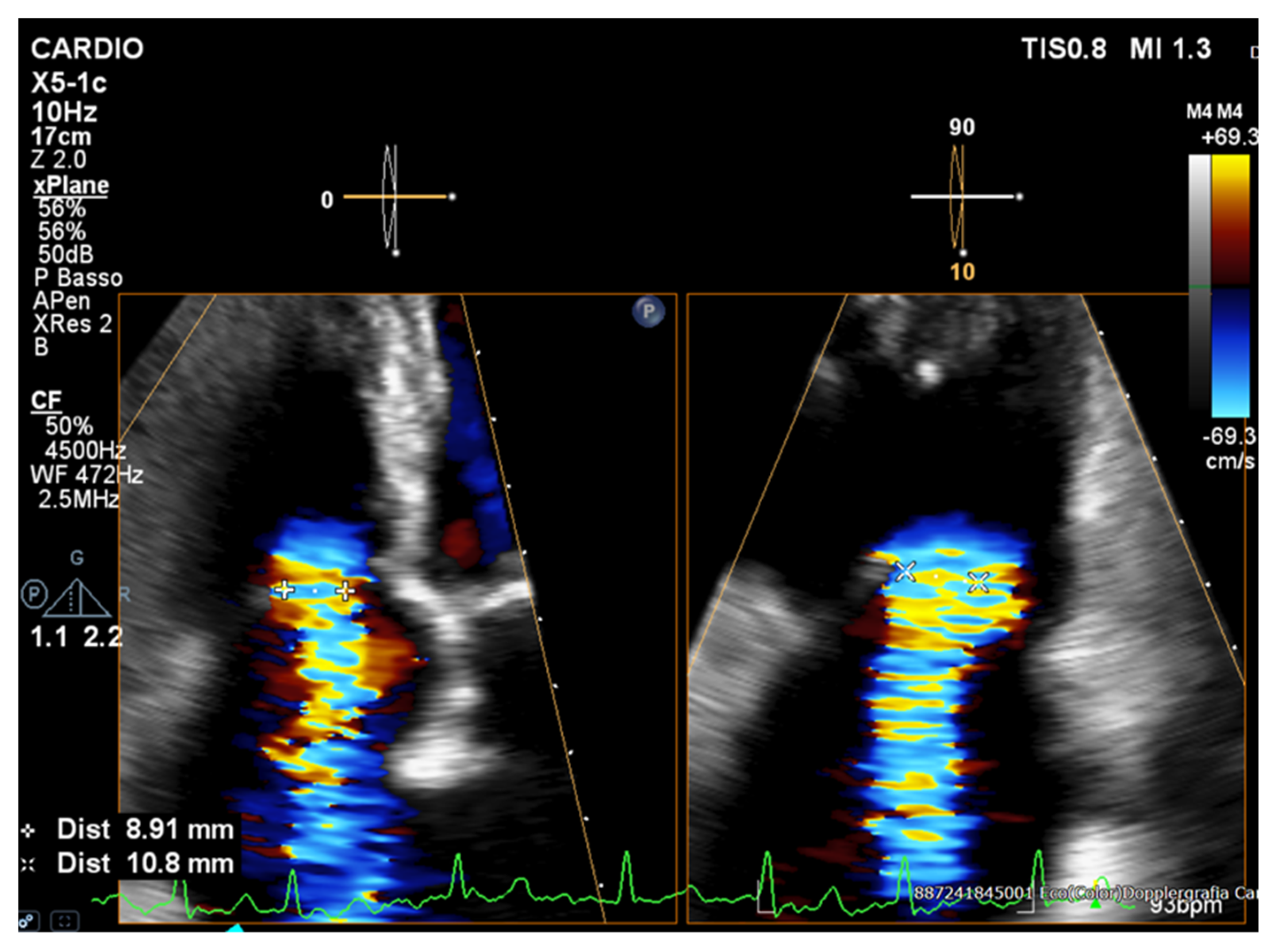
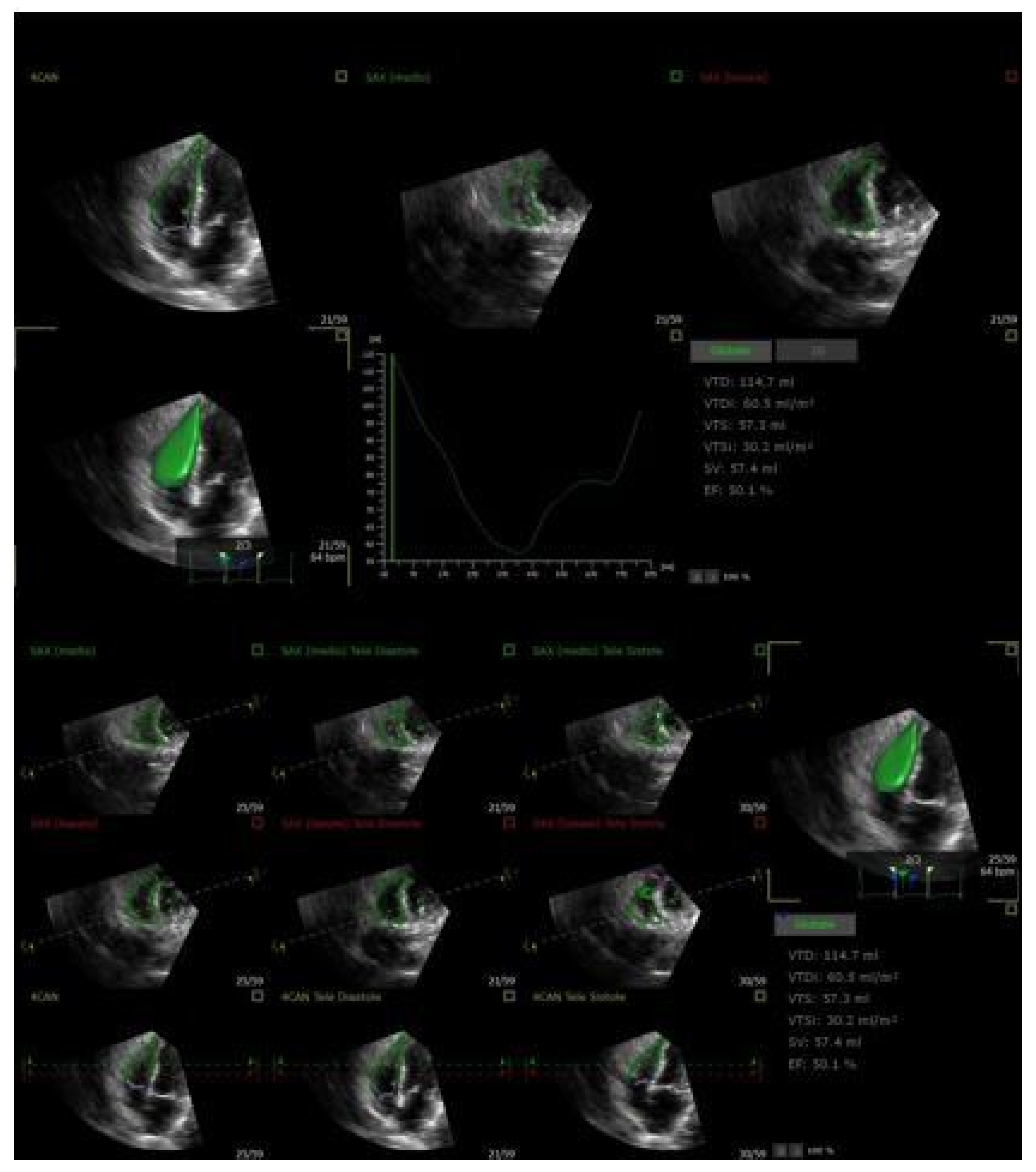
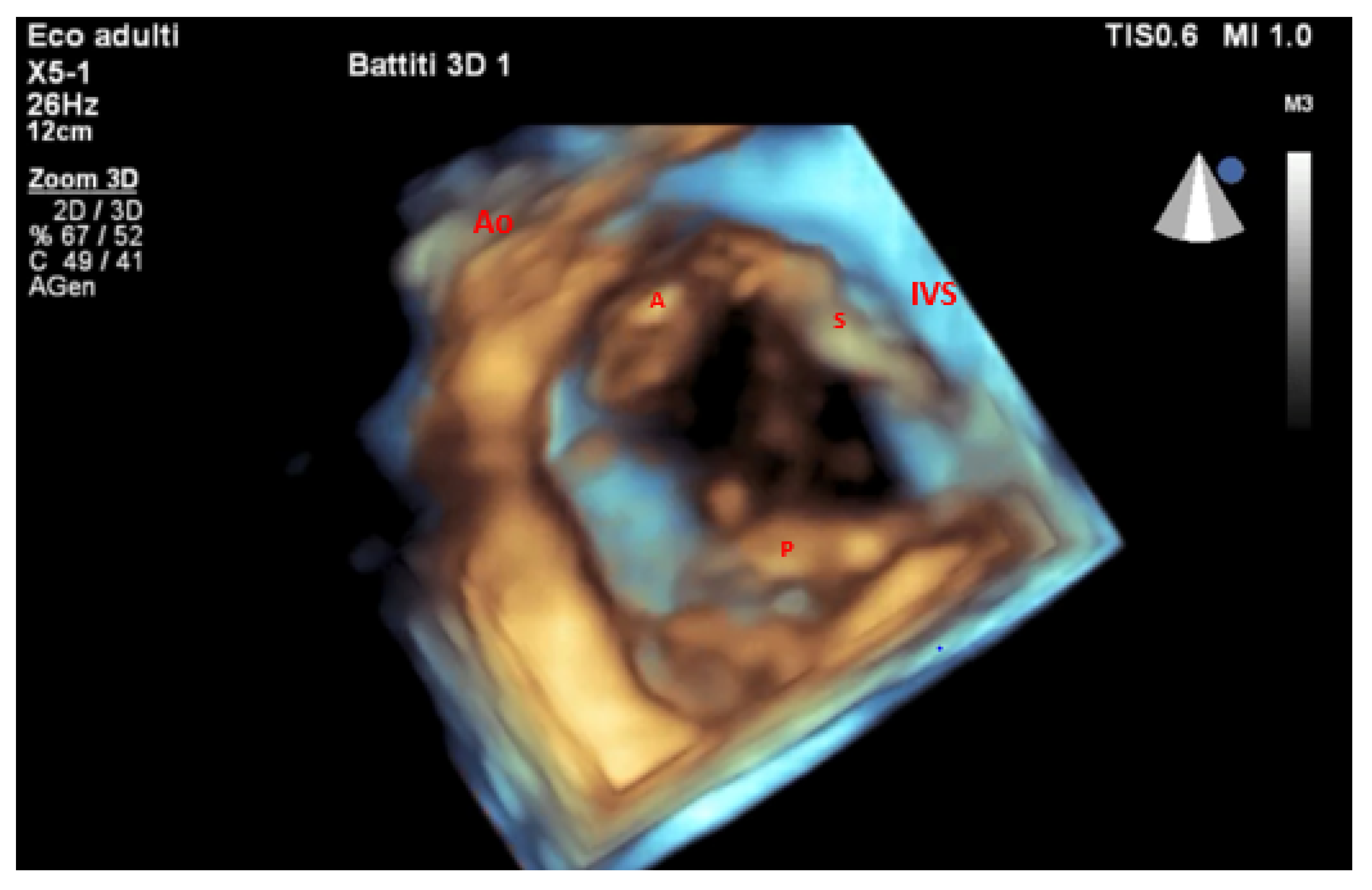

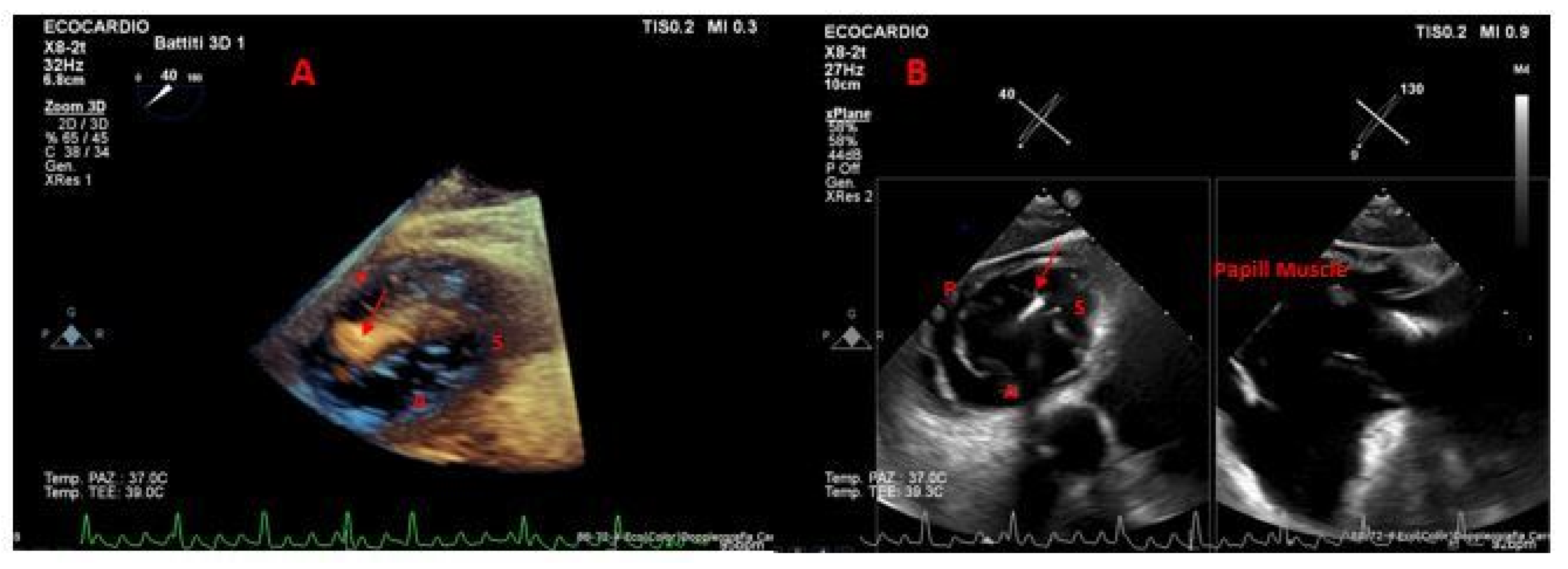
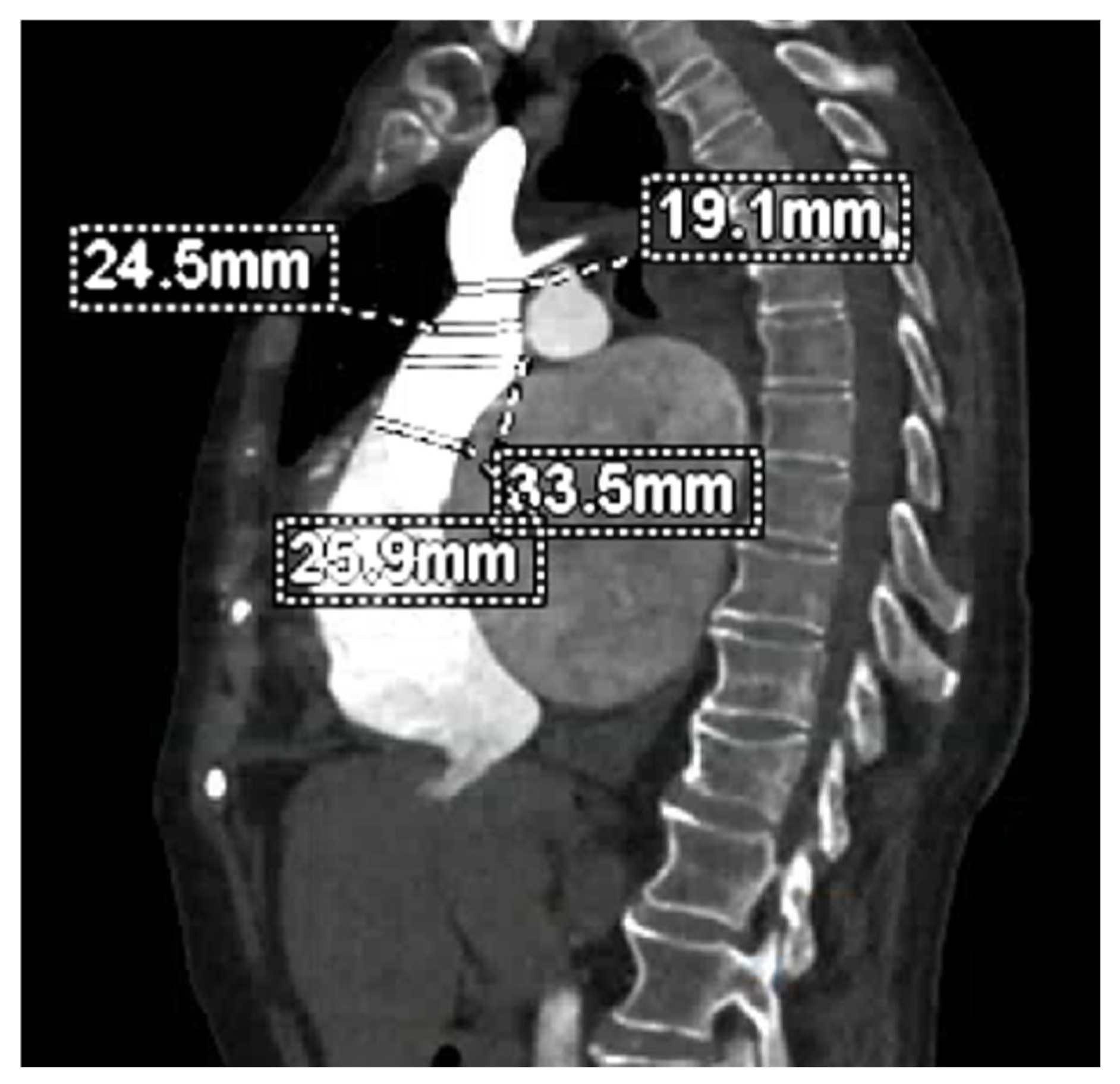
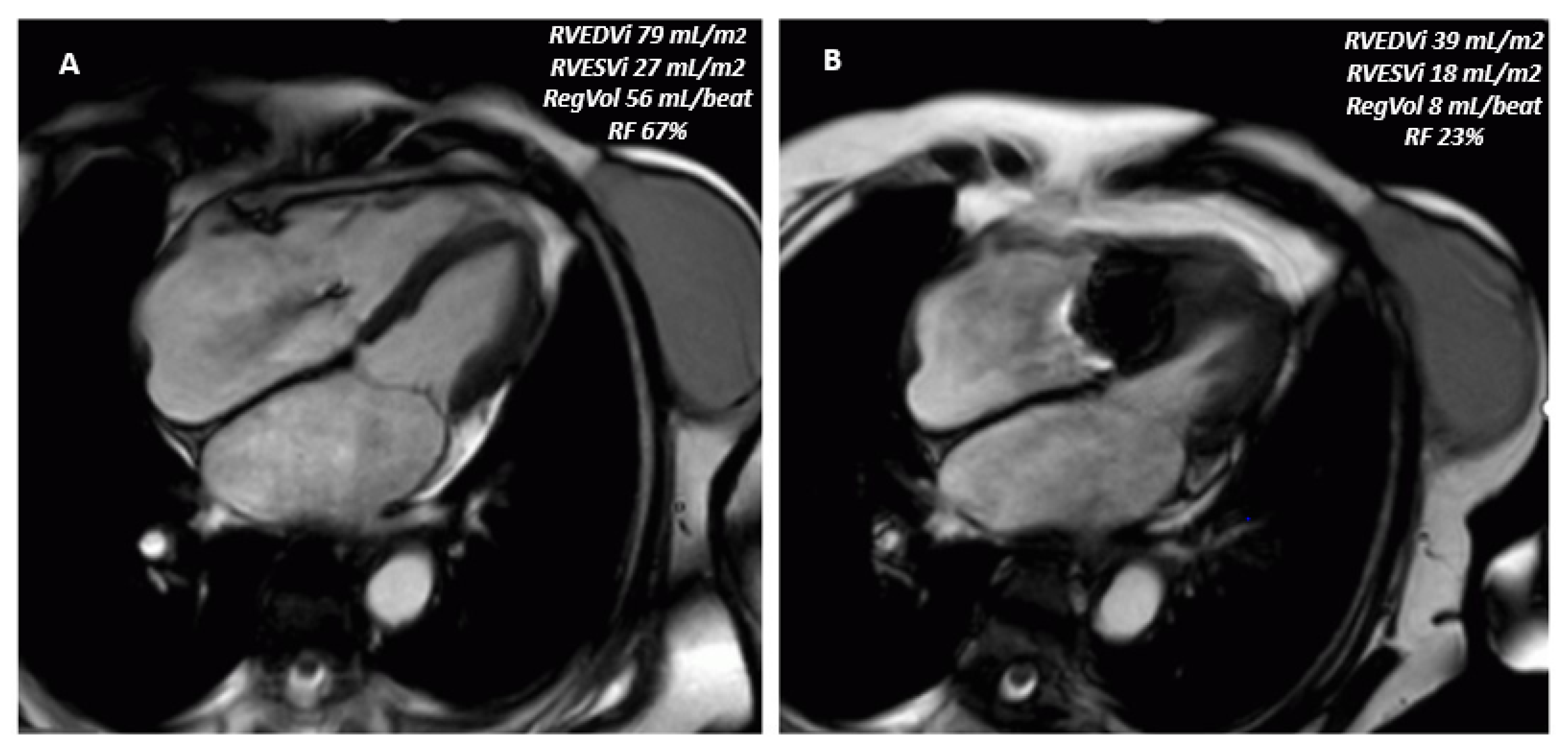
| Method | Parameter(s) | Advantages | Limitations |
|---|---|---|---|
| 2D Echocardiography | TAPSE (tricuspid annular plane systolic excursion) | Simple, widely available, reproducible | Can be pseudo-normal in severe TR |
| S′ (tissue Doppler systolic velocity) | Easy, less load-dependent than TAPSE | Influenced by angle and operator skill | |
| FAC (fractional area change) | Evaluates global RV function | Suboptimal image quality may limit accuracy; in a severe TR setting, it cannot distinguish between anterograde and retrograde flow. | |
| Strain Imaging (Speckle Tracking) | RV longitudinal strain | Early marker of dysfunction; higher prognostic significance in contrast to traditional metrics | Limited standardization, inter-vendor variability |
| 3D Echocardiography | RV ejection fraction (RVEF), RV volumes | Volumetric, less geometrically biased | Requires good acoustic window, technically demanding, poorly characterized prognostic impact in the context of TTVIs |
| Cardiac CMR | RVEF, RV volumes, fibrosis detection | Gold standard for RV volumes and function; not operator-dependent | Limited availability, contraindications (e.g., devices), expensive, does not consider the direction of the flow |
| Cardiac CT | RV volumes (limited functional info) | High spatial resolution; useful for anatomy and procedural planning | Not ideal for function, radiation exposure, no direct contractility assessment |
| Hemodynamic Assessment | Right atrial pressure, pulmonary pressures | Direct measurement; useful during the procedure | Invasive |
| Pulmonary Artery Coupling | TAPSE/PASP ratio | Reflects RV–arterial interaction; prognostic value | Requires accurate PASP estimation |
| Treatment Strategy | Imaging Considerations for Patient Selection |
|---|---|
| T-TEER | Coaptation gap < 8.5 mm; Not suitable in case of TV stenosis or LTR-A. |
| Annuloplasty | Tricuspid annulus diameter < 55 mm; Not suitable in case of severe tethering, RCA proximity, or LTR-A. |
| Coaptation Device | Coaptation gap < 18 mm; Ensure adequate venous access and sufficient distance between papillary muscles, tricuspid annulus, and septum to allow for safe and effective device delivery and deployment |
| TTVR | Not suitable in case of severe RV dysfunction, extreme annular dilation, or unfavorable device access angle. |
| Heterotopic Valve Implantation | Requires adequate caval diameters and intercaval distance; Contraindicated if right atrium to hepatic veins distance is <10–12 mm. |
| Criteria | T-TEER | TTVR |
|---|---|---|
| TR Etiology | Preferably functional TR | Both functional, PMK-associated and primary TR |
| TR Jet | Preferably central TR jet origin | Effective regardless of TR jet origin |
| Anatomical Suitability | Requires adequate leaflet tissue for grasping or coaptation | Less dependent on native leaflet morphology |
| Coaptation Gap | <8.5 mm generally preferred | Larger coaptation gaps acceptable |
| Pacemaker/ICD Leads | Not effective if TR is lead-induced | Challenging if patient recently received lead implantation (within 3 months) |
| Durability Considerations | Long-term durability uncertain | Device durability under investigation |
| Imaging Requirements | High-quality TEE or ICE for guidance | Requires comprehensive multimodality imaging (TEE and/or ICE, and CT) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, V.M.; Censi, S.; Conti, R.; Nerla, R.; Bombace, S.; Ruf, T.F.; von Bardeleben, R.S.; Lurz, P.; Castriota, F.; Squeri, A. Tricuspid Regurgitation in the Era of Transcatheter Interventions: The Pivotal Role of Multimodality Imaging. J. Clin. Med. 2025, 14, 5011. https://doi.org/10.3390/jcm14145011
De Luca VM, Censi S, Conti R, Nerla R, Bombace S, Ruf TF, von Bardeleben RS, Lurz P, Castriota F, Squeri A. Tricuspid Regurgitation in the Era of Transcatheter Interventions: The Pivotal Role of Multimodality Imaging. Journal of Clinical Medicine. 2025; 14(14):5011. https://doi.org/10.3390/jcm14145011
Chicago/Turabian StyleDe Luca, Valeria Maria, Stefano Censi, Rita Conti, Roberto Nerla, Sara Bombace, Tobias Friedrich Ruf, Ralph Stephan von Bardeleben, Philipp Lurz, Fausto Castriota, and Angelo Squeri. 2025. "Tricuspid Regurgitation in the Era of Transcatheter Interventions: The Pivotal Role of Multimodality Imaging" Journal of Clinical Medicine 14, no. 14: 5011. https://doi.org/10.3390/jcm14145011
APA StyleDe Luca, V. M., Censi, S., Conti, R., Nerla, R., Bombace, S., Ruf, T. F., von Bardeleben, R. S., Lurz, P., Castriota, F., & Squeri, A. (2025). Tricuspid Regurgitation in the Era of Transcatheter Interventions: The Pivotal Role of Multimodality Imaging. Journal of Clinical Medicine, 14(14), 5011. https://doi.org/10.3390/jcm14145011





