Concomitant Transcatheter Edge-to-Edge Repair and Left Atrial Appendage Occlusion
Abstract
1. Introduction
2. Materials and Methods
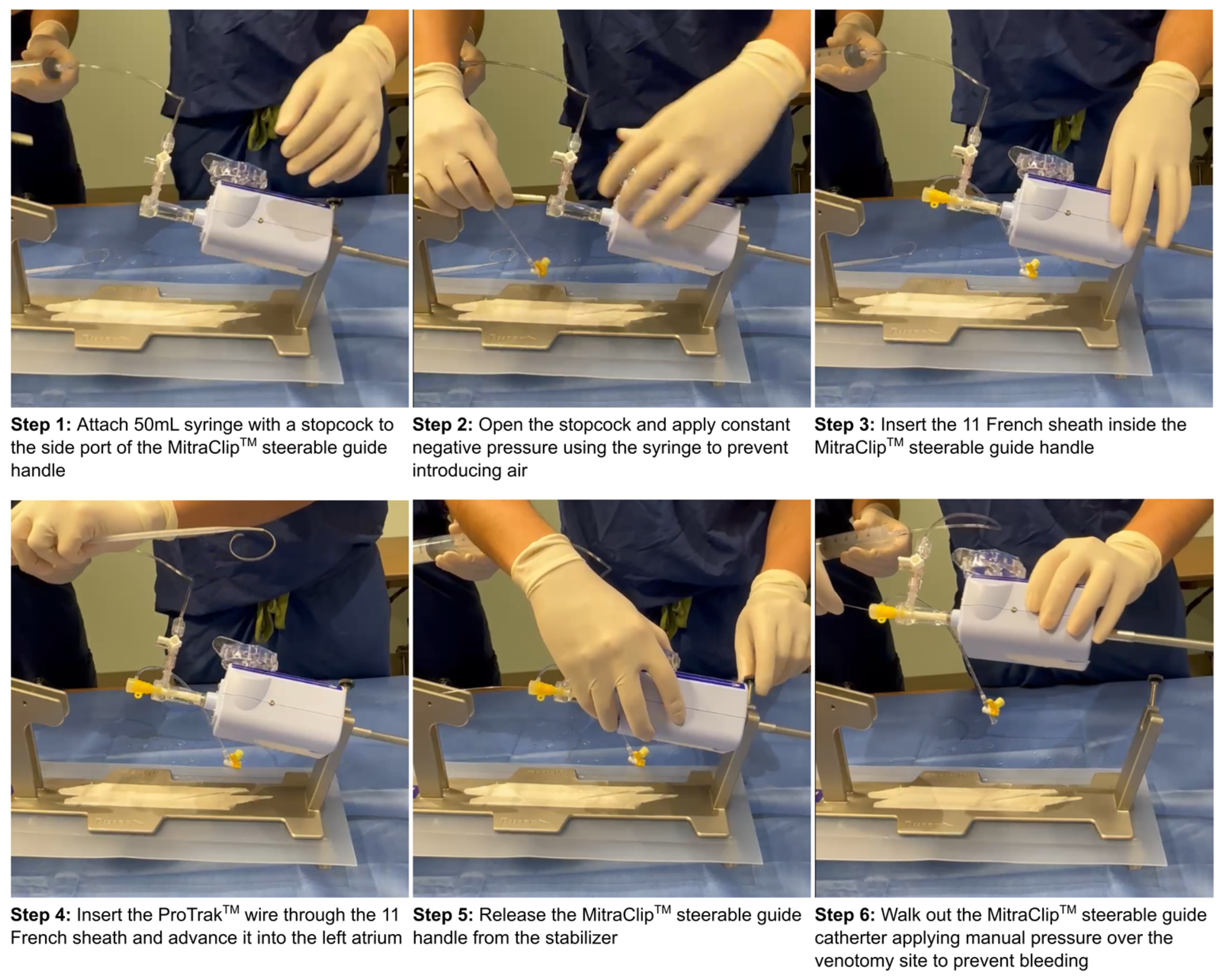
2.1. Procedural Characteristics
2.2. Outcomes
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| DRT | Device related thrombus |
| LAAO | Left atrial appendage occlusion |
| M-TEER | Mitral transcatheter edge-to-edge repair |
| MR | Mitral regurgitation |
| OAC | Oral anticoagulant |
| PDL | Peri-device leak |
| TEE | Transesophageal echocardiogram |
References
- Stone, G.W.; Abraham, W.T.; Lindenfeld, J.; Kar, S.; Grayburn, P.A.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Rinaldi, M.; Kapadia, S.R.; et al. Five-Year Follow-up after Transcatheter Repair of Secondary Mitral Regurgitation. N. Engl. J. Med. 2023, 388, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef]
- Anker, S.D.; Friede, T.; Bardeleben, R.-S.v.; Butler, J.; Khan, M.-S.; Diek, M.; Heinrich, J.; Geyer, M.; Placzek, M.; Ferrari, R.; et al. Transcatheter Valve Repair in Heart Failure with Moderate to Severe Mitral Regurgitation. N. Engl. J. Med. 2024, 391, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.; Carroll, J.D.; Thourani, V.; Vemulapalli, S.; Squiers, J.; Manandhar, P.; Deeb, G.M.; Batchelor, W.; Herrmann, H.C.; Cohen, D.J.; et al. Transcatheter Mitral Valve Therapy in the United States: A Report From the STS-ACC TVT Registry. J. Am. Coll. Cardiol. 2021, 78, 2326–2353. [Google Scholar] [CrossRef]
- Arora, S.; Vemulapalli, S.; Stebbins, A.; Ramm, C.J.; Kosinski, A.S.; Sorajja, P.; Piccini, J.P.; Cavender, M.A.; Vavalle, J.P. The Prevalence and Impact of Atrial Fibrillation on 1-Year Outcomes in Patients Undergoing Transcatheter Mitral Valve Repair: Results From the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. JACC Cardiovasc. Interv. 2019, 12, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Pamporis, K.; Siontis, K.C.; Theofilis, P.; Samaras, A.; Patoulias, D.; Stachteas, P.; Karagiannidis, E.; Stavropoulos, G.; Tzikas, A.; et al. Major clinical outcomes in symptomatic vs. asymptomatic atrial fibrillation: A meta-analysis. Eur. Heart J. 2024, ehae694. [Google Scholar] [CrossRef]
- Fernandes, J.M.; Pinheiro, R.P.S.; Serpa, F.; de Andrade, N.M.; Pereira, V.; Sbardelotto, Â.E.E.; Gomes, W.F. Left atrial appendage occlusion devices vs direct oral anticoagulants for atrial fibrillation: An updated systematic review and meta-analysis. Curr. Probl. Cardiol. 2025, 50, 102880. [Google Scholar] [CrossRef]
- Wazni, O.M.; Saliba, W.I.; Nair, D.G.; Marijon, E.; Schmidt, B.; Hounshell, T.; Ebelt, H.; Skurk, C.; Oza, S.; Patel, C.; et al. Left Atrial Appendage Closure after Ablation for Atrial Fibrillation. N. Engl. J. Med. 2024. [Google Scholar] [CrossRef]
- Ismayl, M.; Ahmed, H.; Freeman, J.V.; Alkhouli, M.; Lakkireddy, D.; Goldsweig, A.M. Safety and Efficacy of Combining Left Atrial Appendage Occlusion With Another Cardiac Procedure. JACC Cardiovasc. Interv. 2024, 17, 262–273. [Google Scholar] [CrossRef]
- D’Amico, G.; Estèvez-Loureiro, R.; Rofastes, X.F.; Ronco, F.; Nombela-Franco, L.; Melica, B.; Bedogni, F.; Saia, F.; Cruz-Gonzalez, I.; Tarantini, G. Combined Procedure of Percutaneous Mitral Valve Repair and Left Atrial Appendage Occlusion: A Multicenter Study. JACC Cardiovasc. Interv. 2021, 14, 590–592. [Google Scholar] [CrossRef]
- Freeman James, V.; Varosy, P.; Price Matthew, J.; Slotwiner, D.; Kusumoto Fred, M.; Rammohan, C.; Kavinsky Clifford, J.; Turi Zoltan, G.; Akar, J.; Koutras, C.; et al. The NCDR Left Atrial Appendage Occlusion Registry. J. Am. Coll. Cardiol. 2020, 75, 1503–1518. [Google Scholar] [CrossRef] [PubMed]
- von Bardeleben, R.S.; Mahoney, P.; Morse, M.A.; Price, M.J.; Denti, P.; Maisano, F.; Rogers, J.H.; Rinaldi, M.; De Marco, F.; Rollefson, W.; et al. 1-Year Outcomes With Fourth-Generation Mitral Valve Transcatheter Edge-to-Edge Repair From the EXPAND G4 Study. JACC Cardiovasc. Interv. 2023, 16, 2600–2610. [Google Scholar] [CrossRef]
- Kapadia, S.R.; Krishnaswamy, A.; Whisenant, B.; Potluri, S.; Iyer, V.; Aragon, J.; Gideon, P.; Strote, J.; Leonardi, R.; Agarwal, H.; et al. Concomitant Left Atrial Appendage Occlusion and Transcatheter Aortic Valve Replacement Among Patients With Atrial Fibrillation. Circulation 2024, 149, 734–743. [Google Scholar] [CrossRef]
- Saw, J.; Inohara, T.; Gilhofer, T.; Uchida, N.; Pearce, C.; Dehghani, P.; Kass, M.; Ibrahim, R.; Morillo, C.; Wardell, S.; et al. The Canadian WATCHMAN Registry for Percutaneous Left Atrial Appendage Closure. CJC Open 2023, 5, 522–529. [Google Scholar] [CrossRef]
- Kuwata, S.; Taramasso, M.; Zuber, M.; Suetsch, G.; Attinger-Toller, A.; Wicki, D.; Maisano, F.; Nietlispach, F. Feasibility of concomitant MitraClip and left atrial appendage occlusion. EuroIntervention 2017, 12, 1940–1945. [Google Scholar] [CrossRef]
- Tichelbäcker, T.; Puls, M.; Jacobshagen, C.; Hasenfuß, G.; Schillinger, W.; Hünlich, M.; Schroeter, M.R. MitraClip® and Amplatzer® cardiac plug implantation in a single procedure: A reasonable approach? Int. J. Cardiol. 2016, 220, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Francisco, A.R.G.; Infante de Oliveira, E.; Nobre Menezes, M.; Carrilho Ferreira, P.; Canas da Silva, P.; Nobre, Â.; Pinto, F.J. Combined MitraClip implantation and left atrial appendage occlusion using the Watchman device: A case series from a referral center. Rev. Port. Cardiol. 2017, 36, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Freixa, X.; Estévez-Loureiro, R.; Carrasco-Chinchilla, F.; Arzamendi, D.; Jiménez-Quevedo, P.; Nombela-Franco, L.; Cruz-González, I.; Amat-Santos, I.J.; Sabaté, M. Initial Results of Combined MitraClip® Implantation and Left Atrial Appendage Occlusion. J. Heart Valve Dis. 2017, 26, 169–174. [Google Scholar]
- Al-Abcha, A.; Di Santo, P.; Rihal, C.S.; Simard, T.; Hibbert, B.; Alkhouli, M. Outcomes of Combined Left Atrial Appendage Occlusion and Transcatheter Mitral Edge-to-Edge Repair: The WATCH-TEER Study. JACC Adv. 2025, 4, 101541. [Google Scholar] [CrossRef]
- Afzal Muhammad, R.; Gabriels James, K.; Jackson Gregory, G.; Chen, L.; Buck, B.; Campbell, S.; Sabin Dawn, F.; Goldner, B.; Ismail, H.; Liu Christopher, F.; et al. Temporal Changes and Clinical Implications of Delayed Peridevice Leak Following Left Atrial Appendage Closure. JACC Clin. Electrophysiol. 2022, 8, 15–25. [Google Scholar] [CrossRef]
- Alkhouli, M.; Busu, T.; Shah, K.; Osman, M.; Alqahtani, F.; Raybuck, B. Incidence and Clinical Impact of Device-Related Thrombus Following Percutaneous Left Atrial Appendage Occlusion. JACC Clin. Electrophysiol. 2018, 4, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Price, M.J.; Valderrábano, M.; Zimmerman, S.; Friedman, D.J.; Kar, S.; Curtis, J.P.; Masoudi, F.A.; Freeman, J.V. Periprocedural Pericardial Effusion Complicating Transcatheter Left Atrial Appendage Occlusion: A Report From the NCDR LAAO Registry. Circ. Cardiovasc. Interv. 2022, 15, e011718. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.B.; Khan, M.Z.; Darden, D.; Pasupula, D.K.; Balla, S.; Han, F.T.; Reeves, R.; Hsu, J.C. Pericardial effusion requiring intervention in patients undergoing percutaneous left atrial appendage occlusion: Prevalence, predictors, and associated in-hospital adverse events from 17,700 procedures in the United States. Heart Rhythm. 2021, 18, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.G.; Abdel-Razek, O.; Di Santo, P.; Gillmore, T.; Stotts, C.; Makwana, D.; Soriano, J.; Moreland, R.; Verreault-Julien, L.; Goh, C.Y.; et al. Impact of atrial fibrillation on the risk of major adverse cardiac events following coronary revascularisation. Open Heart 2022, 9, e002012. [Google Scholar] [CrossRef]
- Urena, M.; Webb, J.G.; Eltchaninoff, H.; Munoz-Garcia, A.J.; Bouleti, C.; Tamburino, C.; Nombela-Franco, L.; Nietlispach, F.; Moris, C.; Ruel, M.; et al. Late cardiac death in patients undergoing transcatheter aortic valve replacement: Incidence and predictors of advanced heart failure and sudden cardiac death. J. Am. Coll. Cardiol. 2015, 65, 437–448. [Google Scholar] [CrossRef]
- Feldman, T.; Foster, E.; Glower, D.D.; Kar, S.; Rinaldi, M.J.; Fail, P.S.; Smalling, R.W.; Siegel, R.; Rose, G.A.; Engeron, E.; et al. Percutaneous repair or surgery for mitral regurgitation. N. Engl. J. Med. 2011, 364, 1395–1406. [Google Scholar] [CrossRef]
- Friberg, L.; Rosenqvist, M.; Lip, G.Y. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: The Swedish Atrial Fibrillation cohort study. Eur. Heart J. 2012, 33, 1500–1510. [Google Scholar] [CrossRef]
- Cameli, M.; Lunghetti, S.; Mandoli, G.E.; Righini, F.M.; Lisi, M.; Curci, V.; Tommaso, C.D.; Solari, M.; Nistor, D.; Gismondi, A.; et al. Left Atrial Strain Predicts Pro-Thrombotic State in Patients with Non-Valvular Atrial Fibrillation. J. Atr. Fibrillation 2017, 10, 1641. [Google Scholar] [CrossRef]
- Jung, R.G.; Stotts, C.; Gupta, A.; Prosperi-Porta, G.; Dhaliwal, S.; Motazedian, P.; Abdel-Razek, O.; Santo, P.D.; Parlow, S.; Belley-Cote, E.; et al. Prognostic Factors Associated with Mortality in Cardiogenic Shock—A Systematic Review and Meta-Analysis. NEJM Evid. 2024, 3, EVIDoa2300323. [Google Scholar] [CrossRef]
| M-TEER + LAAO (n = 15) | |
|---|---|
| Age, Years (IQR) | 80.0 (76.0–85.0) |
| Female, n (%) | 3 (20%) |
| Prior Myocardial Infarction, n (%) | 4 (27%) |
| Diabetes, n (%) | 5 (33%) |
| Hypertension, n (%) | 10 (67%) |
| Dyslipidemia, n (%) | 6 (40%) |
| Smoking, n (%) | 3 (20%) |
| Creatinine (mg/dL) | 1.4 (1.1–2.5) |
| Prior Stroke/CVA, n (%) | 3 (20%) |
| Peripheral Arterial Disease, n (%) | 1 (7%) |
| Chronic Obstructive Pulmonary Disease, n (%) | 2 (13%) |
| Left Ventricular Ejection Fraction, % (IQR) | 45% (40–55%) |
| Mitral Regurgitation Mechanism | |
| Degenerative, n (%) | 8 (53%) |
| Functional, n (%) | 7 (47%) |
| Mitral Regurgitation Severity | |
| 3+ | 5 (33%) |
| 4+ | 10 (67%) |
| Atrial Fibrillation | |
| Permanent, n (%) | 10 (67%) |
| Paroxysmal, n (%) | 5 (33%) |
| NYHA Class | |
| II, n (%) | 9 (6%) |
| III, n (%) | 6 (40%) |
| IV, n (%) | 0 (0%) |
| CHA2DS2-VaSC Score | 5 (4–6) |
| HAS-BLED Score | 3 (3–3) |
| M-TEER + LAAO (n = 15) | |
|---|---|
| Total procedure time, min (IQR) | 111 (93–124) |
| Total fluoroscopy time, min (IQR) | 31 (22–45) |
| General anesthesia | 15 (100%) |
| Transesophageal guidance | 15 (100%) |
| M-TEER Procedure | |
| Procedure completed | 15 (100%) |
| Duration of procedure, min (IQR) | 85 (80–104) |
| #of MitraClip™ deployed per case | |
| 1 | 9 (60) |
| 2 | 3 (20) |
| 3 | 3 (20) |
| Type of devices deployed | |
| XT | 0 (0%) |
| NT | 5 (21%) |
| XTW | 7 (29%) |
| NTW | 12 (50%) |
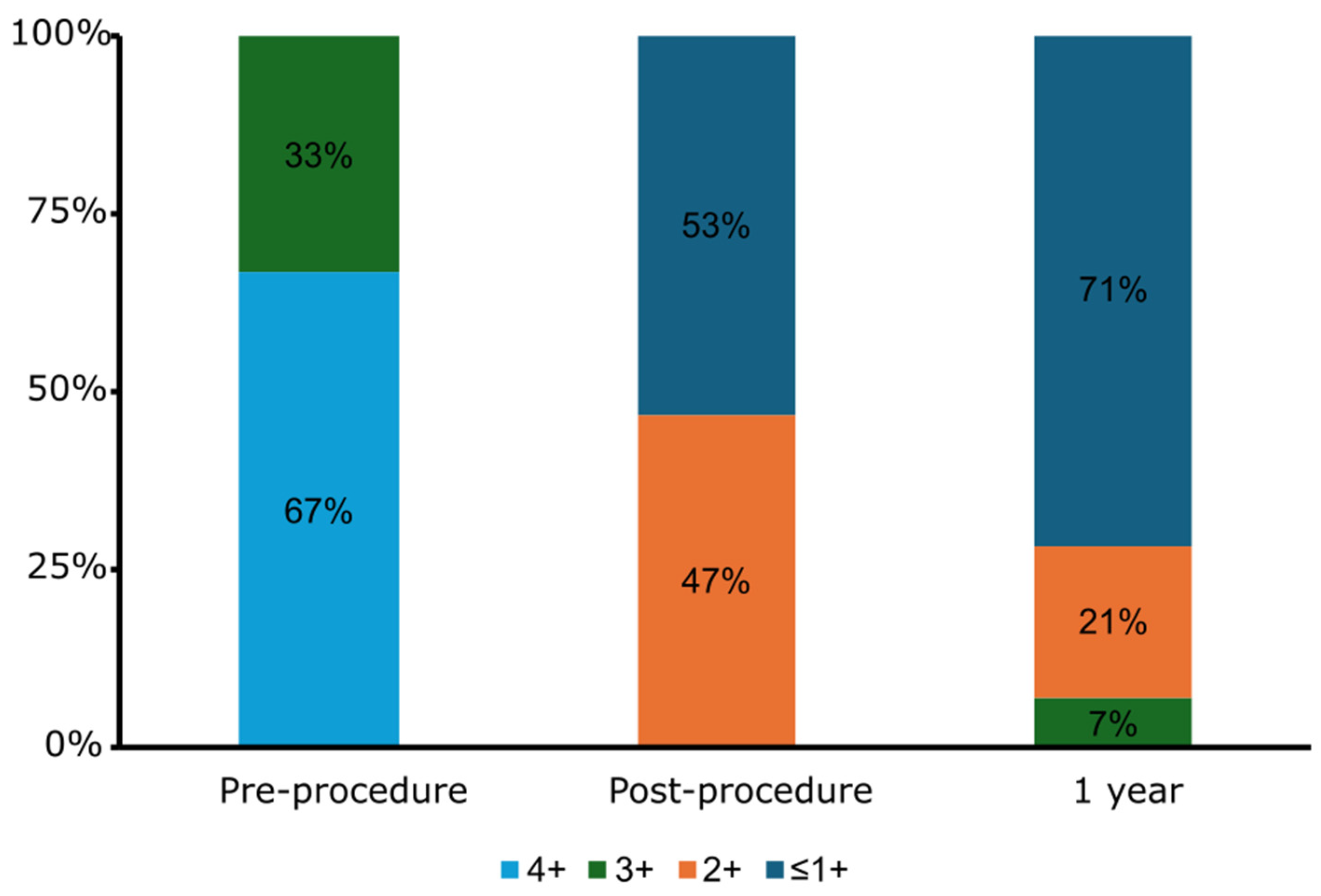
| Residual MR | |
| ≤1+ | 9 (53%) |
| 2+ | 6 (47%) |
| 3+ | 0 (0%) |
| 4+ | 0 (0%) |
| Post-M-TEER mitral valve gradient, mmHg (IQR) | 3.9 (3.4–4.5) |
| LAAO Procedure | |
| MitraClip™ sheath removal to LAAO deployment, min (IQR) | 15 (11–29) |
| LAAO device used | |
| Amplatzer™ Cardiac Plug | 2 (13.3) |
| WATCHMAN™ FLX | 2 (13.3) |
| Amulet™ | 11 (73.3) |
| Device margin leak > 3 mm | 0 (0%) |
| ASD Closure | 4 (27%) |
| Baseline (n = 15) | Post-Procedure (n = 15) | 1 Year (n = 14) | |
|---|---|---|---|
| Aspirin | 2 (13%) | 0 | 5 (36%) |
| P2Y12 | 0 | 0 | 0 |
| DAPT | 2 (13%) | 9 (60%) | 1 (7%) |
| Warfarin | 0 | 0 | 0 |
| LMWH | 0 | 0 | 1 (7%) |
| DOAC | 8 (53%) | 6 (40%) | 3 (21%) |
| No antithrombotic therapy | 3 (20%) | 0 | 4 (29%) |
| 0–45 Days a (n = 15) | 45 Days–1 Year (n = 15) | Cumulative Insidence at 1 Year (n = 15) | |
|---|---|---|---|
| All-cause death | 0 | 1 (7%) | 1 (7%) |
| Cardiac death | 0 | 1 (7%) | 1 (7%) |
| Non-cardiac death | 0 | 0 | 0 |
| HF hospitalization | 0 | 0 | 0 |
| Major bleeding | 0 | 1 (7%) | 1 (7%) |
| Life threatening bleeding | 1 (7%) | 0 | 1 (7%) |
| Stroke or TIA | 0 | 0 | 0 |
| Hemorrhagic stroke | 0 | 0 | 0 |
| Vascular complications | 0 | 0 | 0 |
| Myocardial infarction | 0 | 0 | 0 |
| New dialysis | 0 | 0 | 0 |
| Endocarditis | 0 | 0 | 0 |
| Mitral valve-related intervention | 0 | 0 | 0 |
| Unplanned cardiac intervention or surgery | 0 | 0 | 0 |
| M-TEER embolization | 0 | 0 | 0 |
| LAAO-related intervention | 0 | 0 | 0 |
| LAAO thrombus | 0 | 0 | 0 |
| LAAO migration | 0 | 0 | 0 |
| LAAO embolization | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prosperi-Porta, G.; Dryden, A.; Nicholson, D.; Hynes, M.; Chan, V.; Jung, R.G.; Di Santo, P.; Simard, T.; Labinaz, M.; Hibbert, B.; et al. Concomitant Transcatheter Edge-to-Edge Repair and Left Atrial Appendage Occlusion. J. Clin. Med. 2025, 14, 2257. https://doi.org/10.3390/jcm14072257
Prosperi-Porta G, Dryden A, Nicholson D, Hynes M, Chan V, Jung RG, Di Santo P, Simard T, Labinaz M, Hibbert B, et al. Concomitant Transcatheter Edge-to-Edge Repair and Left Atrial Appendage Occlusion. Journal of Clinical Medicine. 2025; 14(7):2257. https://doi.org/10.3390/jcm14072257
Chicago/Turabian StyleProsperi-Porta, Graeme, Adam Dryden, Donna Nicholson, Mark Hynes, Vincent Chan, Richard G. Jung, Pietro Di Santo, Trevor Simard, Marino Labinaz, Benjamin Hibbert, and et al. 2025. "Concomitant Transcatheter Edge-to-Edge Repair and Left Atrial Appendage Occlusion" Journal of Clinical Medicine 14, no. 7: 2257. https://doi.org/10.3390/jcm14072257
APA StyleProsperi-Porta, G., Dryden, A., Nicholson, D., Hynes, M., Chan, V., Jung, R. G., Di Santo, P., Simard, T., Labinaz, M., Hibbert, B., & Abdel-Razek, O. (2025). Concomitant Transcatheter Edge-to-Edge Repair and Left Atrial Appendage Occlusion. Journal of Clinical Medicine, 14(7), 2257. https://doi.org/10.3390/jcm14072257





