Real-World Effectiveness and Safety of Upadacitinib in Patients with Ulcerative Colitis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
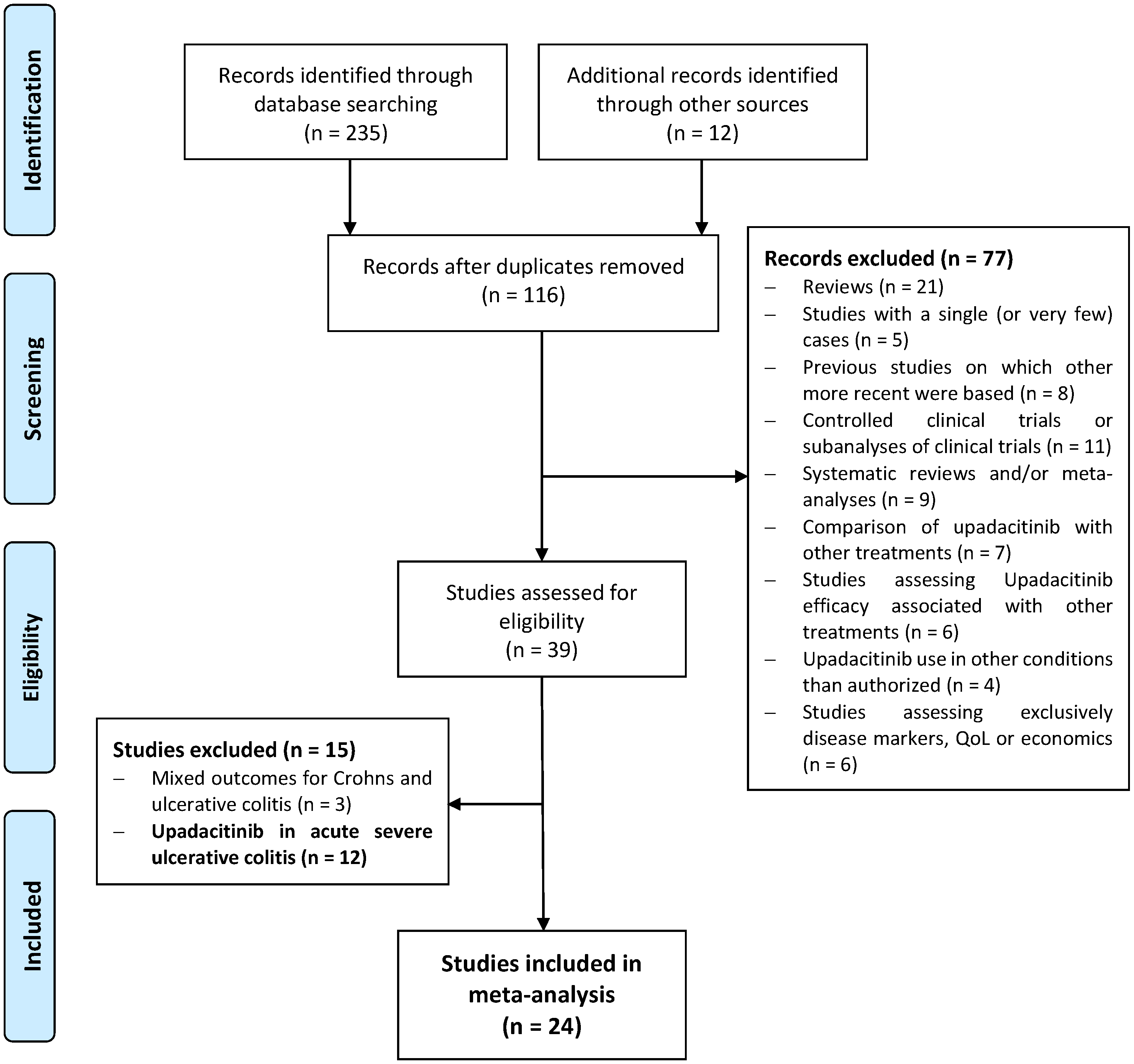
2.1. Search Strategy and Study Selection
2.2. Data Extraction and Outcome Measures
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Demographics and Characteristics of UC Populations
3.2. Primary Endpoint: Clinical Remission
3.3. Clinical Response
3.4. Steroid-Free Clinical Remission
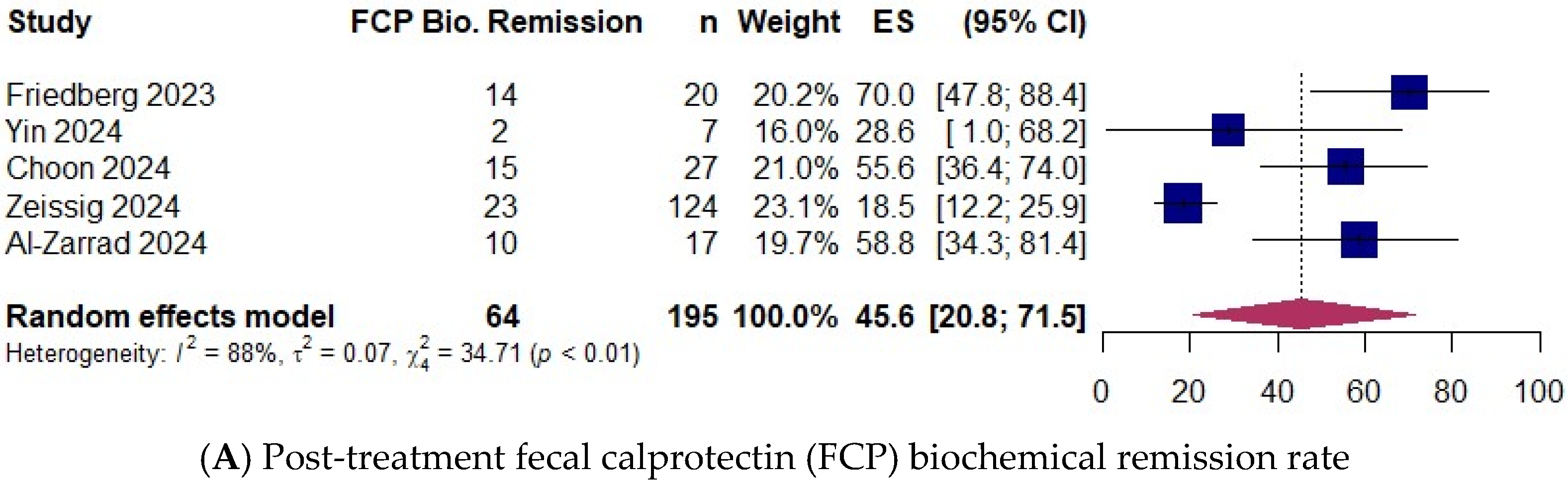
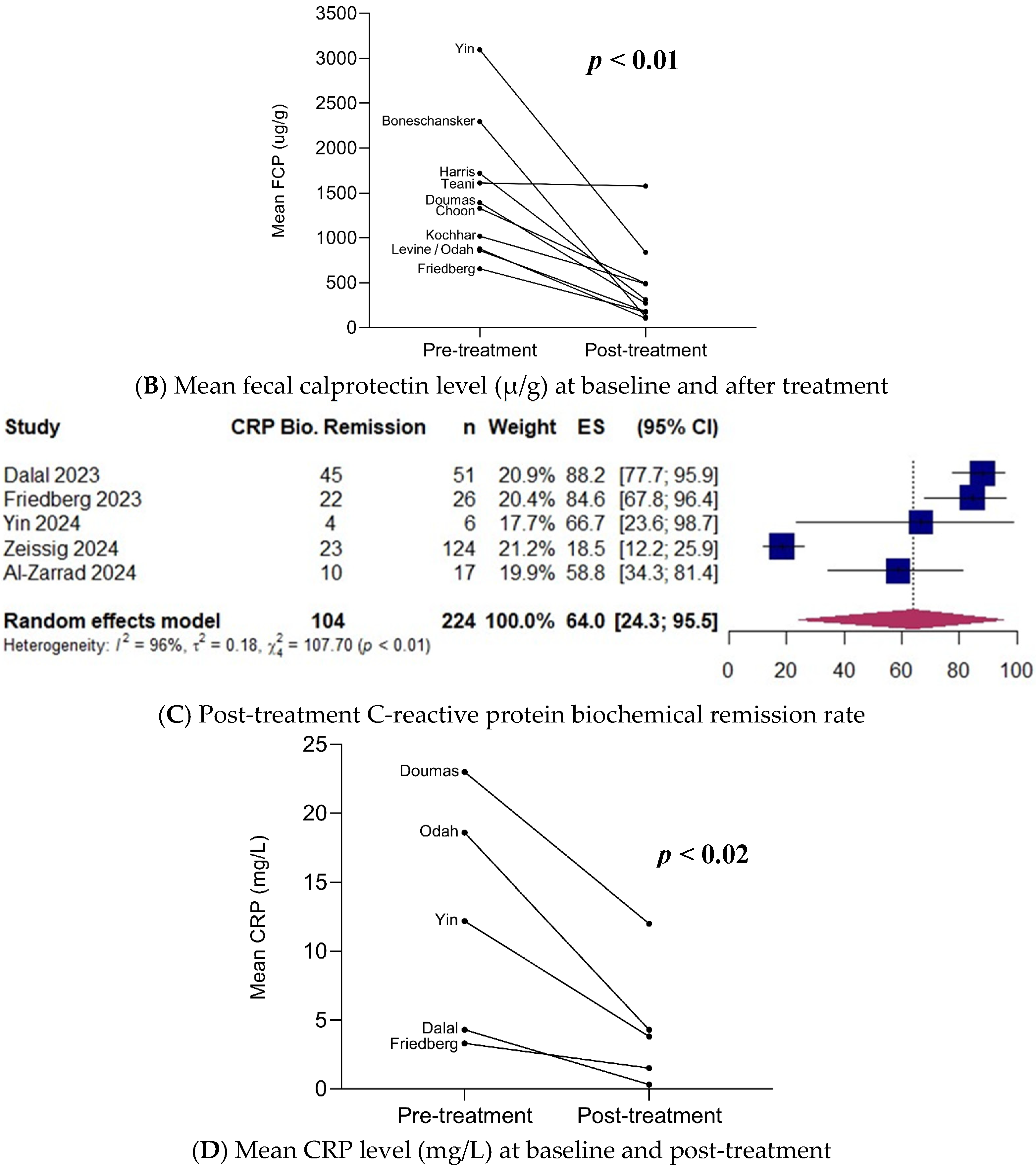
3.5. Biochemical Remission
3.6. Clinical Remission with Upadacitinib as Second-Line JAKi
3.7. Subgroup Analysis
3.8. Colectomy Rates
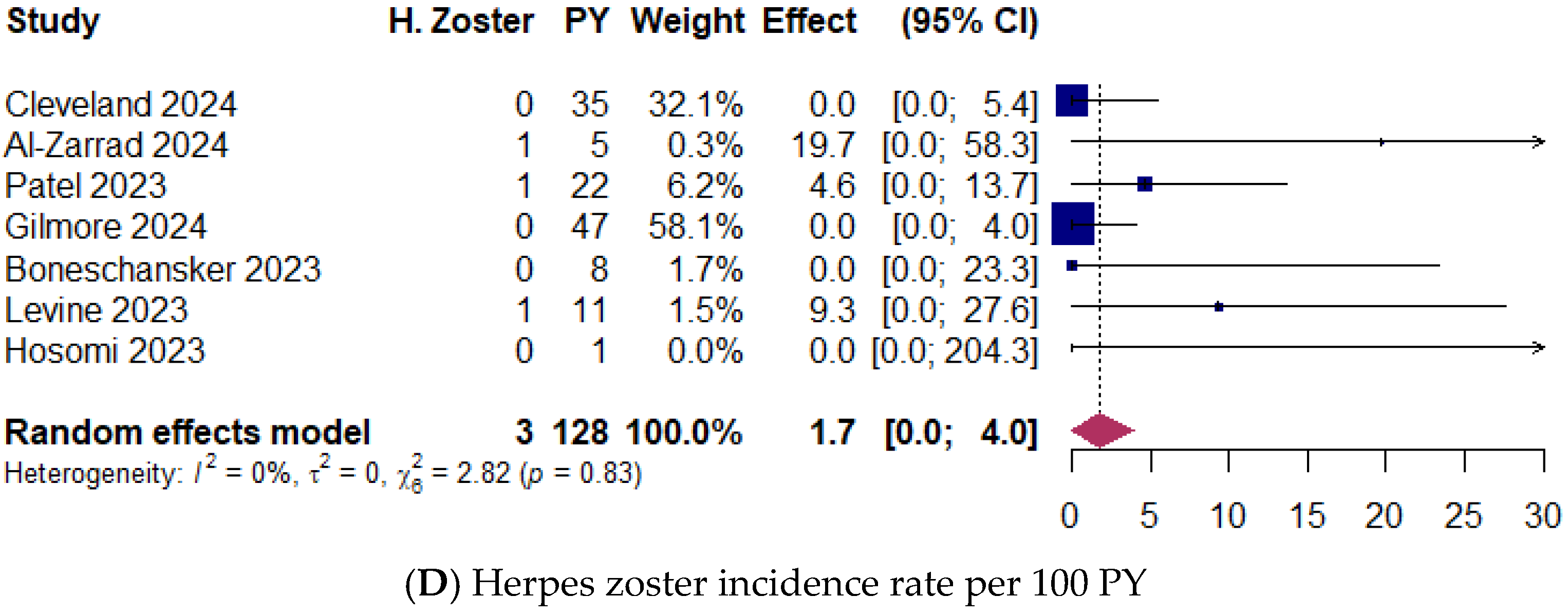
3.9. Upadacitinib Safety
3.10. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, C.M.; Cooles, F.A.; Isaacs, J.D. Basic Mechanisms of JAK Inhibition. Mediterr. J. Rheumatol. 2020, 31 (Suppl. 1), 100–104. [Google Scholar] [CrossRef]
- Clark, J.D.; Flanagan, M.E.; Telliez, J.B. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J. Med. Chem. 2014, 57, 5023–5038. [Google Scholar] [CrossRef]
- Boland, B.S.; Sandborn, W.J.; Chang, J.T. Update on Janus kinase antagonists in inflammatory bowel disease. Gastroenterol. Clin. N. Am. 2014, 43, 603–617. [Google Scholar] [CrossRef]
- Danese, S.; Vermeire, S.; Zhou, W.; Pangan, A.L.; Siffledeen, J.; Greenbloom, S.; Hébuterne, X.; D’Haens, G.; Nakase, H.; Panés, J.; et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: Results from three phase 3, multicentre, double-blind, randomised trials. Lancet 2022, 399, 2113–2128. [Google Scholar] [PubMed]
- Panaccione, R.; Lichtenstein, G.; Nakase, H.; Armuzzi, A.; Kucharzik, T.; Levy, G.; Palac, H.; Kujawski, M.; Klaff, J.; Cheon, J.H. Safety of upadacitinib in ulcerative colitis: Long-term data from the phase 3 open-label extension study (U-ACTIVATE). Gastroenterology 2023, 164, S-1100. [Google Scholar]
- Ha, C.; Ullman, T.A.; Siegel, C.A.; Kornbluth, A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin. Gastroenterol. Hepatol. 2012, 10, 1002–1007. [Google Scholar] [CrossRef]
- Dalal, R.S.; Kallumkal, G.; Cabral, H.J.; Bachour, S.; Barnes, E.L.; Allegretti, J.R. Clinical and Endoscopic Outcomes After Upadacitinib Induction for Ulcerative Colitis: A Multicenter Retrospective Cohort Study. Inflamm. Bowel Dis. 2024, 30, 1207–1210. [Google Scholar]
- Friedberg, S.; Choi, D.; Hunold, T.; Choi, N.K.; Garcia, N.M.; Picker, E.A.; Cohen, N.A.; Cohen, R.D.; Dalal, S.R.; Pekow, J.; et al. Upadacitinib Is Effective and Safe in Both Ulcerative Colitis and Crohn’s Disease: Prospective Real-World Experience. Clin. Gastroenterol. Hepatol. 2023, 21, 1913–1923.e2. [Google Scholar]
- Cleveland, N.K.; Choi, N.K.; Klein, J.A.; Fear, E.N.; Fine, Z.D.; Garcia, N.M.; Picker, E.A.; Friedberg, S.; Cohen, R.D.; Dalal, S.R.; et al. Mo1848 Upadacitinib is effective and safe for the treatment of ulcerative colitis and crohn’s disease: 1-year prospective real-world experience. Gastroenterology 2024, 166, S1141–S1142. [Google Scholar]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Metaanalysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Based Health 2015, 13, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xu, C. Arcsine-based transformations for meta-analysis of proportions: Pros, cons, and alternatives. Health Sci. Rep. 2020, 3, e178. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, Version 6.0; 2019. Available online: https://training.cochrane.org/handbook/archive/v6 (accessed on 12 July 2024).
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Meta-Analysis with R; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-21415-3. [Google Scholar] [CrossRef]
- Yin, J.; El-Najjar, Y.; Cordova, N.; Touma, M.J.; Nguyen, N.; Boktor, M.; Burstein, E.; Fudman, D.I. Short-Term Use of Upadacitinib in Combination With Biologic Therapy for Inducing Clinical Remission in Patients With Active Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2024, 30, 1914–1916. [Google Scholar] [CrossRef]
- Choon, X.Y.; Patel, C.; White, C.; Centritto, A.; Lal, N.; Sharma, E.; Chester, D.; Dart, R.; Anderson, S.; Ray, S.; et al. P1072 Upadacitinib appears effective in inducing clinical, biochemical, endoscopic and histologic improvements in previously treatment refractory Ulcerative Colitis. J. Crohn’s Colitis 2024, 18 (Suppl. 1), i1926. [Google Scholar] [CrossRef]
- Kaniewska, M.; Krogulecki, M.; Lewandowski, K.; Filipiuk, A.; Gonciarz, M.; Rydzewska, G. P1031 Intestinal ultrasound for monitoring therapeutic response in patients with ulcerative colitis treated with upadacitinib. J. Crohn’s Colitis 2024, 18 (Suppl. 1), i1860. [Google Scholar]
- Zeissing, S.; Schmelz, R.; Helwig, U.; Moschen, A.R.; Greuter, T.; Fischer, I.; Hammer, L.; Rath, S.; Kucharzik, T.; Maaser, C. Sa1765 Symptomatic remission and IUS improvements in a multinational real-world cohort of CU patients treated with Upadacitinib—First results from the IBD-DACH study EUROPE. Gastroenterology 2024, 166, S513. [Google Scholar]
- Al-Zarrad, D.; Yeung, K.; Arebi, N.; Dyall, L.; Kamperidis, N. P718 Observational real-world evidence on the efficacy and safety of Janus Kinase inhibitors (JAKi) in the treatment of moderate to severe Active Ulcerative Colitis (UC). J. Crohn’s Colitis 2024, 18 (Suppl. 1), i1350. [Google Scholar] [CrossRef]
- Bhatia, K.; Mahadevan, U. Real world effectiveness of jak inhibitor upadacitinib in ulcerative colitis versus Crohn’s disease in an IBD tertiary care center. Gastroenterology 2024, 166, S10. [Google Scholar] [CrossRef]
- Teani, I.; Bevilacqua, N.; Gabbiadini, R.; Bezzio, C.; Ferronato, A.; Saibeni, S.; Armuzzi, A.; Comberlato, M.; Desideri, F. P535 Effectiveness of upadacitinib in patients with Ulcerative Colitis: A real-life, multicenter, Italian report. J. Crohn’s Colitis 2024, 18 (Suppl. 1), i1052–i1053. [Google Scholar] [CrossRef]
- García, M.; Brenes, Y.; Vicuña, M.; Bermejo, F.; Sierra-Ausín, M.; Cuadro, C.P.; Arroyo, M.T.; Montiel, P.M.; Villoria, A.; Pérez-Calle, J.L.; et al. P569 Persistence and safety of upadacitinib in Crohn’s disease and ulcerative colitis in real life: Results from a Spanish nationwide study. J. Crohn’s Colitis 2024, 18 (Suppl. 1), i1112–i1113. [Google Scholar]
- Harris, C.; Gee, T.; Barcan, A.; Yanagisawa, Y.; Brown, M.; Gordon, J.N. P589 Real-world data on upadacitinib in the treatment of inflammatory bowel disease: Safe and highly effective with extremely positive patient feedback. J. Crohn’s Colitis 2024, 18 (Suppl. 1), i1147. [Google Scholar]
- Annadurai, V.; Axenfeld, E.; Faye, A.; Axelrad, J. Mo1859 Treatment outcomes of prolonged induction or re-escalation dosing of upadacitinib in patients with inflammatory bowel disease. Gastroenterology 2024, 166, S1147. [Google Scholar]
- Patel, A.; Johnson, A.M.; Berinstein, J.; Dulaney, D.; Fenster, M.; Ayoub, M.; Huang, K.; Lieto, S.; Scalzo, N.; Loftus, E.V.; et al. Tu1806 Real-world effectiveness and safety of upadacitinib in ulcerative colitis: A multicenter study. Gastroenterology 2023, 164, S-1136–S-1137. [Google Scholar]
- Chowla, N.; Tariq, R.; Aggarwal, M.; Loftus, E.V. S1075 Real-World Experience of Upadacitinib in Treatment of Adults with Moderate to Severe Ulcerative Colitis. Am. J. Gastroenterol. 2023, 118, S820. [Google Scholar]
- Doumas, S.; Dahmani, S.; Singh, H.; Chi, P.; Mattar, M. S999 Upadacitinib in Ulcerative Colitis: Early Experience from a Large Regional Health System. Am. J. Gastroenterol. 2023, 118, S756. [Google Scholar]
- Kochhar, G.S.; Khataniar, H.; Jairath, V.; Farraye, F.A.; Desai, A. Comparative Effectiveness of Upadacitinib and Tofacitinib in Ulcerative Colitis: A U.S. Propensity-Matched Cohort Study. Am. J. Gastroenterol. 2024, 19, 2471–2479. [Google Scholar]
- Gilmore, R.; Fernandes, R.; Hartley, I.; Arzivian, A.; Leong, R.; Andrew, B.; Vasudevan, A.; Greeve, T.; Moore, G.; Kim, S.; et al. P1064 Upadacitinib is safe and effective in Ulcerative Colitis patients with prior exposure to Tofacitinib. J. Crohn’s Colitis 2024, 18 (Suppl. 1), i1913. [Google Scholar]
- Boneschansker, L.; Ananthakrishnan, A.N. Comparative Effectiveness of Upadacitinib and Tofacitinib in Inducing Remission in Ulcerative Colitis: Real-World Data. Clin. Gastroenterol. Hepatol. 2023, 21, 2427–2429.e1. [Google Scholar] [CrossRef]
- Levine, J.; McKibbin, J.; Ham, R.; Cohen-Mekelburg, S.; Bishu, S.; Tang, K.; Higgins, P.D.; Berinstein, J.A. Use of Upadacitinib in 16 Tofacitinib-refractory Ulcerative Colitis Patients: A Single-center Case 2 Series. Inflamm. Bowel Dis. 2023, 30, 2232–2235. [Google Scholar] [CrossRef]
- Hosomi, S.; Nishida, Y.; Fujiwara, Y. Efficacy of Upadacitinib as a Second-line JAK Inhibitor in Ulcerative Colitis: A Case Series. Intern. Med. 2024, 63, 1882–1885. [Google Scholar]
- Radia, C.; Abdel-Aziz, S.; Ratcliff, S.; Blake, T.; Medcalf, L.; Dubois, P.; Kent, A.; Pavlidis, P. P113 Upadacitinib in patients with steroid dependent ulcerative colitis refractory to advanced therapies including tofacitinib: A case series. Gut 2023, 72 (Suppl. 2), A112–A113. [Google Scholar]
- Cleveland, N.K.; Friedberg, S.; Choi, D.; Hunold, T.; Choi, N.K.; Garcia, N.M.; Picker, E.A.; Cohen, N.A.; Cohen, R.D.; Dalal, S.R.; et al. P724 Upadacitinib is Effective and Safe in Tofacitinib-Experienced Patients with Ulcerative Colitis: A Prospective Real-World Experience. J. Crohn’s Colitis 2023, 17 (Suppl. 1), i854–i856. [Google Scholar]
- Odah, T.; Karime, C.; Desai, A.; Picco, M.F.; Kinnucan, J.A.; Hashash, J.G.; Farraye, F.A. Response to Upadacitinib in Patients with Inflammatory Bowel Disease Previously Treated with Tofacitinib. Dig. Dis. Sci. 2024, 69, 3911–3919. [Google Scholar]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [PubMed]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar]
- Sandborn, W.J.; Su, C.; Sands, B.E.; Vermeire, S.; Schreiber, S.; Danese, S.; Feagan, B.G.; Reinisch, W.; Niezychowski, W.; Friedman, G.; et al. Tofacitinib as Induction and Maintenance Therapy for UC. N. Engl. J. Med. 2017, 376, 1723–1736. [Google Scholar]
- Taxonera, C.; Olivares, D.; Alba, C. Real-world effectiveness and safety of tofacitinib in patients with ulcerative colitis: Systematic review with meta-analysis. Inflamm. Bowel Dis. 2022, 28, 32–40. [Google Scholar]
- Taxonera, C.; Olivares, D.; López-García, O.N.; Alba, C. Meta-analysis: Real-world effectiveness and safety of ustekinumab in patients with ulcerative colitis. Aliment. Pharmacol. Ther. 2023, 57, 610–619. [Google Scholar]
- Schreiber, S.; Dignass, A.; Peyrin-Biroulet, L.; Hather, G.; Demuth, D.; Mosli, M.; Curtis, R.; Khalid, J.M.; Loftus, E.V., Jr. Systematic review with meta-analysis: Real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J. Gastroenterol. 2018, 53, 1048–1064. [Google Scholar]
- Lasa, J.S.; Olivera, P.A.; Danese, S.; Peyrin-Biroulet, L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: A systematic review and network meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 161–170. [Google Scholar] [PubMed]
- Loftus, E.V., Jr.; Colombel, J.F.; Takeuchi, K.; Gao, X.; Panaccione, R.; Danese, S.; Dubinsky, M.; Schreiber, S.; Ilo, D.; Finney-Hayward, T.; et al. Upadacitinib therapy reduces ulcerative colitis symptoms as early as day 1 of induction treatment. Clin. Gastroenterol. Hepatol. 2023, 21, 2347–2358. [Google Scholar] [PubMed]
- Lee, H.H.; Solitano, V.; Singh, S.; Ananthakrishnan, A.N.; Jairath, V.; Syal, G.; Boland, B.S.; Ghosh, P.; Chang, J.T.; Singh, S. Differential Efficacy of Advanced Therapies in Inducing Remission in Ulcerative Colitis Based on Prior Exposure to TNF Antagonists. Clin. Gastroenterol. Hepatol. 2024, 26. [Google Scholar] [CrossRef]
- Farkas, B.; Bessissow, T.; Limdi, J.K.; Sethi-Arora, K.; Kagramanova, A.; Knyazev, O.; Bezzio, C.; Armuzzi, A.; Lukas, M.; Michalopoulos, G.; et al. Real-World Effectiveness and Safety of Selective JAK Inhibitors in Ulcerative Colitis and Crohn’s Disease: A Retrospective, Multicentre Study. J. Clin. Med. 2024, 13, 7804. [Google Scholar] [CrossRef]
- Ma, C.; Panaccione, R.; Xiao, Y.; Khandelwal, Y.; Murthy, S.K.; Wong, E.C.L.; Narula, N.; Tsai, C.; Peerani, F.; Reise-Filteau, M.; et al. REMIT-UC: Real-World Effectiveness and Safety of Tofacitinib for Moderate-to-Severely Active Ulcerative Colitis: A Canadian IBD Research Consortium Multicenter National Cohort Study. Am. J. Gastroenterol. 2023, 118, 861–871. [Google Scholar]


| Study | No. Patients | Mean LFU (Weeks) | Sex Female (%) | Mean Age | Smokers (%) | Mean BMI (kg/m2) | Mean UC Duration (Years) | Extension Montreal Classification (%) | Prior Treatment (%) | Current Steroids | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E1 | E2 | E3 | Total Bio | =1 Bio | ≥2 Bio | Anti-TNF | VED | UST | TOF | |||||||||
| Dalal et al. [7] | 76 | 33.3 | 47.4 | 39.3 | 1.3 | 25.0 | 9.0 | - | - | - | 100 | - | - | 90.8 | 73.7 | 30.3 | 30.3 | 53.9 |
| Friedberg et al. [8] | 44 | 6.9 | 47.7 | 38.9 | 4.5 | - | 12.4 | 7.5 | 32.5 | 60.0 | 100 | 18.2 | 81.8 | 100 | 61.4 | 36.4 | 38.6 | 31.8 |
| Cleveland et al. [9] | 57 | 31.7 | 43.9 | 40.7 | - | - | 11.0 | 7.0 | 35.1 | 57.9 | 98.2 | 8.8 | 89.5 | - | - | - | 42.1 | - |
| Yin et al. [16] | 8 | 35.0 | 37.5 | 34.5 | - | - | 9.1 | 0.0 | 25.0 | 75.0 | 100 | 12.5 | 87.5 | - | 50.0 | 25.0 | - | 12.5 |
| Choon et al. [17] | 42 | 5.3 | 47.6 | 39.0 | - | - | - | 6.7 | 73.8 | 19.0 | 92.9 | 40.5 | 52.4 | - | - | - | - | - |
| Kaniewska et al. [18] | 27 | 8.0 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Zeissig et al. [19] | 124 | 8.0 | 39.5 | 38.6 | 4.8 | 23.9 | 7.0 | 5.6 | 46.0 | 48.4 | 85.5 | 20.2 | 52.4 | - | - | - | - | 46.0 |
| Al-Zarrad et al. [20] | 22 | 12.0 | 27.3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Bhatia et al. [21] | 34 | - | 47.1 | 37.4 | - | - | 12.0 | - | - | - | 100 | 29.4 | 70.6 | - | - | - | - | - |
| Teani et al. [22] | 12 | 13.1 | - | - | - | - | - | - | - | - | 100 | 16.7 | 83.3 | 100 | - | - | 25.0 | 25.0 |
| Garcia et al. [23] | 32 | - | - | - | - | - | - | - | - | - | 100 | - | - | - | - | - | 78.1 | - |
| Harris et al. [24] | 34 | 8.0 | - | - | - | - | - | - | - | - | 67.6 | - | - | - | - | - | - | - |
| Annadurai et al. [25] | 11 | - | - | - | - | - | - | 0.0 | 18.2 | 81.8 | - | - | - | - | - | - | - | - |
| Patel et al. [26] | 98 | 11.5 | 35.7 | 26.3 | - | - | 6.0 | - | - | 68.4 | 69.4 | 13.3 | 56.1 | - | - | - | 31.6 | - |
| Chowla et al. [27] | 87 | 15.4 | 37.9 | 40.5 | - | - | - | - | - | - | 100 | - | - | - | - | 19.5 | - | |
| Doumas et al. [28] | 15 | 10.8 | 46.7 | 33.9 | - | 24.9 | 7.8 | 13.3 | 0.0 | 86.7 | 100 | - | - | - | - | - | - | - |
| Kochhar et al. [29] | 526 | 52.0 | 44.9 | 40.4 | 4.8 | - | - | 3.4 | 16.2 | 80.4 | - | - | - | 34.4 | 15.4 | 21.5 | - | 27.9 |
| Gilmore et al. [30] | 152 | 16.0 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 27.6 | - |
| Boneschansker et al. [31] | 35 | 12.0 | 54.3 | 39.0 | - | - | 9.6 | 11.4 | 28.6 | 60.0 | - | - | - | 97.1 | 74.3 | 40.0 | 34.3 | 40.0 |
| Levine et al. [32] | 16 | 34.9 | 43.8 | 37.5 | - | - | 7.9 | 0.0 | 25.0 | 75.0 | 100 | 0.0 | 100 | - | 43.8 | 25.0 | 100 | 50.0 |
| Hosomi et al. [33] | 6 | 8.0 | 33.3 | 45.2 | - | - | 6.4 | 0.0 | 0.0 | 100 | 100 | 16.7 | 83.3 | 50.0 | 33.3 | 33.3 | 50.0 | - |
| Radia et al. [34] | 5 | 4.0 | 0.0 | - | - | - | 6.0 | - | - | - | 100 | - | - | - | - | - | 100 | 100 |
| Cleveland et al. [35] | 18 | 4.4 | 38.9 | 39.3 | 5.6 | - | 15.2 | 6.3 | 37.5 | 56.3 | 100 | 0.0 | 100 | - | - | - | 100 | 44.4 |
| Odah et al. [36] | 26 | 52.8 | 53.8 | 35.2 | 0.0 | - | - | 3.8 | 15.4 | 80.8 | 100 | - | - | - | 76.9 | 69.2 | 100 | 57.7 |
| Total of patients | 1388 | |||||||||||||||||
| Mean | 19.6 | 40.0 | 37.7 | 3.1 | 24.6 | 8.3 | 4.7 | 25.8 | 69.5 | 94.6 | 17.6 | 75.0 | 78.7 | 53.6 | 35.1 | 53.2 | 44.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taxonera, C.; García-Brenes, M.A.; Machín, M.; Olivares, D.; López-García, O.N.; Zapater, R.; Alba, C. Real-World Effectiveness and Safety of Upadacitinib in Patients with Ulcerative Colitis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 2232. https://doi.org/10.3390/jcm14072232
Taxonera C, García-Brenes MA, Machín M, Olivares D, López-García ON, Zapater R, Alba C. Real-World Effectiveness and Safety of Upadacitinib in Patients with Ulcerative Colitis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(7):2232. https://doi.org/10.3390/jcm14072232
Chicago/Turabian StyleTaxonera, Carlos, Miguel A. García-Brenes, María Machín, David Olivares, Olga N. López-García, Raúl Zapater, and Cristina Alba. 2025. "Real-World Effectiveness and Safety of Upadacitinib in Patients with Ulcerative Colitis: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 7: 2232. https://doi.org/10.3390/jcm14072232
APA StyleTaxonera, C., García-Brenes, M. A., Machín, M., Olivares, D., López-García, O. N., Zapater, R., & Alba, C. (2025). Real-World Effectiveness and Safety of Upadacitinib in Patients with Ulcerative Colitis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(7), 2232. https://doi.org/10.3390/jcm14072232







