Patterns of Disease Progression and Outcomes of Inferior ST-Elevation Myocardial Infarction Complicated by Cardiogenic Shock: The Multicenter INSTINCT Registry
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Definitions and Outcomes
2.3. Statistical Analysis
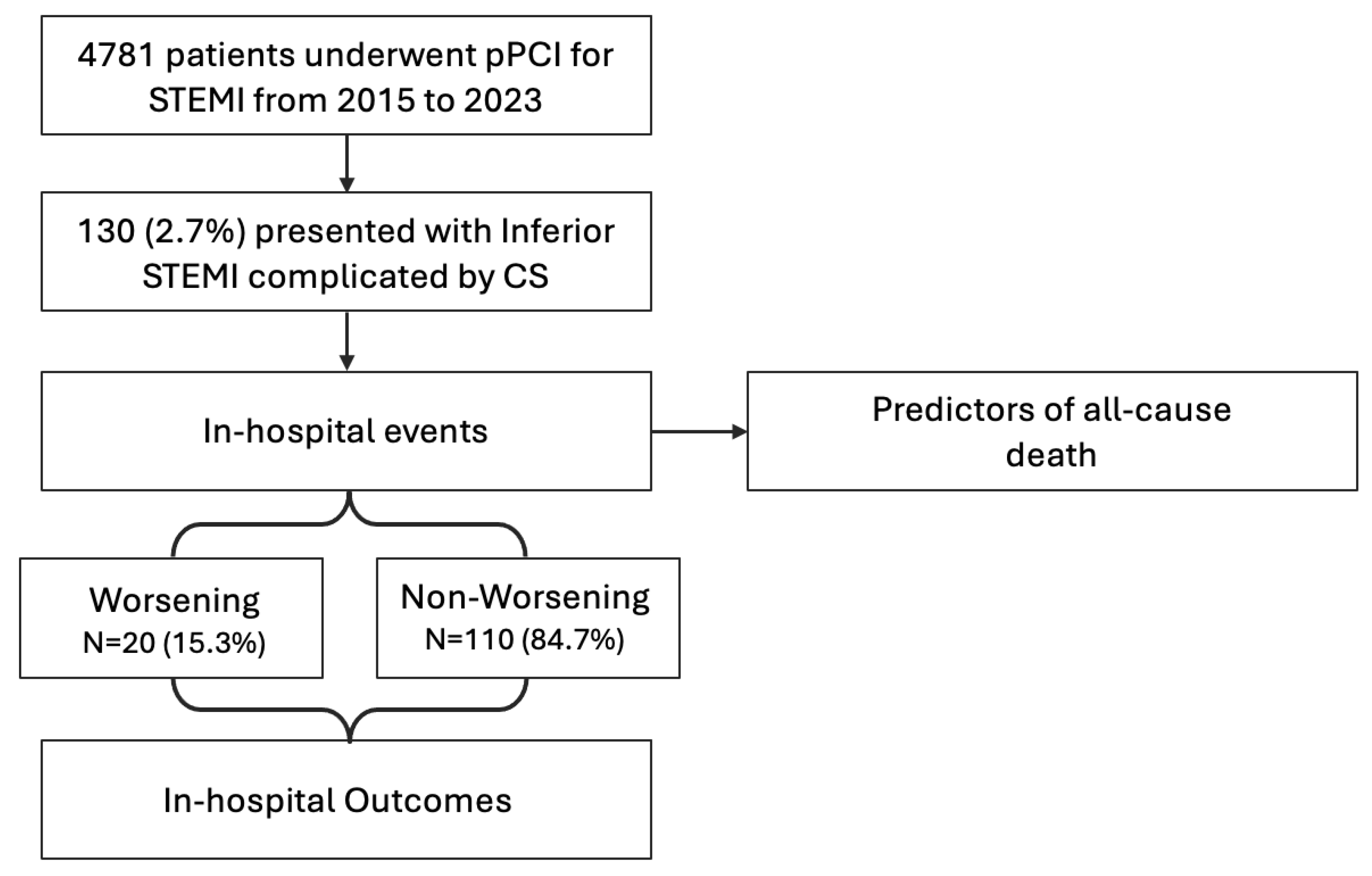
3. Results
3.1. Study Population and Clinical Presentation at Diagnosis of Shock
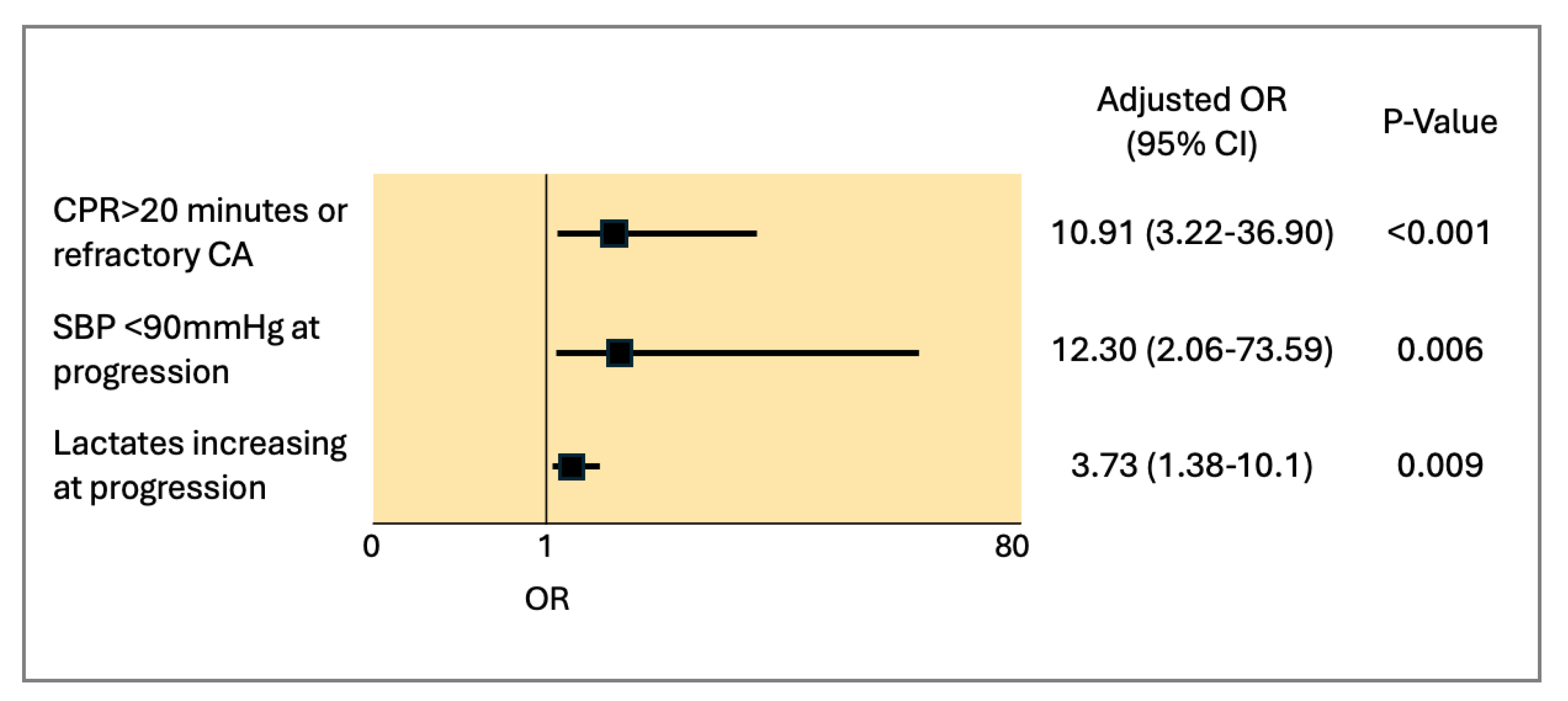
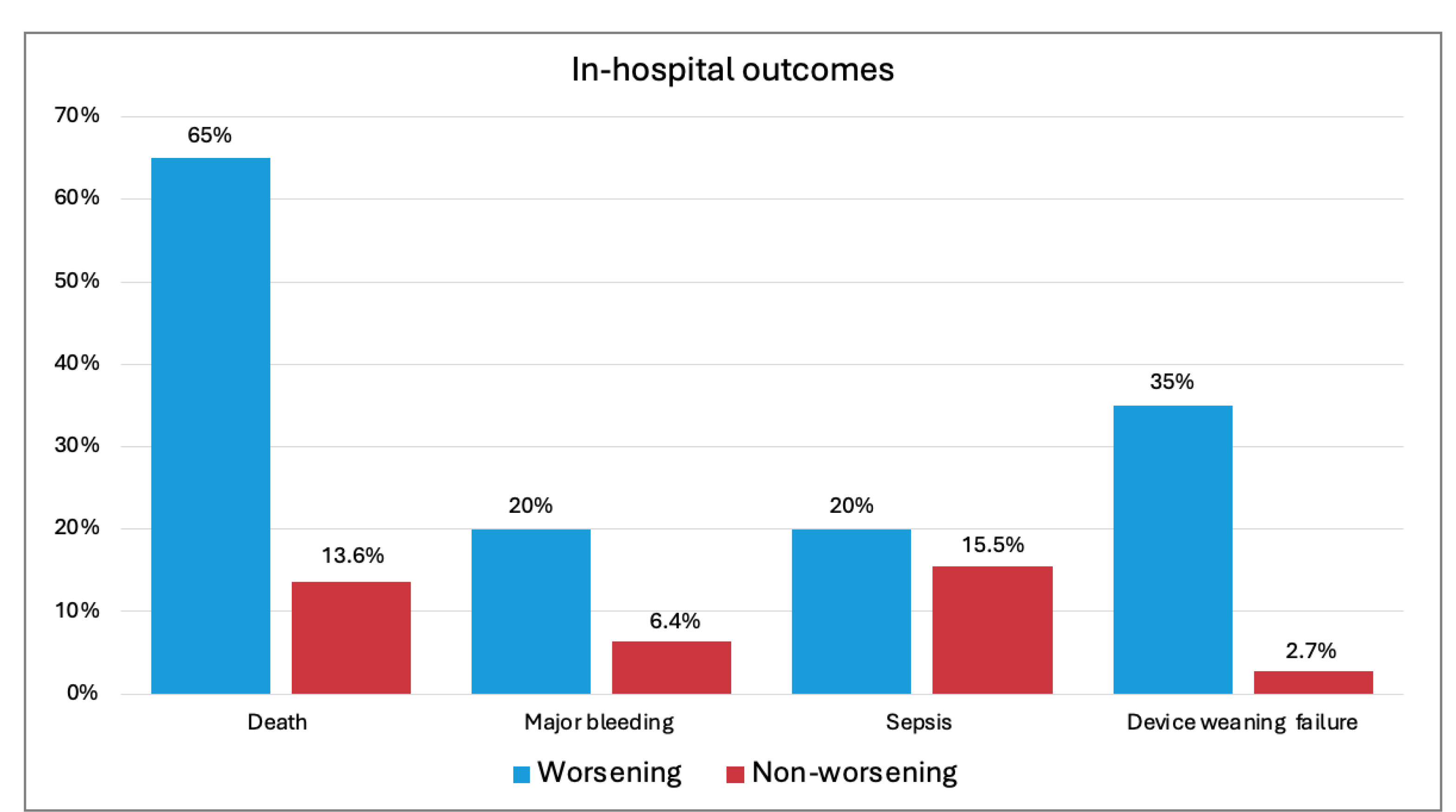
3.2. In-Hospital Outcomes and Predictors of All-Cause Mortality
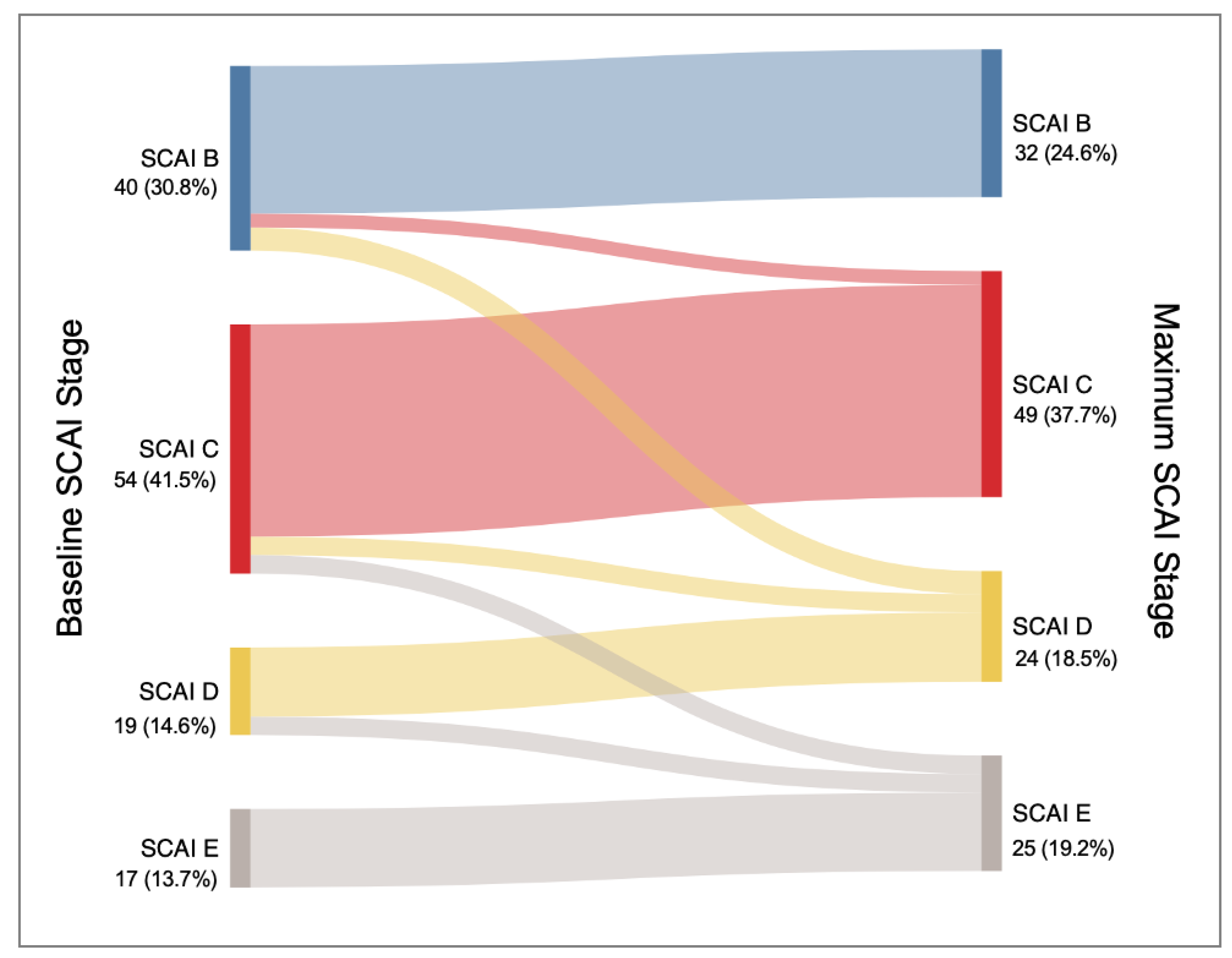
3.3. Patterns of Progression of Cardiogenic Shock
4. Discussion
- The prevalence of inferior STEMI complicated by cardiogenic shock among patients undergoing pPCI was low (2.7%). In-hospital mortality reached 22.3%, and complication rates of bleeding, device access site issues, and sepsis were not negligible, being, respectively, 8.5%, 4.6%, and 16.2%.
- One-third of patients presented with cardiac arrest (CA), and, although CA alone was not a predictor of mortality, prolonged CPR (>20 min) or refractory CA significantly increased the odds. Other predictors included persistently low systolic blood pressure during management and elevated lactate levels.
- Deterioration in CSWG-SCAI stage, although rare, was associated with higher risks of mortality and complications.
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | acute coronary syndrome (s) |
| AMICS | acute myocardial infarction with cardiogenic shock |
| CA | cardiac arrest |
| CAD | coronary artery disease |
| CMR | cardiac magnetic resonance |
| CPR | cardiopulmonary resuscitation |
| CS | cardiogenic shock |
| ICU | intensive care unit |
| HF | heart failure |
| LV | left ventricle/ventricular |
| LVEF | left ventricular ejection fraction |
| OHCA | out-of-hospital cardiac arrest |
| (p) PCI | (primary) percutaneous coronary intervention |
| RCT | randomized controlled trial |
| RV | right ventricle/ventricular |
| SBP | systolic blood pressure |
| SCAI | Society of Cardiovascular Angiography and Interventions |
| STEMI | ST-elevation myocardial infarction |
| TAPSE | tricuspid annular plane systolic excursion |
| WPs | worsening patients |
References
- Aissaoui, N.; Puymirat, E.; Delmas, C.; Ortuno, S.; Durand, E.; Bataille, V.; Drouet, E.; Bonello, L.; Bonnefoy-Cudraz, E.; Lesmeles, G.; et al. Trends in cardiogenic shock complicating acute myocardial infarction. Eur. J. Heart Fail. 2020, 22, 664–672. [Google Scholar] [CrossRef]
- Babaev, A.; Frederick, P.D.; Pasta, D.J.; Every, N.; Sichrovsky, T.; Hochman, J.S.; NRMI Investigators. Trends in Management and Outcomes of Patients with Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA 2005, 294, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.K.; Kanwar, M.; Sinha, S.S.; Thayer, K.L.; Garan, A.R.; Hernandez-Montfort, J.; Zhang, Y.; Li, B.; Baca, P.; Dieng, F.; et al. Criteria for Defining Stages of Cardiogenic Shock Severity. J. Am. Coll. Cardiol. 2022, 80, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Jeger, R.V.; Radovanovic, D.; Hunziker, P.R.; Pfisterer, M.E.; Stauffer, J.-C.; Erne, P.; Urban, P.; AMIS Plus Registry Investigators. Ten-Year Trends in the Incidence and Treatment of Cardiogenic Shock. Ann. Intern. Med. 2008, 149, 618–626. [Google Scholar] [CrossRef]
- van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2017, 136, E232–E268. [Google Scholar] [CrossRef]
- Gupta, T.; Weinreich, M.; Kolte, D.; Khera, S.; Villablanca, P.A.; Bortnick, A.E.; Wiley, J.M.; Menegus, M.A.; Kirtane, A.J.; Bhatt, D.L.; et al. Comparison of Incidence and Outcomes of Cardiogenic Shock Complicating Posterior (Inferior) Versus Anterior ST-Elevation Myocardial Infarction. Am. J. Cardiol. 2020, 125, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.K.; Paruchuri, V.; Jagannathan, A.; Steinberg, D.; Chakrabarti, A.K.; Pinto, D.; Aghili, N.; Najjar, S.; Finley, J.; Orr, N.M.; et al. Mechanical Circulatory Support for Right Ventricular Failure. JACC Heart Fail. 2013, 1, 127–134. [Google Scholar] [CrossRef]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Sanborn, T.A.; White, H.D.; Talley, J.D.; Christopher, E.B.; Jacobs, A.K.; Slater, J.N.; Col, J.; et al. Early Revascularization in Acute Myocardial Infarction Complicated by Cardiogenic Shock. N. Engl. J. Med. 1999, 341, 625–634. [Google Scholar] [CrossRef]
- Farooq, V.; van Klaveren, D.; Steyerberg, E.W.; Meliga, E.; Vergouwe, Y.; Chieffo, A.; Kappetein, A.P.; Colombo, A.; Holmes, D.R.; Mack, M.; et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: Development and validation of SYNTAX score II. Lancet 2013, 381, 639–650. [Google Scholar] [CrossRef]
- Naidu, S.S.; Baran, D.A.; Jentzer, J.C.; Hollenberg, S.M.; van Diepen, S.; Basir, M.B.; Grines, C.L.; Diercks, D.B.; Hall, S.; Kapur, N.K.; et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies. J. Am. Coll. Cardiol. 2022, 79, 933–946. [Google Scholar] [CrossRef]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Akin, I.; Behnes, M.; Rassaf, T.; Mahabadi, A.A.; Lehmann, R.; Eitel, I.; Graf, T.; Seidler, T.; et al. Extracorporeal Life Support in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2023, 389, 1286–1297. [Google Scholar] [CrossRef]
- Møller, J.E.; Engstrøm, T.; Jensen, L.O.; Eiskjær, H.; Mangner, N.; Polzin, A.; Schulze, P.C.; Skurk, C.; Nordbeck, P.; Clemmensen, P.; et al. Microaxial Flow Pump or Standard Care in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2024, 390, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography: Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef] [PubMed]
- Vakil, D.; Soto, C.; D’costa, Z.; Volk, L.; Kandasamy, S.; Iyer, D.; Ikegami, H.; Russo, M.J.; Lee, L.Y.; Lemaire, A. Short-term and intermediate outcomes of cardiogenic shock and cardiac arrest patients supported by venoarterial extracorporeal membrane oxygenation. J. Cardiothorac. Surg. 2021, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Konstam, M.A.; Kiernan, M.S.; Bernstein, D.; Bozkurt, B.; Jacob, M.; Kapur, N.K.; Kociol, R.D.; Lewis, E.F.; Mehra, M.R.; Pagani, F.D.; et al. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e578–e622. [Google Scholar] [CrossRef]
- Bergamaschi, L.; Arangalage, D.; Maurizi, N.; Pizzi, C.; Valgimigli, M.; Iglesias, J.F.; Landi, A.; Leo, L.A.; Eeckhout, E.; Schwitter, J.; et al. Hepatic T1 mapping as a novel cardio-hepatic axis imaging biomarker early after ST-elevation myocardial infarction. Eur. Heart J.-Cardiovasc. Imaging 2024, 26, 229–238. [Google Scholar] [CrossRef]
- Voelkel, N.F.; Quaife, R.A.; Leinwand, L.A.; Barst, R.J.; McGoon, M.D.; Meldrum, D.R.; Dupuis, J.; Long, C.S.; Rubin, L.J.; Smart, F.W.; et al. Right Ventricular Function and Failure. Circulation 2006, 114, 1883–1891. [Google Scholar] [CrossRef]
- Gramegna, M.; Beneduce, A.; Bertoldi, L.F.; Pagnesi, M.; Marini, C.; Pazzanese, V.; Camici, P.G.; Chieffo, A.; Pappalardo, F. Impella RP support in refractory right ventricular failure complicating acute myocardial infarction with unsuccessful right coronary artery revascularization. Int. J. Cardiol. 2020, 302, 135–137. [Google Scholar] [CrossRef]
- Harjola, V.; Mebazaa, A.; Čelutkienė, J.; Bettex, D.; Bueno, H.; Chioncel, O.; Crespo-Leiro, M.G.; Falk, V.; Filippatos, G.; Gibbs, S.; et al. Contemporary management of acute right ventricular failure: A statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 226–241. [Google Scholar] [CrossRef]
- Belletti, A.; Lerose, C.C.; Zangrillo, A.; Landoni, G. Vasoactive-Inotropic Score: Evolution, Clinical Utility, and Pitfalls. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3067–3077. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, M.K.; Everett, K.D.; Gulati, G.; Brener, M.I.; Kapur, N.K. Epidemiology and management of right ventricular-predominant heart failure and shock in the cardiac intensive care unit. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Ostadal, P.; Rokyta, R.; Karasek, J.; Kruger, A.; Vondrakova, D.; Janotka, M.; Naar, J.; Smalcova, J.; Hubatova, M.; Hromadka, M.; et al. Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock: Results of the ECMO-CS Randomized Clinical Trial. Circulation 2023, 147, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Baran, D.A.; Grines, C.L.; Bailey, S.; Burkhoff, D.; Hall, S.A.; Henry, T.D.; Hollenberg, S.M.; Kapur, N.K.; O’Neill, W.; Ornato, J.P.; et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter. Cardiovasc. Interv. 2019, 94, 29–37. [Google Scholar] [CrossRef]
- Chieffo, A.; Dudek, D.; Hassager, C.; Combes, A.; Gramegna, M.; Halvorsen, S.; Huber, K.; Kunadian, V.; Maly, J.; Møller, J.E.; et al. Joint EAPCI/ACVC expert consensus document on percutaneous ventricular assist devices. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 570–583. [Google Scholar] [CrossRef]
- Spadafora, L.; Betti, M.; D’ascenzo, F.; De Ferrari, G.; De Filippo, O.; Gaudio, C.; Collet, C.; Sabouret, P.; Agostoni, P.; Zivelonghi, C.; et al. Impact of In-Hospital Bleeding on Post-Discharge Therapies and Prognosis in Acute Coronary Syndromes. J. Cardiovasc. Pharmacol. 2025. [Google Scholar] [CrossRef]
| n = 130 | |
|---|---|
| Baseline Characteristics | |
| Female gender | 41 (31.5) |
| Age | 69.8 ± 12.4 |
| BMI | 25.5 ± 3.8 |
| Smoker | 68 (52.3) |
| Diabetes | 39 (30.0) |
| Hypertension | 85 (65.4) |
| CAD | 20 (15.4) |
| Heart failure | 4 (3.1) |
| Previous AMI | 13 (10.0) |
| Previous CABG | 7 (5.4) |
| Previous PCI | 16 (12.3) |
| Atrial fibrillation | 11 (8.5) |
| COPD | 17 (13.1) |
| CVA | 11 (8.5) |
| CKD | 38 (29.2) |
| Dialysis | 4 (3.1) |
| Clinical presentation | |
| Cardiac arrest | 42 (32.3) |
| CPR < 20 min | 25 (19.2) |
| CPR < 20 min | 9 (6.9) |
| Refractory CA | 8 (6.2) |
| SBP (mmHg) | 87.7 ± 20.4 |
| DBP (mmHg) | 53.4 ± 13.7 |
| n = 130 | |
|---|---|
| Laboratory workup | |
| Creatinine (mg/dL) | 1.08 [0.9–1.5] |
| BUN (mg/dL) | 40 [28–57] |
| Leucocytes (cells/mm3) | 14,950 [11,155–18,660] |
| Platelets (cells/mm3) | 249,417 ± 95,258 |
| Hemoglobin (g/dL) | 13.30 ± 2.30 |
| AST (mU/mL) | 106 [40–216] |
| ALT (mU/mL) | 42 [22–83] |
| Bilirubin (mg/dL) | 0.57 [0.35–0.90] |
| CRP (mg/dL) | 4.4 [1.3–40.7] |
| Blood gas | |
| SpO2 (%) | 98.75 ± 40.71 |
| PCO2 (mmHg) | 39.44 ± 13.79 |
| HCO3− (mEq/L) | 20.29 ± 5.11 |
| pH | 7.32 ± 0.15 |
| Lactates (mmol/L) | 3.65 [2.6–5.8] |
| Glucose (mg/dL) | 220.15 ± 106.52 |
| Echocardiography | |
| LVEF (%) | 43.17 ± 11.10 |
| RVD1 (mm) | 37.5 ± 6.96 |
| TAPSE (mm) | 14.03 ± 4.68 |
| S’ TDI (cm/s) | 8.26 ± 3.61 |
| PAPs (mmHg) | 34.69 ± 9.06 |
| IVC (mm) | 20.14 ± 4.40 |
| Biventricular Dysfunction | 44 (33.8) |
| n = 130 | |
|---|---|
| PCI | 130 (100) |
| Multivessel disease | 83 (63.8) |
| Left main | 12 (9.2) |
| Left anterior descending | 75 (57.7) |
| Circumflex | 50 (38.5) |
| Concomitant multivessel PCI | 19 (14.6) |
| Inotropes | 99 (76.2) |
| Mechanical ventilation | 40 (30.8) |
| Mechanical circulatory support | 49 (37.8) |
| VA-ECMO | 9 (6.9) |
| IABP | 41 (31.5) |
| Impella LV | 9 (6.9) |
| Impella RP | 9 (6.9) |
| CVVH/Dialysis | 19 (14.6) |
| Cytosorb | 7 (5.4) |
| Blood transfusion | 30 (23.1) |
| Successful device weaning/explantation | 39 (30.0) |
| A | At Diagnosis of Shock | Peak | p-Value | ||
|---|---|---|---|---|---|
| Lactates (mmol/L) | 3.65 [2.6–5.8] | 4.1 [2.6–7.3] | <0.001 | ||
| Creatinine (mg/dL) | 1.08 [0.9–1.5] | 1.24 [0.9–2.3] | <0.001 | ||
| AST (mU/mL) | 106 [40–216] | 238 [165–401] | 0.007 | ||
| ALT (mU/mL) | 42 [22–83] | 70 [41–131] | <0.001 | ||
| Bilirubin (mg/dL) | 0.57 [0.35–0.90] | 0.9 [0.5–1.3] | 0.027 | ||
| B | At Diagnosis of Shock | Start Treatment | Progression | Recovery/last Available | p-Value |
|
SBP (mmHg) | 87.7 ± 20.4 | 111.23 ± 21.1 | 116.0 ± 10.1 | 116.2 ± 20.5 | <0.001 |
| DBP (mmHg) | 53.4 ± 13.7 | 59.7 ± 12.0 | 62.0 ± 11.9 | 64.2 ± 13.2 | <0.001 |
| Inotropic score | - | 5.0 [3.0–10.0] | 3.0 [.0–6.9] | 0 | <0.001 |
| Lactates (mmol/L) | 3.65 [2.6–5.8] | 3.2 [1.9–5.7] | 1.94 [1.1–3.2] | 1 [0.8–1.7] | <0.001 |
| CVP (mmHg) | - | 11.6 ± 5.6 | 10.4 ± 4.3 | 8.8 ± 5.3 | <0.001 |
| n = 130 | |
|---|---|
| Death | 29 (22.3) |
| Major bleeding | 11 (8.5) |
| Device access site complication | 6 (4.6) |
| Hemolysis | 8 (6.2) |
| Stroke | 3 (2.3) |
| Sepsis | 21 (16.2) |
| Duration of inotropic support (days) | 3.0 [2.0–5.0] |
| Duration of MCS (days) | 3.0 [2.0–5.0] |
| ICU stay (days) | 4.5 [3.0–8.0] |
| Hospital stay (days) | 10.0 [7.0–15.3] |
| Echocardiography at recovery/discharge | |
| LVEF (%) | 48.2 ± 10.6 |
| RVD1 (mm) | 33.3 ± 8.9 |
| TAPSE (mm) | 17.9 ± 4.2 |
| S’ TDI (cm/s) | 10.8 ± 2.6 |
| Tricuspid regurgitation (≥2/4) | 11 (8.5) |
| PAPs (mmHg) | 30.1 ± 8.7 |
| IVC (mm) | 15.1 ± 5.5 |
| Residual RV dysfunction | 26 (20.0) |
| Survivors n = 101 | Non-Survivors n = 29 | OR (CI) | p-Value | |
|---|---|---|---|---|
| Cardiac arrest | 30 (29.7) | 12 (41.4) | 1.69 (0.72–3.98) | 0.226 |
| CPR < 20 min or Refractory CA | 11 (10.9) | 6 (20.7) | 9.67 (3.17–29.51) | <0.001 |
| Mechanical ventilation | 26 (25.7) | 14 (48.3) | 3.28 (1.34–8.0) | 0.009 |
| PCI failure | 14 (13.9) | 13 (44.8) | ||
| Multivessel disease | 64 (63.3) | 18 (62.1) | 0.93 (0.39–2.18) | 0.870 |
| SBP | ||||
| <90 (mmHg) baseline | 56 (55.4) | 15 (51.7) | 0.84 (0.37–1.93) | 0.691 |
| <60 (mmHg) baseline | 9 (8.9) | 5 (17.2) | 2.15 (0.66–7.02) | 0.203 |
| <90 (mmHg) at progression | 2 (2.0) | 6 (20.7) | 12.91 (2.47–68.82) | 0.003 |
| Lactates | ||||
| >5 (mmol/L) baseline | 14 (13.9) | 5 (17.2) | 1.31 (0.43–3.99) | 0.636 |
| Increasing at progression | 21 (20.8) | 7 (24.1) | 3.53 (1.42–7.87) | 0.049 |
| Echocardiography at diagnosis | ||||
| LVEF (%) | 44.1 ± 10.7 | 39.2 ± 12.1 | 0.96 (0.92–1.0) | 0.066 |
| TAPSE (mm) | 14.5 ± 4.6 | 11.6 ± 4.2 | 0.84 (0.72–0.98) | 0.026 |
| S’TDI (cm/s) | 8.7 ± 3.6 | 6.0 ± 2.8 | 0.75 (0.52–1.09) | 0.104 |
| Biventricular Dysfunction | 33 (32.7) | 11 (37.9) | 1.57 (0.60–4.11) | 0.362 |
| Worsening n = 20 | Non-Worsening n = 110 | OR (CI) | p-Value | |
|---|---|---|---|---|
| In-Hospital Clinical Characteristics | ||||
| Cardiac arrest | 6 (30.0) | 35 (31.8) | 0.91 (0.33–2.59) | 0.872 |
| PCI failure | 10 (50%) | 17 (15.4) | 5.41 (1.96–14.97) | <0.001 |
| MCS | ||||
| VA-ECMO | 4 (20.0) | 5 (4.5) | 5.25 (1.27–21.63) | 0.022 |
| IABP | 5 (25.0) | 36 (32.7) | 0.68 (0.23–2.03) | 0.494 |
| Impella LV | 5 (25.0) | 4 (3.6) | 8.83 (2.13–36.6) | 0.003 |
| Impella RP | 1 (7.3) | 8 (5.0) | 0.67 (0.079–5.67) | 0.713 |
| Peak lactates (mmol/L) | 9.05 [4.5–13.5] | 3.60 [2.4–5.9] | 1.15 (1.05–1-26) | 0.003 |
| Peak creatinine (mg/dL) | 2.39 [1.3–3.7] | 1.13 [0.9–1.8] | 1.32 (1.02–1.70) | 0.031 |
| Peak AST (mU/mL) | 411 [213–1713] | 236 [162–362] | 1.5 (1.1–3.2) | 0.019 |
| Peak ALT (mU/mL) | 118 [54–698] | 63 [40–109] | 1.02 (1.01–1.09) | 0.043 |
| Outcomes | ||||
| Death | 13 (65.0) | 15 (13.6) | 11.63 (4.0–33.87) | <0.001 |
| Major bleeding | 4 (20.0) | 7 (6.4) | 3.70 (2.2–14.0) | 0.044 |
| Sepsis | 4 (20.0) | 17 (15.5) | 1.37 (0.41–4.59) | 0.611 |
| Device weaning failure | 7 (35.0) | 3 (2.7) | 3.53 (1.16–11.2) | 0.032 |
| ICU stay (days) | 5 [3–8] | 4 [3–8] | 0.97 (0.92–1.04) | 0.484 |
| Hospital stays (days) | 7.5 [3.25–13.5] | 10 [7–17] | 0.97 (0.93–1.01) | 0.263 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botti, G.; Pieri, M.; Cappannoli, L.; Munafò, A.R.; Gramegna, M.; Gamardella, M.; Camporotondo, R.; Aurigemma, C.; Ferlini, M.; Guida, S.; et al. Patterns of Disease Progression and Outcomes of Inferior ST-Elevation Myocardial Infarction Complicated by Cardiogenic Shock: The Multicenter INSTINCT Registry. J. Clin. Med. 2025, 14, 2231. https://doi.org/10.3390/jcm14072231
Botti G, Pieri M, Cappannoli L, Munafò AR, Gramegna M, Gamardella M, Camporotondo R, Aurigemma C, Ferlini M, Guida S, et al. Patterns of Disease Progression and Outcomes of Inferior ST-Elevation Myocardial Infarction Complicated by Cardiogenic Shock: The Multicenter INSTINCT Registry. Journal of Clinical Medicine. 2025; 14(7):2231. https://doi.org/10.3390/jcm14072231
Chicago/Turabian StyleBotti, Giulia, Marina Pieri, Luigi Cappannoli, Andrea Raffaele Munafò, Mario Gramegna, Marco Gamardella, Rita Camporotondo, Cristina Aurigemma, Marco Ferlini, Stefania Guida, and et al. 2025. "Patterns of Disease Progression and Outcomes of Inferior ST-Elevation Myocardial Infarction Complicated by Cardiogenic Shock: The Multicenter INSTINCT Registry" Journal of Clinical Medicine 14, no. 7: 2231. https://doi.org/10.3390/jcm14072231
APA StyleBotti, G., Pieri, M., Cappannoli, L., Munafò, A. R., Gramegna, M., Gamardella, M., Camporotondo, R., Aurigemma, C., Ferlini, M., Guida, S., Cascone, A., Russo, F., Lanzillo, G., Burzotta, F., Montorfano, M., Scandroglio, A. M., & Chieffo, A. (2025). Patterns of Disease Progression and Outcomes of Inferior ST-Elevation Myocardial Infarction Complicated by Cardiogenic Shock: The Multicenter INSTINCT Registry. Journal of Clinical Medicine, 14(7), 2231. https://doi.org/10.3390/jcm14072231






