Association Between Arterial Stiffness, High Blood Pressure, and Hypertensive Phenotypes: Insights from the PAMELA Study
Abstract
1. Introduction
2. The PAMELA Study
3. Relationships Between CAVI and Other Variables
Arterial Stiffness and Demographic Variables
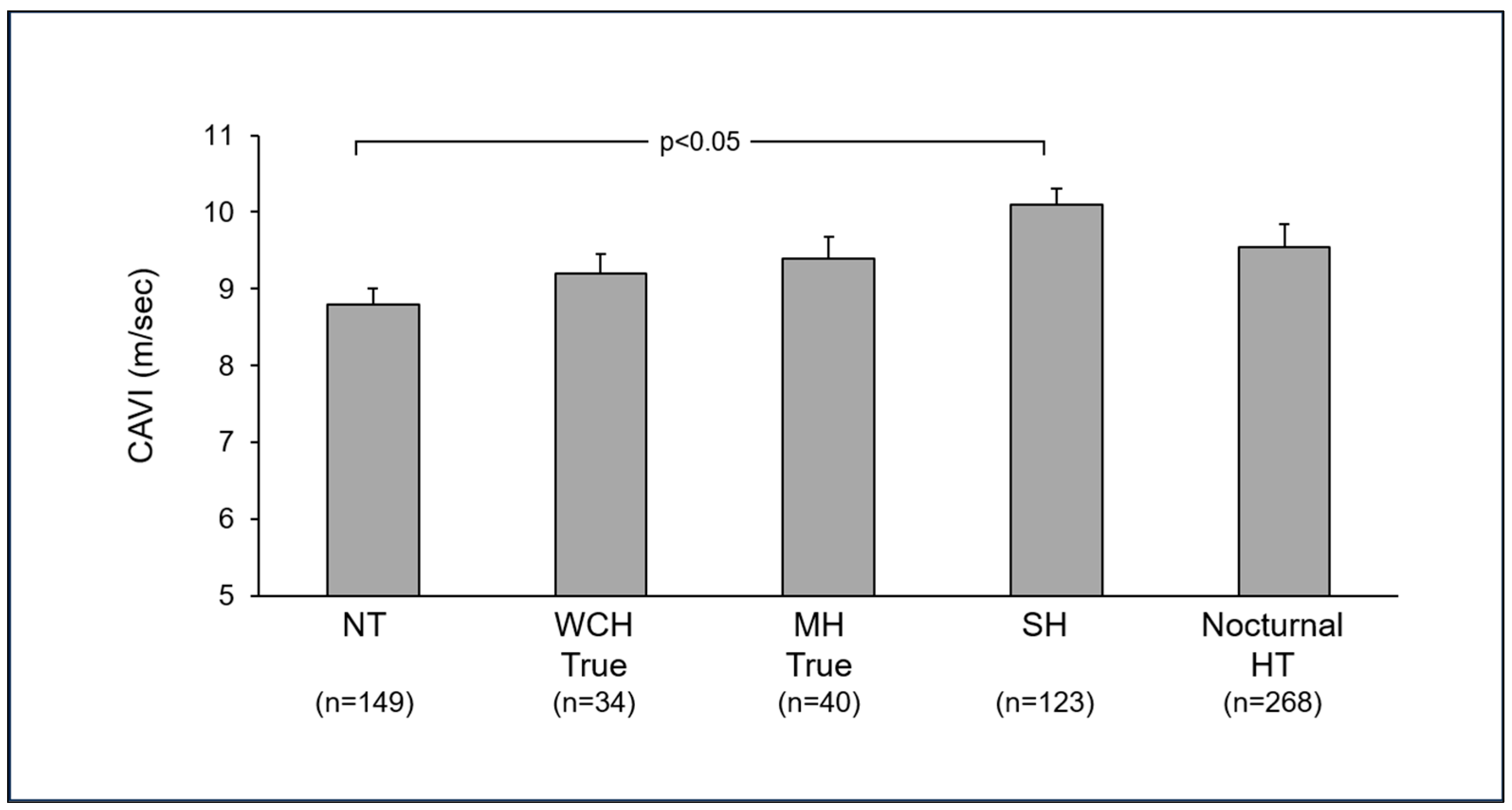
4. Arterial Stiffness in Hypertensive Phenotypes
4.1. White Coat Hypertension
4.2. Masked Hypertension
4.3. Isolated Systolic Hypertension
4.4. Nocturnal Hypertension
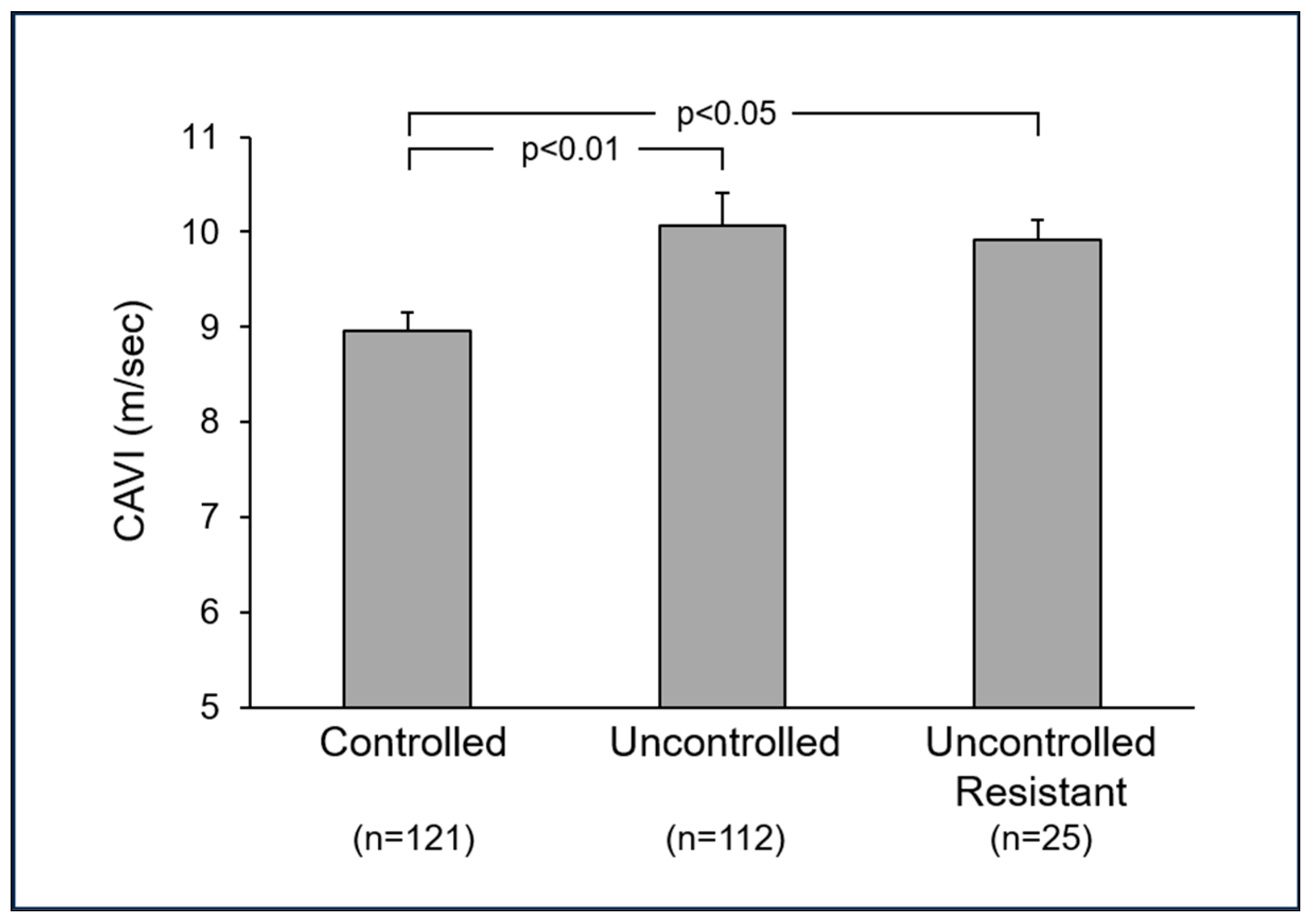
5. Arterial Stiffness and Blood Pressure Control
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large-artery stiffness in health and disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1237–1263. [Google Scholar] [PubMed]
- Fortini, F.; Vieceli Dalla Sega, F.; Marracino, L.; Severi, P.; Rapezzi, C.; Rizzo, P.; Ferrari, R. Well-known and novel players in endothelial dysfunction: Updates on a notch(ed) landscape. Biomedicines 2021, 9, 997. [Google Scholar] [CrossRef] [PubMed]
- Saiki, A.; Ohira, M.; Yamaguchi, T.; Nagayama, D.; Shimizu, N.; Shirai, K.; Tatsuno, I. New horizons of arterial stiffness developed using cardio-ankle vascular index (CAVI). J. Atheroscler. Thromb. 2020, 27, 732–748. [Google Scholar] [PubMed]
- Grassi, G.; Quarti-Trevano, F.; Dell’Oro, R.; Cuspidi, C.; Mancia, G. The PAMELA research project: A 25-year long journey. Panminerva Med. 2021, 63, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Tomiyama, H.; Maruhashi, T.; Matsuzawa, Y.; Miyoshi, T.; Kabutoya, T.; Kario, K.; Sugiyama, S.; Munakata, M.; Ito, H.; et al. Physiological diagnosis criteria for vascular failure committee. Physiological diagnostic criteria for vascular failure. Hypertension 2018, 72, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Pechlaner, R.; Cai, J.; Yuan, H.; Huang, Z.; Yang, G.; Wang, J.; Chen, Z.; Kiechl, S.; Xu, Q. Trajectories of age-related arterial stiffness in chinese men and women. J. Am. Coll. Cardiol. 2020, 75, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Kiechl, S.J.; Wang, J.; Xu, Q.; Kiechl, S.; Pechlaner, R.; Global Pulse Wave Velocity Study Group. Global distribution of age- and sex-related arterial stiffness: Systematic review and meta-analysis of 167 studies with 509,743 participants. EBioMedicine 2023, 32, 104618. [Google Scholar]
- Fantini, F.; Giani, A.; Trentin, M.; Rossi, A.P.; Zoico, E.; Mazzali, G.; Micciolo, R.; Zamboni, M. The correlation of arterial stiffness parameters with aging and comorbidity burden. J. Clin. Med. 2022, 11, 5761. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Facchetti, R.; Gherbesi, E.; Quarti-Trevano, F.; Vanoli, J.; Mancia, G.; Grassi, G. Ambulatory blood pressure phenotypes, arterial stiffness, and cardiac remodeling. Am. J. Hypertens. 2024, 37, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Boutouyrie, P.; Chowienczyk, P.; Humphrey, J.D.; Mitchell, G.F. Arterial stiffness and cardiovascular risk in hypertension. Circ. Res. 2021, 128, 864–886. [Google Scholar] [CrossRef] [PubMed]
- Steinsaltz, D.; Patten, H.; Bester, D.; Rehkopf, D. Short-term and mid-term blood pressure variability and long-term mortality. J. Am. Coll. Cardiol. 2025, 234, 71–78. [Google Scholar]
- Kase, M.; Iijima, T.; Niitani, T.; Sagara, M.; Sakurai, S.; Tomaru, T.; Jojima, T.; Usui, I.; Aso, Y. Relationship between reduced heart rate variability and increased arterial stiffness evaluated by cardio-ankle vascular index in people with type diabetes. Diabetol. Int. 2022, 14, 94–102. [Google Scholar] [PubMed]
- Wang, H.; Liu, J.; Zhao, H.; Zhao, X.; Li, L.; Shi, H.; Zhan, S.; Liu, J. Relationships between cardio-ankle vascular index and plasma lipids in hypertension subjects. J. Hum. Hypertens. 2015, 29, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, D.; Watanabe, Y.; Saiki, A.; Shirai, K.; Tatsuno, I. Lipid parameters are independently associated with cardio-ankle vascular index in healthy japanese subjects. J. Atheroscler. Throm 2018, 25, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Townsend, R.R.; Cohen, J.B. White-coat hypertension & cardiovascular outcomes. Curr. Hypertens. Rep. 2024, 26, 399–407. [Google Scholar] [PubMed]
- AlGhatrif, M.; Lakatta, E.G. The conundrum of arterial stiffness, elevated blood pressure, and aging. Curr. Hypertens. Rep. 2015, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Shin, C.; Kim, S.; Kim, J.S.; Lim, A.Y.; Seo, H.S.; Lim, H.E.; Sung, K.C.; Cho, G.Y.; Lee, S.; et al. Prevalence of isolated nocturnal hypertension and development of arterial stiffness, left ventricular hypertrophy, and silent cerebrovascular lesions: The KoGES (Korean Genome and Epidemiology Study). J. Am. Heart Assoc. 2022, 11, e025641. [Google Scholar] [PubMed]
- Kario, K.; Hoshide, S.; Mizuno, H.; Kabutoya, T.; Nishizawa, M.; Yoshida, T.; Abe, H.; Katsuya, T.; Fujita, Y.; Okazaki, O.; et al. Nighttime blood pressure phenotype and cardiovascular prognosis: Practitioner-based nationwide JAMP study. Circulation 2020, 142, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, T.; Kubozono, T.; Ojima, S.; Kawasoe, S.; Akasaki, Y.; Salim, A.A.; Ikeda, Y.; Miyata, M.; Takenaka, T.; Ohishi, M. Insufficient blood pressure control is independently associated with increased arterial stiffness. Hypertens. Res. 2022, 45, 1861–1868. [Google Scholar] [PubMed]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.A.; Algharably, E.A.E.; et al. 2023 ESH guidelines for the management of arterial hypertension. J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [PubMed]

| Variable | r Value | Adjusted p-Value |
|---|---|---|
| Body mass index (kg/m2) | −0.1799 | 0.3323 |
| Waist circumference (cm) | −0.05034 | 0.2184 |
| Office SBP (mmHg) | 0.28363 | 0.0003 |
| Office DBP (mmHg) | 0.11589 | 0.0024 |
| Office HR (b/min) | 0.03674 | 0.6693 |
| Home SBP (mmHg) | 0.28937 | 0.0003 |
| Home DBP (mmHg) | 0.0732 | 0.0035 |
| Home HR (b/min) | 0.00982 | 0.5401 |
| 24 h SBP (mmHg) | 0.23004 | 0.0005 |
| 24 h DBP (mmHg) | 0.12726 | 0.0002 |
| 24 h SBP SD | 0.02594 | 0.5588 |
| 24 h DBP SD | 0.02913 | 05116 |
| 24 h HR (b/min) | −0.02834 | 0.5676 |
| Total cholesterol (mg/dL) | −0.09252 | 0.6976 |
| HDL cholesterol (mg/dL) | −0.02894 | 0.6684 |
| Serum glucose (mg/dL) | 0.12326 | 0.1259 |
| Type 2 diabetes (%) | 0.13813 | 0.1049 |
| Triglycerides (mg/dL) | 0.03829 | 0.5157 |
| Uric acid (mg/dL) | 0.02524 | 0.5536 |
| Serum creatinine (mg/dL) | 0.03636 | 0.3835 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quarti-Trevano, F.; Cuspidi, C.; Dell’Oro, R.; Ambrosino, P.; Grassi, G. Association Between Arterial Stiffness, High Blood Pressure, and Hypertensive Phenotypes: Insights from the PAMELA Study. J. Clin. Med. 2025, 14, 2230. https://doi.org/10.3390/jcm14072230
Quarti-Trevano F, Cuspidi C, Dell’Oro R, Ambrosino P, Grassi G. Association Between Arterial Stiffness, High Blood Pressure, and Hypertensive Phenotypes: Insights from the PAMELA Study. Journal of Clinical Medicine. 2025; 14(7):2230. https://doi.org/10.3390/jcm14072230
Chicago/Turabian StyleQuarti-Trevano, Fosca, Cesare Cuspidi, Raffaella Dell’Oro, Pasquale Ambrosino, and Guido Grassi. 2025. "Association Between Arterial Stiffness, High Blood Pressure, and Hypertensive Phenotypes: Insights from the PAMELA Study" Journal of Clinical Medicine 14, no. 7: 2230. https://doi.org/10.3390/jcm14072230
APA StyleQuarti-Trevano, F., Cuspidi, C., Dell’Oro, R., Ambrosino, P., & Grassi, G. (2025). Association Between Arterial Stiffness, High Blood Pressure, and Hypertensive Phenotypes: Insights from the PAMELA Study. Journal of Clinical Medicine, 14(7), 2230. https://doi.org/10.3390/jcm14072230








