Abstract
Background: Transthyretin amyloidosis is a multi-system disease that may manifest as cardiomyopathy (ATTR-CM) and/or polyneuropathy. Both disease manifestations are associated with autonomic dysfunction. However, the prevalence of autonomic dysfunction in ATTR-CM remains to be evaluated. Methods: Within the scope of a prospective ATTR-CM registry, the Composite Autonomic Symptom Score-31 (COMPASS-31) questionnaire was applied to consecutive patients between November 2022 and November 2024. Baseline characteristics are described, and associations of the COMPASS-31 score with markers of disease severity were assessed. Kaplan–Meier analysis was utilized to assess the COMPASS-31 score’s association with a combined endpoint of all-cause mortality and heart failure-related hospitalizations. Results: A total of 129 ATTR-CM patients [81.7 years (IQR: 77.4–84.3), 108 male (83.7%)] were included in the final study cohort. After stratification using the COMPASS-31 median [14 points, interquartile range (IQR): 6–29], statistically significant differences with regard to New York Heart Association (NYHA) stage and the Kansas City Cardiomyopathy Questionnaire (KCCQ) were observed. Furthermore, the COMPASS-31 score was moderately correlated with the KCCQ score in Spearman correlation analysis (r = −0.55, p < 0.001). The primary endpoint occurred in 16 patients (13 HF-hospitalizations/3 deaths) after 6.3 (IQR: 2.8–17.1) months. In Kaplan–Meier analysis, a COMPASS-31 score above the median of 14 was also associated with the primary endpoint of all-cause mortality and HF-related hospitalization (log-rank p = 0.047). Conclusions: Autonomic dysfunction is highly prevalent in ATTR-CM, affecting almost two-thirds of patients. As the presence of autonomic dysfunction is likely associated with more severely impaired quality of life, routine screening for this disease manifestation of transthyretin amyloidosis may be advisable.
1. Introduction
Cardiac amyloidosis (CA) is characterized by myocardial amyloid infiltration [1]. Subsequently, wall thickness increases, and myocardial contractility and relaxation become impaired, resulting in decreased cardiac output and heart failure (HF) [1]. The most important amyloid precursor proteins are light chains and transthyretin [1]. Having undergone steric conformation changes, precursor proteins aggregate and form amyloid light chain or amyloid transthyretin (ATTR), which is deposited in tissues and causes amyloidosis. Light chain amyloidosis evolves due to plasma cell dyscrasia, while transthyretin amyloidosis can be caused either by a pathogenic TTR gene variant (formerly hereditary TTR) or arise as an acquired disease of the elderly [1].
While cardiac involvement is a crucial determinant of outcome in amyloidosis [2,3], amyloidosis remains a systemic disease with manifestations beyond the heart. Patients with light chain amyloidosis will often develop renal involvement resulting in chronic kidney disease or kidney failure, gastrointestinal, vascular, or bone marrow involvement [4]. In contrast, ATTR amyloidosis primarily affects the myocardium and peripheral nervous system and may cause spinal canal stenosis or carpal tunnel syndrome due to infiltration of the ligamentum flavum or the carpal ligament [2,5]. In addition, gastrointestinal, skin, and ocular involvement are occasionally observed [5].
In addition to impaired physical capabilities, CA patients’ quality of life is impaired and associated with adverse outcomes [6,7]. While progressive peripheral sensorimotor polyneuropathy is well described in systemic amyloidosis [8,9], little is known about the prevalence and effects on autonomic dysfunction. We assume that autonomic dysfunction is a crucial determinant of quality of life and is currently not adequately represented in traditional quality of life questionnaires for HF patients. We therefore prospectively applied the Composite Autonomic Symptom Score-31 (COMPASS-31) to our ATTR cardiomyopathy (ATTR-CM) cohort.
2. Materials and Methods
2.1. Setting
The present analysis was performed at the Medical University of Vienna, Vienna, Austria, within the scope of a CA registry, which is approved by the institutional review board (#1079/2023) and implemented in compliance with the Declaration of Helsinki. All patients diagnosed with ATTR-CM according to the guidelines on cardiomyopathies by the European Society of Cardiology [10] were eligible for inclusion. Prior to inclusion, all patients provided written informed consent. Between November 2022 and November 2024, patients who presented to the institution’s dedicated CA outpatient clinic and participated in the registry were asked to complete the COMPASS-31 questionnaire and the Kansas Cardiomyopathy Questionnaire (KCCQ) as described below.
2.2. Quality of Life Questionnaires
The COMPASS-31 questionnaire is a non-disease-specific patient-reported outcomes measurement tool designed to assess signs and symptoms of autonomic dysfunction [11]. Originally developed as an 84-item version, it has been abbreviated to include only 31 questions in six domains, i.e., orthostatic intolerance, vasomotor dysfunction, secretomotor dysfunction, gastrointestinal, bladder, and pupillomotor dysfunction, from which a composite autonomic symptom score is calculated [11]. The abbreviated COMPASS-31 questionnaire was completed self-sufficiently in this study [11]. For the calculation of COMPASS-31 scores, where higher results indicate an increased symptom burden, the method proposed by Sletten et al. [11] was utilized. Currently, there are no established thresholds for autonomic dysfunction in ATTR-CM patients. For the purpose of this study, the screening threshold reported by Meling et al. [12] comprising 10 points was utilized.
The KCCQ is a quality-of-life measurement tool used for HF patients [13]. The KCCQ scale ranges from 100 (indicating ideal health-related quality of life) to 0 (worst imaginable quality of life) [13]. While it has been developed and extensively studied in general HF populations [14,15], we have previously also demonstrated its independent association with adverse outcome in transthyretin amyloid cardiomyopathy [6]. For this analysis, the abbreviated KCCQ-12 [16], comprising 12 items instead of 23 in the original KCCQ, was utilized.
2.3. Further Parameters of Interest
As part of routine baseline or follow-up in-clinic visits in our tertiary referral center, a complete hemogram, blood chemistry, and biomarkers of HF [N-terminal prohormone of brain natriuretic peptide (NT-proBNP), troponin T, and creatinine/estimated glomerular filtration rate (eGFR)] were compiled. All laboratory analyses were performed at the institution’s central laboratory (Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria), and the eGFR was estimated by applying the chronic kidney disease epidemiology collaboration formula [17]. Disease stage was assessed by applying the United Kingdom National Amyloidosis Centre (NAC) staging system’s criteria [18]. Medical history, comorbidities, and concurrent medication were compiled in the course of the in-clinic visits. Additionally, electronic health records were searched and analyzed if applicable. The presence of diabetes mellitus was assumed with evidence of a level of glycated hemoglobin (HbA1c) ≥ 6.5% without anti-diabetic medication [19] or with HbA1c < 6.5% in patients who receive anti-diabetic medication. The primary endpoint of the present analysis was a composite of (1) all-cause mortality and (2) HF-related hospitalization.
2.4. Statistics
Categorial variables are presented as numbers and percentages. Depending on the variables’ distribution, which was assessed using the Shapiro–Wilk test, continuous parameters are presented as mean and standard deviation (SD) or median and interquartile range (IQR). Cohort characteristics were compared between sub-cohorts stratified by the COMPASS-31 score median using the chi-square test and the t-test for independent samples or the Mann–Whitney U test as applicable. In secondary analysis, patients were stratified by sex and ATTRv status. Correlation between COMPASS-31 sub-scores and the KCCQ was assessed using Spearman correlation analysis. The association of parameters with the COMPASS-31 score was assessed using a step-wise linear regression model. Association of the COMPASS-31 score with the primary endpoint was tested by applying Kaplan–Meier analysis and calculation of the log-rank test as well as Cox proportional hazard regression analysis.
Statistical significance was defined as a confidence interval of 95% and a p-value of <0.05, respectively. Statistical analysis was conducted with using BlueSky Statistics 10.3.4, R package version 8.95 (BlueSky Statistics LLC, Chicago, IL, USA).
3. Results
A total of 129 ATTR-CM patients [81.7 years (IQR: 77.4–84.3), 108 males (83.7%)] were included in the final study cohort. The median COMPASS-31 score was 14 (IQR: 6–29) and the full baseline characteristics are depicted in Table 1 and Figure 1. When applying the screening threshold of 10 points, 75 (58.1%) patients presented with COMPASS-31 results indicative of autonomic dysfunction.

Table 1.
Baseline characteristics of the cardiac amyloidosis cohort, stratified by the median COMPASS-31 score.

Figure 1.
Baseline parameters stratified by the COMPASS-31 score median and the presence of autonomic dysfunction stratified by sex. X indicates the mean.
When stratified by the COMPASS-31 score median, 62 patients constituted the patient cohort with more advanced autonomic dysfunction, while 67 patients scored a COMPASS-31 score equal to or less than the median of 14 [30 (IQR: 19–38) vs. 7 (IQR: 3–9), p < 0.001]. Statistically significant differences between these two groups were observed with regard to New York Heart Association (NYHA) functional class, where the patient group with a COMPASS-31 score > 14 reported more severe HF symptoms. In the cohort with higher COMPASS-31 score, a general tendency toward more severely elevated biomarkers of HF and higher loop diuretics doses was observed, and the comparisons failed to surpass the pre-defined level of statistical significance. Between the two cohorts, highly significant differences were observed with regard to all sub-categories of both the COMPASS-31 score as well as the KCCQ.
In our patient cohort, male individuals were less likely to exhibit a ATTR variant in genetic analysis [6 (5.6%) vs. 4 (19.0%), p = 0.036] and were more likely to be in less advanced disease stages assessed using the NAC staging system (p = 0.019). However, no significant differences with regard to COMPASS-31 or KCCQ (sub-)scores were observed. Compared to wild-type ATTR-CM patients, those with a pathogenic TTR gene variant were significantly younger [68.6 (IQR: (78.6–84.3) vs. 81.9 (64.5–78.5), p = 0.003] and more likely to be female (40.0% vs. 14.4%, p = 0.036), and no ATTR variant patients suffered from concomitant cardiac light chain amyloidosis (Table 2).

Table 2.
Baseline characteristics of the cardiac amyloidosis cohort, stratified by variant transthyretin gene status.
In a Spearman correlation analysis (Table 3, Figure 2), significant associations were observed between all COMPASS-31 sub-scores and the KCCQ overall score. Interestingly, the associations were generally weak to moderate. In univariable linear regression analysis, only the NYHA stage and KCCQ score demonstrated a significant association with the COMPASS-31 score. In an adjusted multivariate model, only the KCCQ score remained significantly associated with the COMPASS-31 score. All results of the linear regression analysis are shown in Table 4.

Table 3.
Correlation of the Composite Autonomic Symptom Score-31 and Kansas City Cardiomyopathy Questionnaire.
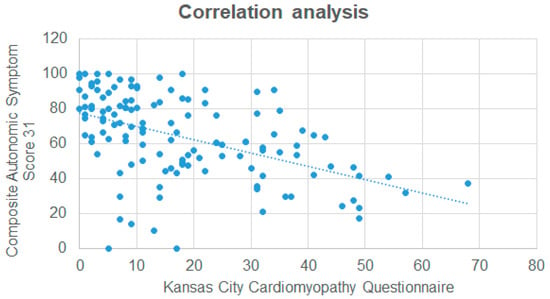
Figure 2.
Correlation analysis between the Kansas City Cardiomyopathy Questionnaire and the Composite Autonomic Symptom Score 31.

Table 4.
Linear regression analysis demonstrating the association between patient characteristics and the COMPASS-31 score.
The primary endpoint, a composite of all-cause mortality and HF-related hospitalizations, occurred in 16 patients (13 HF hospitalizations/3 deaths) after 6.3 (IQR: 2.8–17.1) months. The full observation period was 20.3 (IQR: 10.2–23.0) months. In Kaplan–Meier analysis, a COMPASS-31 score above the median of 14 was also associated with the primary endpoint of all-cause mortality and HF-related hospitalization (Figure 3, log-rank p = 0.047). In Cox regression analysis, the COMPASS-31 score failed to demonstrate significant association with the primary endpoint (p = 0.11).
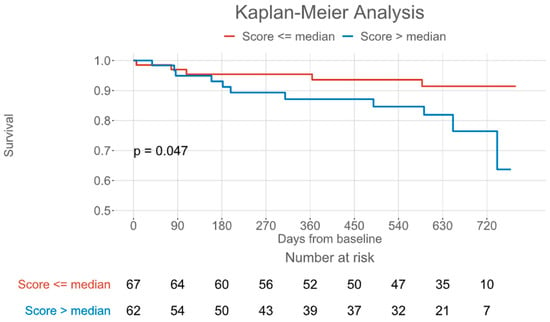
Figure 3.
Kaplan–Meier plot for a composite of all-cause mortality and heart failure-related hospitalization, grouped by the Composite Autonomic Symptom Score 31 median.
4. Discussion
We demonstrated that autonomic dysfunction is highly prevalent in ATTR-CM patients. Relying on previously established thresholds [11], we estimate that almost two-thirds of ATTR-CM patients may be affected by autonomic dysfunction. This study adds to the existing literature as this is the first study to report COMPASS-31 results in wild-type ATTR-CM patients.
Our results suggest that autonomic dysfunction is not significantly associated with traditional assessments of disease severity, e.g., biomarkers of HF, or eGFR, and also not associated with age and sex. Rather, the only entity associated with autonomic dysfunction was quality of life assessed using the KCCQ. Therefore, we suggest that the degree of autonomic dysfunction is among the determinants of overall quality of life and may therefore deserve increased attention from treating physicians.
In clinical practice, symptoms of autonomic dysfunction might easily be overlooked. We speculate that this may be due to the relative difficulty of objectively assessing this aspect of the disease. Furthermore, autonomic dysfunction is diverse and varying in presentation and therefore hard to objectively assess utilizing a single measurement. Previous studies have approached this issue by utilizing heart rate variability in patients with cardiac light chain amyloidosis [20] or by measuring electrochemical skin conductance in ATTR-CM patients [21]. However, these assessments are likely to fail to assess autonomic dysfunction in its entirety. Beyond that, with a high prevalence of cardiac arrhythmia, especially atrial fibrillation [22], heart rate variability alone may not be suitable for assessing autonomic dysfunction in ATTR-CM patients.
While, depending on the genotype, neural involvement is generally a common feature in variant ATTR amyloidosis, in wild-type patients, it seems to appear less frequently, depending on the screening methods [23]. However, as amyloid deposition has been demonstrated to result in both the degeneration of neurons in the sensory and autonomic ganglia as well as atrophy of Schwann cells [24], some degree of neural involvement may be expected in wild-type ATTR patients as well. From findings in a small mixed cohort of patients with ATTR amyloidosis who underwent comprehensive neurological examination including skin biopsy, it can be concluded that 40% of wild-type patients demonstrated findings in line with large and/or small fiber neuropathy that could not be explained by comorbidities [25]. This also supports the notion that neurological involvement and concomitant symptoms are frequently underdiagnosed in these patients.
As a large proportion of ATTR-CM patients are of advanced age, comorbidities and their effect on autonomic dysfunction specifically, but also quality of life more broadly, need to be considered. Most importantly, poorly controlled diabetes mellitus may also lead to autonomic dysfunction and increased COMPASS-31 scores, as previously reported [26]. In our study, however, concomitant diabetes mellitus had no effect on the COMPASS-31 score. This suggests that cohort autonomic dysfunction was primarily due to ATTR amyloidosis in our study.
In a meta-analysis, Pearson and Smart [27] demonstrated that exercise rehabilitation may be suitable to improve heart rate variability, one aspect of autonomic dysfunction. This suggests that autonomic dysfunction is a modifiable aspect of disease and may be utilized as a potential endpoint in rehabilitation trials or quality-of-life focused intervention studies.
Finally, in line with previous studies [21], our results suggest that autonomic dysfunction is also linked to adverse outcome.
4.1. Limitations
As this analysis was conducted in a single tertiary referral center setting, certain biases with regard to patient selection cannot fully be excluded without confirmation of findings in larger, ideally multi-center cohorts.
The majority of participants in our analysis were wild-type patients and were only followed for a median of 20 months. A higher proportion of variant ATTR patients, longer follow-up, and repeated deployment of the COMPASS-31 would have likely increased the robustness of our analysis. As ATTR-CM patients are typically of advanced age, autonomic dysfunction could also be caused by concomitant disease or other unrecognized factors and should ideally be compared to a healthy control group or matched non-ATTR-CM HF patients.
Patient-reported outcome measurements are always susceptible to reporting bias and may fail to adequately represent patients who are not willing to answer questionnaires or fail to recall symptoms or estimate symptom frequency or severity. Finally, there are currently no reliable data on the added clinical benefit with the application of patient-reported outcome measurement tools in ATTR-CM.
4.2. Future Research
Currently, autonomic dysfunction is laborious to evaluate objectively. Objective examinations, e.g., skin conductance measurements, tilt-table testing, or Ewing’s battery, are unlikely to be easily established in clinical practice. Future studies ought to aim to validate tools that are routinely available in practice like the COMPASS-31 questionnaire or heart rate variability utilizing more objective measurements of autonomic dysfunction. In addition, longitudinal assessment of changes in the COMPASS-31 may help to better understand treatment effects, both of specific therapeutics or conventional concomitant therapy, e.g., loop diuretics. Furthermore, as autonomic dysfunction seems to be associated with subjective burden of disease, future randomized controlled trials should include the COMPASS-31 questionnaire to assess potential treatment effects on this aspect of ATTR-CM.
5. Conclusions
Autonomic dysfunction is highly prevalent in ATTR-CM and affects almost two-thirds of this patient population. As autonomic dysfunction is poorly correlated with other markers of disease severity including biomarkers of HF, routine screening for autonomic dysfunction may be considered, especially as the presence of autonomic dysfunction is likely associated with more severely impaired quality of life and worse outcomes.
Author Contributions
Conceptualization, M.P. and F.D.; Data curation, M.P. and K.H.; Investigation, M.P., C.K., N.E., L.M.S., R.R., C.B., and L.C.L.; Methodology, M.P. and F.D.; Supervision, C.H., R.B.E., J.B.-K., J.K., A.A.K., and F.D.; Writing—original draft, M.P.; Writing—review and editing, M.P., K.H., C.K., N.E., L.M.S., R.R., C.B., L.C.L., M.E., C.H., R.B.E., J.B.-K., J.K., A.A.K., and F.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Institutional Review Board at the Medical University of Vienna, Decision No: EK 1079/2023 (27 July 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Conflicts of Interest
F.D. has received research funding from the Austrian Society of Cardiology and Pfizer as well as honoraria and travel support from Bayer, Novartis, Alnylam, Pfizer, and AOP Orphan Pharmaceuticals. R.B.E. has received honoraria from Boehringer Ingelheim and travel support from OrphaCare and participates in an advisory board for Boehringer Ingelheim. All other authors have no relevant financial or non-financial interests to disclose.
Abbreviations
The following abbreviations are used in this manuscript:
| ATTR | amyloid transthyretin |
| ATTR-CM | amyloid transthyretin cardiomyopathy |
| CA | cardiac amyloidosis |
| COMPASS-31 | Composite Autonomic Symptom Score-31 |
| eGFR | estimated glomerular filtration rate |
| HbA1c | glycated hemoglobin |
| HF | heart failure |
| IQR | interquartile range |
| KCCQ | Kansas City Cardiomyopathy Questionnaire |
| NAC | National Amyloidosis Centre |
| NYHA | New York Heart Association functional class |
| NT-proBNP | N-terminal prohormone of brain natriuretic peptide |
References
- Kittleson, M.M.; Maurer, M.S.; Ambardekar, A.V.; Bullock-Palmer, R.P.; Chang, P.P.; Eisen, H.J.; Nair, A.P.; Nativi-Nicolau, J.; Ruberg, F.L. Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement from the American Heart Association. Circulation 2020, 144, E7–E22. [Google Scholar] [CrossRef] [PubMed]
- Grogan, M.; Scott, C.G.; Kyle, R.A.; Zeldenrust, S.R.; Gertz, M.A.; Lin, G.; Klarich, K.W.; Miller, W.L.; Maleszewski, J.J.; Dispenzieri, A. Natural History of Wild-Type Transthyretin Cardiac Amyloidosis and Risk Stratification Using a Novel Staging System. J. Am. Coll. Cardiol. 2016, 68, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Aurich, M.; Bucur, J.; Vey, J.A.; Greiner, S.; Aus Dem Siepen, F.; Hegenbart, U.; Schönland, S.; Katus, H.A.; Frey, N.; Mereles, D. Prognosis of Light Chain Amyloidosis: A Multivariable Analysis for Survival Prediction in Patients with Cardiac Involvement Proven by Endomyocardial Biopsy. Open Heart 2023, 10, e002310. [Google Scholar] [CrossRef] [PubMed]
- Sanchorawala, V. Systemic Light Chain Amyloidosis. N. Engl. J. Med. 2024, 390, 2295–2307. [Google Scholar] [CrossRef]
- Damy, T.; Costes, B.; Hagège, A.A.; Donal, E.; Eicher, J.C.; Slama, M.; Guellich, A.; Rappeneau, S.; Gueffet, J.P.; Logeart, D.; et al. Prevalence and Clinical Phenotype of Hereditary Transthyretin Amyloid Cardiomyopathy in Patients with Increased Left Ventricular Wall Thickness. Eur. Heart J. 2016, 37, 1826–1834. [Google Scholar] [CrossRef]
- Poledniczek, M.; Kronberger, C.; Willixhofer, R.; Ermolaev, N.; Cherouny, B.; Dachs, T.-M.; Rettl, R.; Binder-Rodriguez, C.; Camuz Ligios, L.; Gregshammer, B.; et al. Health-Related Quality of Life Is an Independent Predictor of Mortality and Hospitalisations in Transthyretin Amyloid Cardiomyopathy: A Prospective Cohort Study. Qual. Life Res. 2024, 33, 2743–2753. [Google Scholar] [CrossRef]
- Clerc, O.F.; Vijayakumar, S.; Cuddy, S.A.M.; Bianchi, G.; Canseco Neri, J.; Taylor, A.; Benz, D.C.; Datar, Y.; Kijewski, M.F.; Yee, A.J.; et al. Functional Status and Quality of Life in Light-Chain Amyloidosis: Advanced Imaging, Longitudinal Changes, and Outcomes. JACC Heart Fail. 2024, 12, 1994–2006. [Google Scholar] [CrossRef]
- Poli, L.; Labella, B.; Cotti Piccinelli, S.; Caria, F.; Risi, B.; Damioli, S.; Padovani, A.; Filosto, M. Hereditary Transthyretin Amyloidosis: A Comprehensive Review with a Focus on Peripheral Neuropathy. Front. Neurol. 2023, 14, 1242815. [Google Scholar] [CrossRef]
- Louwsma, J.; Brunger, A.F.; Bijzet, J.; Kroesen, B.J.; Roeloffzen, W.W.H.; Bischof, A.; Kuhle, J.; Drost, G.; Lange, F.; Kuks, J.B.M.; et al. Neurofilament Light Chain, a Biomarker for Polyneuropathy in Systemic Amyloidosis. Amyloid 2021, 28, 50–55. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the Management of Cardiomyopathies Developed by the Task Force on the Management of Cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Sletten, D.M.; Suarez, G.A.; Low, P.A.; Mandrekar, J.; Singer, W. COMPASS 31: A Refined and Abbreviated Composite Autonomic Symptom Score. Mayo Clin. Proc. 2012, 87, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Meling, S.; Tjora, E.; Eichele, H.; Ejskjaer, N.; Carlsen, S.; Njølstad, P.R.; Brock, C.; Søfteland, E. The Composite Autonomic Symptom Score 31 Questionnaire: A Sensitive Test to Detect Risk for Autonomic Neuropathy. J. Diabetes Res. 2023, 2023, 4441115. [Google Scholar] [CrossRef] [PubMed]
- Green, C.P.; Porter, C.B.; Bresnahan, D.R.; Spertus, J.A. Development and Evaluation of the Kansas City Cardiomyopathy Questionnaire: A New Health Status Measure for Heart Failure. J. Am. Coll. Cardiol. 2000, 35, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Ravera, A.; Santema, B.T.; Sama, I.E.; Meyer, S.; Lombardi, C.M.; Carubelli, V.; Ferreira, J.P.; Lang, C.C.; Dickstein, K.; Anker, S.D.; et al. Quality of Life in Men and Women with Heart Failure: Association with Outcome, and Comparison between the Kansas City Cardiomyopathy Questionnaire and the EuroQol 5 Dimensions Questionnaire. Eur. J. Heart Fail. 2021, 23, 567–577. [Google Scholar] [CrossRef]
- Greene, S.J.; Butler, J.; Spertus, J.A.; Hellkamp, A.S.; Vaduganathan, M.; Devore, A.D.; Albert, N.M.; Duffy, C.I.; Patterson, J.H.; Thomas, L.; et al. Comparison of New York Heart Association Class and Patient-Reported Outcomes for Heart Failure With Reduced Ejection Fraction. JAMA Cardiol. 2021, 6, 522–531. [Google Scholar] [CrossRef]
- Spertus, J.A.; Jones, P.G. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ. Cardiovasc. Qual. Outcomes 2015, 8, 469–476. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Lente, F.V.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Damy, T.; Fontana, M.; Hutchinson, M.; Lachmann, H.J.; Martinez-Naharro, A.; Quarta, C.C.; Rezk, T.; Whelan, C.J.; Gonzalez-Lopez, E.; et al. A New Staging System for Cardiac Transthyretin Amyloidosis. Eur. Heart J. 2018, 39, 2799–2806. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on Diabetes, Pre-Diabetes, and Cardiovascular Diseases Developed in Collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- Yamada, S.; Yoshihisa, A.; Hijioka, N.; Kamioka, M.; Kaneshiro, T.; Yokokawa, T.; Misaka, T.; Ishida, T.; Takeishi, Y. Autonomic Dysfunction in Cardiac Amyloidosis Assessed by Heart Rate Variability and Heart Rate Turbulence. Ann. Noninvasive Electrocardiol. 2020, 25, e12749. [Google Scholar] [CrossRef]
- Kharoubi, M.; Roche, F.; Bézard, M.; Hupin, D.; Silva, S.; Oghina, S.; Chalard, C.; Zaroui, A.; Galat, A.; Guendouz, S.; et al. Prevalence and Prognostic Value of Autonomic Neuropathy Assessed by Sudoscan® in Transthyretin Wild-type Cardiac Amyloidosis. ESC Heart Fail. 2020, 8, 1656. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.; Oliveros, E.; Parekh, H.; Farmakis, D. Epidemiology, Mechanisms, and Management of Atrial Fibrillation in Cardiac Amyloidosis. Curr. Probl. Cardiol. 2023, 48, 101571. [Google Scholar] [CrossRef] [PubMed]
- Ruberg, F.L.; Grogan, M.; Hanna, M.; Kelly, J.W.; Maurer, M.S. Transthyretin Amyloid Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2872–2891. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Katsuno, M. The Ultrastructure of Tissue Damage by Amyloid Fibrils. Molecules 2021, 26, 4611. [Google Scholar] [CrossRef]
- Papagianni, A.; Ihne, S.; Zeller, D.; Morbach, C.; Üçeyler, N.; Sommer, C. Clinical and Apparative Investigation of Large and Small Nerve Fiber Impairment in Mixed Cohort of ATTR-Amyloidosis: Impact on Patient Management and New Insights in Wild-Type. Amyloid 2022, 29, 14–22. [Google Scholar] [CrossRef]
- Singh, R.; Arbaz, M.; Rai, N.K.; Joshi, R. Diagnostic Accuracy of Composite Autonomic Symptom Scale 31 (COMPASS-31) in Early Detection of Autonomic Dysfunction in Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2019, 12, 1735–1742. [Google Scholar] [CrossRef]
- Pearson, M.J.; Smart, N.A. Exercise Therapy and Autonomic Function in Heart Failure Patients: A Systematic Review and Meta-Analysis. Heart Fail. Rev. 2018, 23, 91–108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).