Impact of High Intensity Contact Physical Activity During a Match on Echocardiographic Parameters and High-Sensitivity Troponin I in Competitive Rugby Players
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants Population
2.2. Data Collection
2.3. Blood Tests
2.4. Echocardiography
2.5. Study Endpoint
2.6. Statistical Analysis
3. Results
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| hs-TnI | high-sensitivity troponin I |
| CMIA | chemiluminescence microparticle immunoassay |
| ECG | electrocardiogram |
| ROC | receiver operating characteristic |
| ROS | radical oxygen species |
| 2D | two-dimensional |
| 3D | three-dimensional |
| LV | left ventricle |
| GLS | global longitudinal strain |
References
- Arem, H.; Moore, S.C.; Patel, A.; Hartge, P.; De Gonzalez, A.B.; Visvanathan, K.; Campbell, P.T.; Freedman, M.; Weiderpass, E.; Adami, H.O.; et al. Leisure time physical activity and mortality. JAMA Intern. Med. 2015, 175, 959. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.P.; Wai, J.P.M.; Tsai, M.K.; Yang, Y.C.; Cheng, T.Y.D.; Lee, M.-C.; Chan, H.T.; Tsao, C.K.; Tsai, S.P.; Wu, X. Minimum amount of physical activity for reduced mortality and extended life expectancy: A prospective cohort study. Lancet 2011, 378, 1244–1253. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. ESC Scientific Document Group. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Fortescue, E.B.; Shin, A.Y.; Greenes, D.S.; Mannix, R.C.; Agarwal, S.; Feldman, B.J.; Shah, M.I.; Rifai, N.; Landzberg, M.J.; Newburger, J.W.; et al. Cardiac troponin increases among runners in the Boston Marathon. Ann. Emerg. Med. 2007, 49, 137–143.e1. [Google Scholar] [CrossRef] [PubMed]
- Scharhag, J.; Herrmann, M.; Urhausen, A.; Haschke, M.; Herrmann, W.; Kindermann, W. Independent elevations of N-terminal pro–brain natriuretic peptide and cardiac troponins in endurance athletes after prolonged strenuous exercise. Am. Heart J. 2005, 150, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.D.; Baggish, A.L.; Kovacs, R.J.; Link, M.S.; Maron, M.S.; Mitchell, J.H. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task force 1: Classification of sports: Dynamic, static and impact. J. Am. Coll. Cardiol. 2015, 66, 2350–2355. [Google Scholar] [CrossRef]
- Sigurdardottir, F.D.; Lyngbakken, M.N.; Holmen, O.L.; Dalen, H.; Hveem, K.; Røsjø, H.; Omland, T. Relative prognostic value of cardiac troponin I and C-reactive protein in the general population (from the Nord-Trøndelag Health [HUNT] Study). Am. J. Cardiol. 2018, 121, 949–955. [Google Scholar] [CrossRef]
- Gresslien, T.; Agewall, S. Troponin and exercise. Int. J. Cardiol. 2016, 221, 609–621. [Google Scholar] [CrossRef]
- Eijsvogels, T.M.H.; Fernandez, A.B.; Thompson, P.D. Are there deleterious cardiac effects of acute and chronic endurance exercise? Physiol. Rev. 2016, 96, 99–125. [Google Scholar] [CrossRef]
- Evangelista, A.; Flachskampf, F.; Lancellotti, P.; Badano, L.; Aguilar, R.; Monaghan, M.; Zamorano, J.; Nihoyannopoulos, P. European Association of Echocardiography. European Association of Echocardiography recommendations for standardization of performance, digital storage and reporting of echocardiographic studies. Eur. J. Echocardiogr. 2008, 9, 438–448. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. Physiological Society Symposium—The Athlete’s Heart. Exp. Physiol. 2003, 88, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Dawson, E.; George, K.; Shave, R.; Whyte, G.; Ball, D. Does the human heart fatigue subsequent to prolonged exercise? Sports Med. 2003, 33, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Middleton, N.; Shave, R.; George, K.; Whyte, G.; Hart, E.; Atkinson, G. Left ventricular function immediately following prolonged exercise. Med. Sci. Sports Exerc. 2006, 38, 681–687. [Google Scholar] [CrossRef]
- Eysmann, S.B.; Gervino, E.; Vatner, D.E.; Katz, S.E.; Decker, L.; Douglas, P.S. Prolonged exercise alters beta-adrenergic responsiveness in healthy sedentary humans. J. Appl. Physiol. 1996, 80, 616–622. [Google Scholar] [CrossRef]
- Gerdts, E.; Bjornstad, H.; Toft, S.; Devereux, R.B.; Omvik, P. Impact of diastolic doppler indices on exercise capacity in hypertensive patients with electrocardiographic left ventricular hypertrophy (a LIFE substudy). J. Hypertens. 2002, 20, 1223–1229. [Google Scholar] [CrossRef]
- Sharifov, O.F.; Gupta, H. What is the evidence that the tissue doppler index E/e′ reflects left ventricular filling pressure changes after exercise or pharmacological intervention for evaluating diastolic function? A systematic review. J. Am. Heart Assoc. 2017, 6, e004766. [Google Scholar] [CrossRef]
- Murray, J.; Bennett, H.; Bezak, E.; Perry, R.; Boyle, T. The effect of exercise on left ventricular global longitudinal strain. Eur. J. Appl. Physiol. 2022, 122, 1397–1408. [Google Scholar] [CrossRef]
- Baradaran, A.; Nasri, H.; Rafieian-Kopaei, M. Oxidative stress and hypertension: Possibility of hypertension therapy with antioxidants. J. Res. Med. Sci. 2014, 19, 358–367. [Google Scholar]
- Rodrigo, R.; Gonzalez, J.; Paoletto, F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens. Res. 2011, 34, 431–440. [Google Scholar] [CrossRef]
- Moreno-Ruíz, L.A.; Ibarra-Quevedo, D.; Rodríguez-Martínez, E.; Maldonado, P.D.; Sarabia-Ortega, B.; Hernández-Martínez, J.G.; Espinosa-Caleti, B.; Mendoza-Pérez, B.; Rivas-Arancibia, S. Oxidative Stress State Is Associated with Left Ventricular Mechanics Changes, Measured by Speckle Tracking in Essential Hypertensive Patients. Oxidative Med. Cell Longev. 2015, 2015, 502107. [Google Scholar] [CrossRef]
- Ji, L.L.; Kang, C.; Zhang, Y. Exercise-induced hormesis and skeletal muscle health. Free Radic. Biol. Med. 2016, 98, 113–122. [Google Scholar] [CrossRef]
- Lindner, G.; Pfortmueller, C.A.; Braun, C.T.; Exadaktylos, A.K. Non-acute myocardial infarction-related causes of elevated high-sensitive troponin T in the emergency room: A cross-sectional analysis. Intern. Emerg. Med. 2014, 9, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Prasada, S.; Rivera, A.; Nishtala, A.; Pawlowski, A.E.; Sinha, A.; Bundy, J.D.; Chadha, S.A.; Ahmad, F.S.; Khan, S.S.; Achenbach, C.; et al. Differential Associations of Chronic Inflammatory Diseases with Incident Heart Failure. JACC Heart Fail. 2020, 8, 489–498. [Google Scholar] [CrossRef]
- Chaulin, A.M.; Karslyan, L.S.; Bazyuk, E.V.; Nurbaltaeva, D.A.; Duplyakov, D.V. Clinical and Diagnostic Value of Cardiac Markers in Human Biological Fluids. Kardiologiia 2019, 59, 66–75. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Katrukha, I.A.; Katrukha, A.G. Myocardial Injury and the Release of Troponins I and T in the Blood of Patients. Clin. Chem. 2021, 67, 124–130. [Google Scholar] [CrossRef]
- Aengevaeren, V.L.; Baggish, A.L.; Chung, E.H.; George, K.; Kleiven, Ø.; Mingels, A.M.; Ørn, S.; Shave, R.E.; Thompson, P.D.; Eijsvogels, T.M. Exercise-Induced Cardiac Troponin Elevations: From Underlying Mechanisms to Clinical Relevance. Circulation 2021, 144, 1955–1972. [Google Scholar] [CrossRef]
- Aengevaeren, V.L.; Froeling, M.; Hooijmans, M.T.; Monte, J.R.; Berg-Faay, S.v.D.; Hopman, M.T.; Strijkers, G.J.; Nederveen, A.J.; Bakermans, A.; Eijsvogels, T.M. Myocardial Injury and Compromised Cardiomyocyte Integrity Following a Marathon Run. JACC Cardiovasc. Imaging 2020, 13, 1445–1447. [Google Scholar] [CrossRef]
- Vujic, A.; Lerchenmüller, C.; Wu, T.-D.; Guillermier, C.; Rabolli, C.P.; Gonzalez, E.; Senyo, S.E.; Liu, X.; Guerquin-Kern, J.-L.; Steinhauser, M.L.; et al. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nat. Commun. 2018, 9, 1659. [Google Scholar] [CrossRef]
- Weil, B.R.; Suzuki, G.; Young, R.F.; Iyer, V.; Canty, J.M., Jr. Troponin Release and Reversible Left Ventricular Dysfunction After Transient Pressure Overload. J. Am. Coll. Cardiol. 2018, 71, 2906–2916. [Google Scholar] [CrossRef]
- Árnadóttir, Á.; Pedersen, S.; Hasselbalch, R.B.; Goetze, J.P.; Friis-Hansen, L.J.; Bloch-Münster, A.-M.; Jensen, J.S.; Bundgaard, H.; Iversen, K. Temporal Release of High-Sensitivity Cardiac Troponin T and I and Copeptin After Brief Induced Coronary Artery Balloon Occlusion in Humans. Circulation 2021, 143, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, N.; Czarnecki, A.; Kumar, K.; Fallah-Rad, N.; Lytwyn, M.; Han, S.-Y.; Francis, A.; Walker, J.R.; Kirkpatrick, I.D.; Neilan, T.G.; et al. Relation of biomarkers and cardiac magnetic resonance imaging after marathon running. Am. J. Cardiol. 2009, 103, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Shave, R.; George, K.P.; Atkinson, G.; Hart, E.; Middleton, N.; Whyte, G.; Gaze, D.; Collinson, P.O. Exercise-induced cardiac troponin T release: A meta-analysis. Med. Sci. Sports Exerc. 2007, 39, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.; Lee, K.K.; Stewart, S.D.; Wild, A.; Fujisawa, T.; Ferry, A.V.; Stables, C.L.; Lithgow, H.; Chapman, A.R.; Anand, A.; et al. Effect of Exercise Intensity and Duration on Cardiac Troponin Release. Circulation 2020, 141, 83–85. [Google Scholar] [CrossRef]
- Janssen, S.; Berge, K.; Luiken, T.; Aengevaeren, V.L.; Eijsvogels, T.M. Cardiac troponin release in athletes: What do we know and where should we go? Curr. Opin. Physiol. 2023, 31, 100629. [Google Scholar] [CrossRef]
- Eijsvogels, T.M.; Hoogerwerf, M.D.; Maessen, M.F.; Seeger, J.P.; George, K.P.; Hopman, M.T.; Thijssen, D.H. Predictors of cardiac troponin release after a marathon. J. Sci. Med. Sport. 2015, 18, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Aengevaeren, V.L.; Hopman, M.T.; Thompson, P.D.; Bakker, E.A.; George, K.P.; Thijssen, D.H.; Eijsvogels, T.M. Exercise-Induced Cardiac Troponin I Increase and Incident Mortality and Cardiovascular Events. Circulation 2019, 140, 804–814. [Google Scholar] [CrossRef]
- Eijsvogels, T.M.; Hoogerwerf, M.D.; Oudegeest-Sander, M.H.; Hopman, M.T.; Thijssen, D.H. The impact of exercise intensity on cardiac troponin I release. Int. J. Cardiol. 2014, 171, e3–e4. [Google Scholar] [CrossRef]
- Newby, L.K.; Jesse, R.L.; Babb, J.D.; Christenson, R.H.; De Fer, T.M.; Diamond, G.A.; Fesmire, F.M.; Geraci, S.A.; Gersh, B.J.; Larsen, G.C.; et al. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: A report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J. Am. Coll. Cardiol. 2012, 60, 2427–2463. [Google Scholar] [CrossRef]
- Lavallaz, J.d.F.d.; Zehntner, T.; Puelacher, C.; Walter, J.; Strebel, I.; Rentsch, K.; Boeddinghaus, J.; Nestelberger, T.; Twerenbold, R.; Mueller, C. Rhabdomyolysis: A Noncardiac Source of Increased Circulating Concentrations of Cardiac Troponin T? J. Am. Coll. Cardiol. 2018, 72 Pt A, 2936–2937. [Google Scholar] [CrossRef]
- Lippi, G.; Guidi, G.C.; Salvagno, G.L.; Impellizzeri, F.; Schena, F. Highly sensitive cardiac troponin T is not increased by strenuous eccentric exercise. Am. J. Cardiol. 2010, 105, 1043–1044. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.M.C.; Davison, G.W.; McClean, C.M.; Murphy, M.H. A systematic review of the acute effects of exercise on immune and inflammatory indices in untrained adults. Sports Med.-Open 2015, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Guthrie, P.; Growden, G. Rugby for Dummies; John Wiley & Sons: Mississauga, ON, Canada, 2011; pp. 113–126. [Google Scholar]
- Quarrie, K.L.; Hopkins, W.G. Tackle injuries in professional Rugby Union. Am. J. Sports Med. 2008, 36, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E. Coronary artery dissection after a rugby injury. A case report. S. Afr. Med. J. 1990, 77, 586–587. [Google Scholar]
- Yates, M.T.; Aldrete, V. Blunt Trauma Causing Aortic Rupture. Physician Sportsmed. 1991, 19, 96–107. [Google Scholar] [CrossRef]

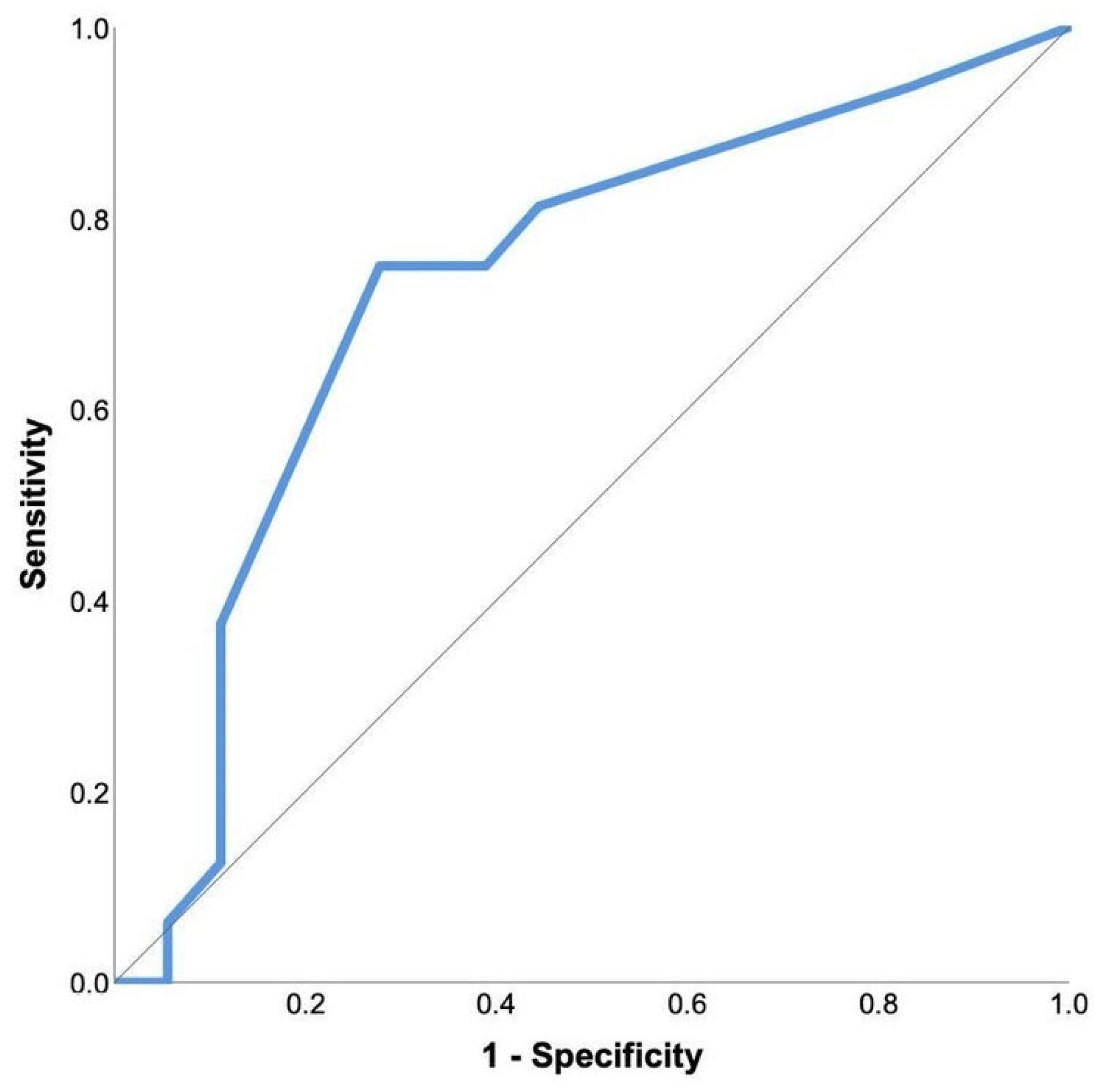
| Percentage (%), Mean (Standard Deviation) or Median (Interquartile Range) | ||
|---|---|---|
| Age | 26 (22–30) | |
| Smokers | 23.52% | |
| Duration of smoking (years) | 9 (6–13) | |
| Number of cigarettes per day | 13 (10–18) | |
| Alcohol consumption | 94.11% | |
| Level of education (higher education/secondary education) | 67.64%/32.35% | |
| Employment status (unemployed/student/employed) | 11.76%/32.35%/55.88% | |
| Chronic therapy † | 5.88% | |
| Duration of rugby training (years) | 13 (9–15) | |
| Forward pack/back line | 47.05%/52.94% | |
| Height (cm) | 182 (175–186) | |
| Mass (kg) | 96.2 (85.4–110.9) | |
| BMI (kg/m2) | 28.9 (26.3–33.1) | |
| BSA (m2) | 2.2 (0.2) | |
| Chest circumference (cm) | 107 (10) | |
| Right hand grip (kg) | 40.6 (9) | |
| Left hand grip (kg) | 36.8 (10.5) | |
| Systolic pressure before the match | Before the match (mmHg) | 134 (16) |
| After the match (mmHg) | 132 (12) | |
| Diastolic pressure | Before the match (mmHg) | 76 (12) |
| After the match (mmHg) | 75 (11) | |
| Pulse | Before the match (/min) | 72 (10) |
| After the match(/min) | 92 (11) | |
| Respiratory rate | Before the match (/min) | 16(14–18) |
| After the match (/min) | 22 (18–24) | |
| Oxygen saturation | Before the match (%) | 98 (97–98) |
| After the match (%) | 98 (97–98) | |
| Body temperature | Before the match (°C) | 36.4 (36.2–36.5) |
| After the match (°C) | 36.4 (36.3–36.6) | |
| PR interval | Before the match (ms) | 156 (25) |
| After the match (ms) | 155 (19) | |
| QRS duration | Before the match (ms) | 97 (9) |
| After the match (ms) | 96 (8) | |
| QT duration | Before the match (ms) | 398 (25) |
| After the match(ms) | 360 (22) | |
| QTc | Before the match (ms) | 397 (390–409) |
| After the match (ms) | 402.9 (12.4) | |
| Mean (Standard Deviation) or Median (Interquartile Range) | |||
|---|---|---|---|
| Before the Match | After the Match | p | |
| LVEF 2D (%) | 55 (51–57) | 50 (47–52) | <0.001 |
| LVEF 3D (%) | 55 (5) | 50 (4) | <0.001 |
| LV diameter (mm) | 51 (4) | 53 (4) | <0.001 |
| Indexed (BSA) LV diameter (mm/m2) | 23.2 (2.1) | 24 (2) | <0.001 |
| E (cm/s) | 0.77 (0.11) | 0.66 (0.11) | <0.001 |
| A (cm/s) | 0.5 (0.09) | 0.52 (0.1) | 0.269 |
| E/A | 1.48 (1.36–1.82) | 1.26(1.18–1.41) | <0.001 |
| E′ septal (m/s) | 0.12 (0.02) | 0.1 (0.02) | <0.001 |
| E′ lateral (m/s) | 0.18 (0.03) | 0.15 (0.03) | <0.001 |
| Mean E′ (m/s) | 0.15 (0.02) | 0.13 (0.02) | <0.001 |
| E/E′ mean | 5.3 (0.84) | 5.37 (1.12) | <0.001 |
| 2D LV volume (end-systolic) (mL) | 64 (8) | 71 (11) | 0.004 |
| 2D LV volume (end-diastolic) (mL) | 140 (131–157) | 138 (129–149) | 0.487 |
| 3D LV volume (end-systolic) (mL) | 64 (9) | 71 (10) | <0.001 |
| 3D LV volume (end-diastolic) (mL) | 142 (20) | 145 (20) | 0.126 |
| Indexed 2D LV volume (end-systolic) (mL/m2) | 29.4 (3.6) | 32.3 (4.9) | 0.005 |
| Indexed 2D LV volume (end-diastolic) (mL/m2) | 65.3 (8.1) | 64.3 (9.1) | 0.479 |
| Indexed 3D LV volume (end-systolic) (mL/m2) | 29 (4.5) | 32.5 (4.6) | <0.001 |
| Indexed 3D LV volume (end-diastolic) (mL/m2) | 64.7 (8.5) | 66.22 (8.2) | 0.174 |
| LV GLS (%) | −17.9 (1.6) | −16 (1.7) | <0.001 |
| 2D stroke volume (mL) | 78 (70–89) | 70 (62–75) | <0.001 |
| 3D stroke volume (mL) | 77 (67–85) | 73 (65–82) | 0.001 |
| Indexed 2D stroke volume (mL/m2) | 36.1 (33.2–40.2) | 32 (29.1–35.3) | <0.001 |
| Indexed 3D stroke volume (mL/m2) | 36 (6.8) | 33.29 (5.5) | 0.001 |
| Basal septum TVI (%) | −20.1 (6.1) | −13.9 (6.2) | <0.001 |
| Mid-septum TVI (%) | −14.5 (6) | −10.3 (−13.6–−7.9) | 0.006 |
| Apical septum TVI (%) | −20.1 (5.4) | −16.4 (5.6) | 0.003 |
| Basal lateral TVI (%) | −13.4 (6.9) | −8.8 (4.7) | <0.001 |
| Mid-lateral TVI (%) | −12.4 (6.2) | −12 (6.5) | 0.782 |
| Apical lateral TVI (%) | −12.5 (7.7) | −12 (7.5) | 0.601 |
| Mean TVI (%) | −15.5 (2.4) | −12.3 (2.7) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radic, P.; Bulj, N.; Car, S.; Cancarevic, M.; Sikic, A.; Delic-Brkljacic, D.; Pavlov, M.; Babic, Z. Impact of High Intensity Contact Physical Activity During a Match on Echocardiographic Parameters and High-Sensitivity Troponin I in Competitive Rugby Players. J. Clin. Med. 2025, 14, 2226. https://doi.org/10.3390/jcm14072226
Radic P, Bulj N, Car S, Cancarevic M, Sikic A, Delic-Brkljacic D, Pavlov M, Babic Z. Impact of High Intensity Contact Physical Activity During a Match on Echocardiographic Parameters and High-Sensitivity Troponin I in Competitive Rugby Players. Journal of Clinical Medicine. 2025; 14(7):2226. https://doi.org/10.3390/jcm14072226
Chicago/Turabian StyleRadic, Petra, Nikola Bulj, Sinisa Car, Martina Cancarevic, Aljosa Sikic, Diana Delic-Brkljacic, Marin Pavlov, and Zdravko Babic. 2025. "Impact of High Intensity Contact Physical Activity During a Match on Echocardiographic Parameters and High-Sensitivity Troponin I in Competitive Rugby Players" Journal of Clinical Medicine 14, no. 7: 2226. https://doi.org/10.3390/jcm14072226
APA StyleRadic, P., Bulj, N., Car, S., Cancarevic, M., Sikic, A., Delic-Brkljacic, D., Pavlov, M., & Babic, Z. (2025). Impact of High Intensity Contact Physical Activity During a Match on Echocardiographic Parameters and High-Sensitivity Troponin I in Competitive Rugby Players. Journal of Clinical Medicine, 14(7), 2226. https://doi.org/10.3390/jcm14072226






