Abstract
Background: The increasing prevalence of severe calcified coronary artery disease has expanded the role of rotational atherectomy (RA) in percutaneous coronary intervention (PCI). In the drug-eluting stent (DES) era, RA remains a key tool for complex lesion modification. This review focuses on its clinical outcomes and evolving indications. Methods: This review was conducted as a narrative review, focusing on the most relevant clinical studies regarding RA in the DES era. Articles were identified through a systematic PubMed search. Results: Comparing to early-generation DES, new-generation DES (NG-DES) demonstrate superior outcomes due to thinner struts and biocompatible polymers. RA plays a critical role in challenging scenarios, including chronic total occlusions and de novo small vessel lesions. Despite these advances, further randomized controlled trials are needed to validate the long-term safety and efficacy of RA-based strategies. Conclusions: This review highlights the clinical outcomes of RA in the DES era and its evolving role in contemporary cardiology. RA has shown promising potential for broader clinical applications in complex coronary artery disease. However, critical knowledge gaps remain. Further research is needed to refine RA-based strategies.
1. Introduction
Since its introduction in 1977, rotational atherectomy (RA) has been widely used to treat heavily calcified coronary artery disease [1]. Coronary artery calcification (CAC) increases with atherosclerosis, aging, chronic hypercalcemia, hyperlipidemia, or chronic renal disease [2,3]. Indeed, a large scale meta-analysis revealed that the prevalence of moderate to severe CAC was approximately one-third of the overall study population (6211 of 13,622) [4]. With the growing prevalence of calcified lesions in patients, the RA has become increasingly important, highlighting the necessity of understanding its evolving role in tandem with technological advancements [5].
Several plaque modification techniques have been introduced to facilitate percutaneous coronary intervention (PCI) in calcified lesions, including orbital atherectomy and intravascular lithotripsy. However, RA remains one of the most widely utilized techniques due to its distinct mechanism of differential cutting and long-standing clinical experience. This study focuses on RA in the drug-eluting stent era, examining its efficacy and clinical implications.
Recent data from a pooled analysis of the two randomized ROTAXUS and PREPARE-CALC trials showed the technical evolution in RA practice and proposed recommendations to improve RA skills [6]. Those studies demonstrated superior clinical outcomes of new-generation drug-eluting stent (NG-DES) compared to early-generation drug-eluting stent (EG-DES). However, randomized trials often fail to fully capture real-world clinical practice due to inherent selection bias. To address this limitation, we reviewed and summarized clinical outcomes from contemporary studies utilizing real-world data in drug-eluting stent (DES) era.
Within the context, particular attention is given to advancements in the treatment of complex coronary pathologies, such as chronic total occlusion (CTO) and de novo small vessel coronary lesions. Development of RA techniques and device technology have expanded its indications to more complex coronary artery lesions such as CTO [7,8] and de novo small vessel coronary lesions.
Therefore, we focused on (1) clinical outcomes of NG-DES in coronary artery calcification (CAC) compared with EG-DES; (2) studies comparing biodegradable polymer (BP) DES and durable polymer (DP) DES; (3) clinical evidence of RA in chronic total occlusion (CTO) or de novo small vessel coronary lesions.
2. Basic Principles of RA
The RA utilizes a metallic burr coated with 20–30 µm diamond crystals. The burr diameter ranges from 1.25 to 2.5 mm. It is performed over a 0.014” guidewire (floppy and extra support) and operates at a high rotational speed of 140,000–180,000 rpm. RA ablates on the principle of differential cutting, selectively applying force to inelastic tissue while sparing the elastic arterial wall. The atheroma component is fragmented into microscopic particles, approximately 5 µm in size, which are smaller than red blood cells, and are cleared via the reticuloendothelial system without causing capillary obstruction. RA enhances lesion preparation by modifying heavily calcified plaques, thereby improving subsequent stent delivery and optimization. However, it carries the risk of complications such as slow flow, perforation, or no-reflow phenomena.
3. Methodology
This review was conducted as a narrative review, focusing on the most relevant clinical studies regarding rotational atherectomy in the DES era. Relevant articles were identified through a PubMed database search, restricted to studies published between 2011 and 2024. Keywords were “drug-eluting stent”, “coronary”, “rotational atherectomy”, and “clinical outcome”.
A total of 98 studies were initially retrieved and screened based on predefined inclusion and exclusion criteria. Studies consisted of registry-based studies or retrospective real-world data in DES era and were included if they reported at least 1-year clinical outcomes.
Exclusion criteria included case reports, pre-DES era studies, animal/in vitro research, and studies focusing on specific disease subsets (such as left main disease, ostial lesions, bifurcation lesions or in-stent restenosis) or focusing on specific study population. To ensure clarity in the selection process, a PRISMA-style flowchart (Figure 1) has been provided to summarize the methodology.
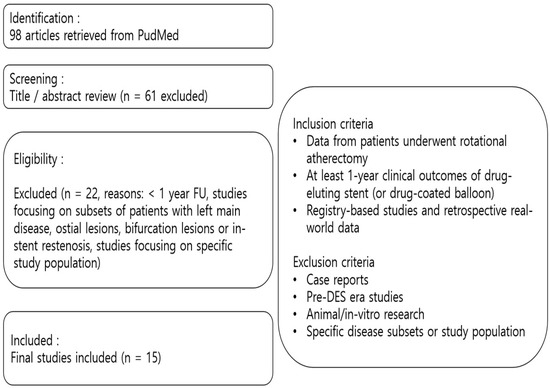
Figure 1.
Flowchart of study selection. FU, follow-up; DES, drug-eluting stent.
4. Clinical Outcomes of RA Treated with DES (Early-Generation DES Versus New-Generation DES)
4.1. Clinical Impact of CAC During Procedure and on Long-Term Outcomes
Significant CAC is associated with worse clinical outcomes in both the general population and patients undergoing PCI. One of the key reasons is that patients with moderate to severe CAC often had higher chances of comorbidity—such as diabetes mellitus, chronic kidney disease, and hypertension—and more complex CAD [9,10,11,12]. The amount of coronary calcification also correlates with overall atherosclerotic plaque burden [13]. Moreover, microcalcifications within the fibrous cap and calcific nodules have been implicated in recurrent plaque rupture and intraplaque hemorrhage, further exacerbating luminal narrowing and contributing to significant CAD progression and adverse clinical outcomes [14,15].
In addition, CAC introduces several technical challenges during PCI, often complicating the procedural outcomes and increasing the risk of suboptimal outcomes [9]. One of the primary concerns is mechanical resistance, which hinders device delivery [16]. This often necessitates aggressive lesion preparation, including high-pressure non-compliant balloon inflation, cutting or scoring balloons, and atherectomy techniques, to ensure successful advancement of balloons and stents [17,18]. Furthermore, rigid calcified plaques can restrict stent expansion, leading to underexpansion or malapposition, both of which are associated with increased risks of stent thrombosis and in-stent restenosis [4,19,20].
Beyond that, the presence of nodular calcium can increase the risk of vessel injury during procedure, including dissections or perforations, which can be life-threatening. Given these procedural challenges, appropriate lesion preparation and modification through techniques such as RA or intravascular lithotripsy are essential to optimize stent deployment, improve procedural success.
4.2. Clinical Performance of EG-DES and NG-DES in CAC Lesions
Guedeney et al. investigated the impact of moderate to severe CAC on long-term clinical outcomes and the respective performance of EG-DES and NG-DES [4]. This meta-analysis of 18 randomized trial demonstrated moderate to severe CAC was independently associated with poorer prognosis. However, patients treated with NG-DES showed significant reduction in the 5-year rates of the patient-oriented clinical endpoint (composite outcome of any death, any revascularization, or any myocardial infarction [MI]) compared with EG-DES.
4.3. Clinical Evidences of RA Treated with NG-DES
To reflect real-world practice, we retrospectively analyzed clinical outcomes of RA in the DES era using the ‘Clinical Outcome of Rotational Atherectomy in Calcified Lesions in Korea (ROCK)’ registry. The registry enrolled 540 consecutive patients with severe coronary artery calcification who underwent percutaneous coronary intervention (PCI) using rotational atherectomy (RA) between January 2010 and October 2019 at nine tertiary centers in Korea [21]. In this study, majority of study population (with the exception for one patient) treated with NG-DES, which reflect the clinical outcomes of RA in the NG-DES era. Target vessel failure (TVF, defined as cardiac death, target-vessel spontaneous MI, or target vessel revascularization [TVR]) occurred in 16.0% at 1.5 years, which showed acceptable clinical outcomes.
Several other real-world data of NG-DES were also listed in Table 1. Jinnouchi H et al. reported the cumulative 2-year incidence of major adverse cardiac event (MACE, defined as a composite of cardiac death, MI, clinically driven target lesion revascularization [TLR]) of 20.3% among 252 patients who received NG-DES after RA [22]. Hachinohe D et al. reported that 51 lesions (6.6%) resulted in TVF at 1 year among 744 patients, indicating relatively better clinical outcomes [23]. Okai I et al. reported 25.7% of MACE at 3 years using data from the J2T ROTA registry [5].

Table 1.
Comparison of clinical outcomes according to early generation DES versus new generation DES after RA.
There were two studies comparing clinical outcomes of NG-DES versus EG-DES that showed better outcomes of NG-DES (Table 1). Kawamoto H et al. suggested a possible advantage of NG-DES compared to EG-DES (2-year of clinically driven MACE [all-cause death, any MI, and TLR]: 12.9% vs. 19.2%, p = 0.04) from subgroup analysis in the ROTATE multicenter registry [24]. Allali A et al. demonstrated that NG-DES showed a lower MACE rate than EG-DES (2 years of MACE [all-cause death, spontaneous MI, and TVR]: 21.1% vs. 31.1%, log-rank p = 0.04) [25]. Based on the studies as represented above, the clinical outcomes of NG-DES implantation after RA have been improved compared to those of EG-DES over the past two decades. In addition to drug-eluting device technology, development of optimal strategy of medications, widespread use of intravascular image during procedure, enhanced procedure skills and optimal recommendations of RA, thinner stent strut platforms and more biocompatible polymers of characteristics of NG-DES compared with EG-DES may account for those differences in real-world practice. This review of real-world data highlights the important role of RA, combined with NG-DES, in optimizing clinical outcomes for patients with heavy calcified complex coronary disease.
5. Studies Comparing Biodegradable Polymer Versus Durable Polymer DES After RA
5.1. Clinical Evidences of BP-DES and DP-DES in CAC Lesions
The novel randomized study, BIO-RESORT trial was conducted by Buiten et al. [26], which was to assess 2-year clinical performance of three thin-strut DES (biodegradable polymer everolimus-eluting stents [BP-EESs] or biodegradable polymer sirolimus-eluting stents [BP-SESs], versus durable polymer zotarolimus-eluting stents [DP-ZESs]) in patients with severely calcified lesions. A total of 783 all-comer patients with severely calcified lesions were assessed for final analysis. Multivariate analysis showed that BP-EESs was independently associated with lower TVR rates compared to DP-ZESs. However, RA was used in 4.4–6.4% of overall patients in the study.
5.2. Clinical Evidences of BP-DES and DP-DES After RA
As shown in Table 2, there were two studies comparing biodegradable polymer drug eluting stent (BP-DES) and durable polymer drug eluting stent (DP-DES) after RA. Mankerious et al. reported comparable outcomes between Orsiro biodegradable sirolimus-eluting stent (SES) and durable polymer everolimus-eluting stent (EES) with 285 patients underwent PCI with RA [27]. The rates of target lesion failure (TLF) at 2 years were not significantly different between two groups (BP-DES:18% vs. DP-DES:15%, log-rank p = 0.98) However, in a subgroup analysis with small-sized (≤3.0 mm) stent group, the incidence rate of TLF was significantly lower in the BP-DES group than that of DP-DES group (3% vs. 19%, log-rank p = 0.004), mainly driven by TLR (2% vs. 12%, log-rank p = 0.022). Because of inherent limitations of study design (single-center, retrospective non-randomized) and the small size of the study population, generalization of the results should be performed with caution.

Table 2.
Comparison of clinical outcomes according to biodegradable polymer versus durable polymer DES after RA.
Recently, Kim et al. aimed to demonstrate the better outcomes of BP-DES compared to DP-DES in patients underwent RA with larger study population (n = 510, Table 2) [28]. BP-DES was associated with lower incidence rates of all-cause mortality and cardiovascular mortality compared to DP-DES (4.7% vs. 10.7%, hazard ratio [HR] 0.41, p = 0.047; 3.4% vs. 8.1%, HR 0.28, p = 0.024, respectively). However, due to higher incidence rates of TVR or TLR in BP-DES than DP-DES, TVF did not show any significant differences between the two groups. Because both studies were designed retrospectively, those different results of the previous studies were enough to explain the need for further randomized study regarding comparison of clinical outcomes of BP-DES versus DP-DES in patients treated with RA.
6. Clinical Impact of RA in CTO
6.1. Case
A 78-year old presented with stable angina. Two months prior, he underwent an unsuccessful CTO-PCI for proximal right coronary artery CTO. Despite attempts using the anchoring balloon technique with a Judkins right 4.0 guiding catheter, the balloon failed to cross the lesion. Right coronary angiogram showed short CTO segment and microchannel formation from the previous intervention (Figure 2B). However, an additional challenge was the severely tortuous brachial artery, which resulted in poor back-up support with a conventional guiding catheter (Figure 2A).
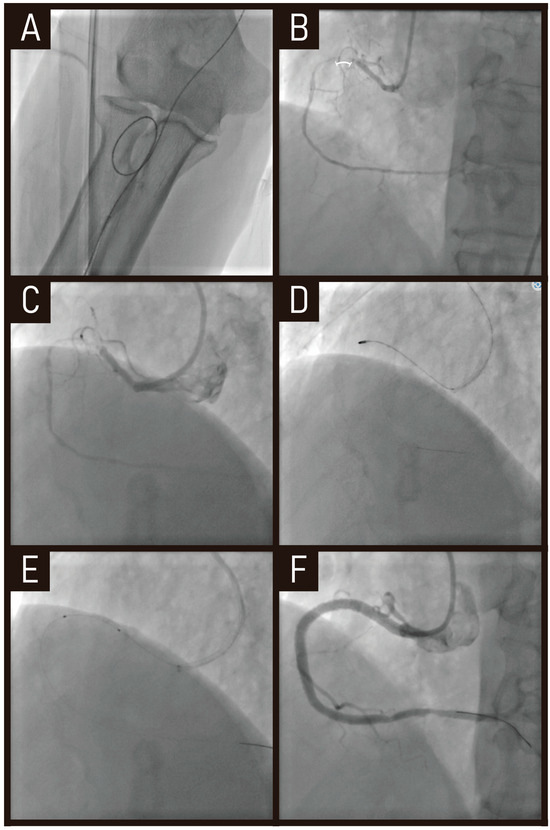
Figure 2.
Case of RA in CTO-PCI. RA with a 1.25 mm burr was performed to modify a calcified CTO lesion, allowing procedural success in this previously failed case. (A) Severely tortuous brachial artery. (B) CTO segment (marked by a white bracket). (C) Ikari right 1.5 guiding catheter providing better engagement and stability. (D) RA with a 1.25 mm burr performed in the CTO lesion. (E,F) balloon inflation and stent implantation. RA, rotational atherectomy; CTO-PCI, chronic total occlusion-percutaneous coronary intervention.
To overcome these limitations, we utilized an Ikari right 1.5 guiding catheter, which provided better engagement and stability (Figure 2C). Additionally, RA with a 1.25 mm burr was performed to modify the calcified lesion (Figure 2D), allowing for successful balloon inflation and stent implantation (Figure 2E,F).
This case highlights how RA, in combination with an optimized guiding catheter selection, can overcome anatomical challenges such as tortuous access and poor back-up support, ultimately enabling successful PCI in complex CTO lesions.
6.2. Prevalence of Calcification in CTO Lesions
Calcification of CTOs are more prevalent in those of longer occlusion duration [29]. On intravascular ultrasound (IVUS) analysis, Guo et al. reported that 64% of CTOs had predominantly fibrocalcific plaque [30]. A moderate or severe calcification was present in 58% of CTO lesions as assessed by angiography [31]. From the PROGRESS registry, Japanese Multicentre registry reported 57–59% of CTOs had angiographically moderate or severe calcification [32,33].
Calcification in CTO lesions is an independent predictor of lower procedural success rates or higher risk of complications [32,34,35]. The presence of calcium at the proximal cap can pose a significant hurdle to the passage of wires or microcatheters, sometimes resulting in deflection into the subintimal space [36]. After successful device crossing, adequate lesion preparation and the modification of calcified plaques are crucial steps prior to stent implantation to ensure stent optimization and better outcomes [37,38]. However, heavily calcific plaques act as a challenge due to their refractory characteristics and rough lesion surface. This can contribute the failure of stent delivery or insufficient stent expansion [39,40,41,42].
6.3. Clinical Usefullness of RA in CTO Lesions
RA has been considered to be the effective procedure in CTO PCI and might enhance the procedure success and the preparation of calcified lesions [43,44]. With debulking the atheromas, RA can enable balloon dilatation, plaque fracture, stent delivery, and expansion [45]. Also, the utilization of RA facilitates a minimally invasive strategy by allowing interventions that typically require a large bore catheter via the femoral approach to be performed through a radial approach with a 6 Fr guiding catheter. This adaptation may benefit patients by reducing procedural invasiveness and the associated risks.
6.4. Clinical Outcomes of RA in CTO Lesions
We demonstrated the usefulness of RA in chronic total occlusion (CTO) lesions compared to non-CTO lesions. Lee et al. reported the feasible success rates of RA in CTO-PCI (90.5 % in CTO-PCI group vs. 96.9 % in non-CTO-PCI group, p = 0.06) and clinical outcomes compared to the non CTO-PCI group (Table 3) [46]. Although 42 CTO lesions were enrolled, which were relatively small study population, any MI or stent thrombosis (ST) did not occur. This finding could be interpolated in our clinical practice for operators adjusting RA during CTO-PCI. Another important finding is higher proportion of IVUS use in CTO-PCI group than non-CTO-PCI group (69.1 % vs. 43.8 %, p = 0.002, respectively).

Table 3.
Clinical outcomes of RA in CTO-PCI.
Another study in the literature showed acceptable long-term clinical outcomes of RA-PCI compared with conventional PCI for CTO. Ayoub et al. reported that RA-PCI had significantly better procedural success rates compared to those treated without RA (93.3% vs. 85.1%, p = 0.0002). Although the 1-year MACCE rate was similar in both groups (18.7% vs. 16.7%, p = 0.49) [47], Huang et al. demonstrated better clinical outcomes of a RA-PCI group over a period of more than 4 years compared to a non-RA-PCI group. In the DES subgroup, long-term MACE, non-fatal MI, and TLR were similar across both groups. However, regarding CV death, RA-PCI showed significant fewer events [8]. Azzalini et al. reported procedural success rates reached by 77% in RA for CTO PCI. During a mean follow-up duration of 658 ± 423 days, the incidence rates of primary endpoint (MACE) were similar between two groups (15% vs. 13%, p = 0.70, Table 3) [48]. In RA group, even 20% was in the setting of dissection/re-entry; thus, no perforation occurred. In an editorial comment, Brilakis et al. emphasized that RA is an important tool for complex CTO-PCI, especially in cases of balloon uncrossable and undilatable lesions, and in such case of dissection/re-entry, subintimal RA may be safe in CTO-PCI [49]. Recently, meta-analysis including seven studies with 5494 patients reported that RA was comparable to conventional percutaneous coronary intervention (PCI) without RA for CTO lesions [50].
7. RA Co-Treated with Drug-Coated Balloon
7.1. Case
A 63-year old male presented with a one-week history of resting chest pain. His past medical history was treated hypertension. Invasive coronary angiography revealed a long-segment, severe (99%) stenosis in the first diagonal branch (Figure 3A,B). An initial attempt with a 2.0 mm plain balloon failed to cross the lesion (Figure 3C). Even after inflating a 1.5 mm plain balloon, the Finecross microcatheter was still unable to advance across the lesion (Figure 3D).
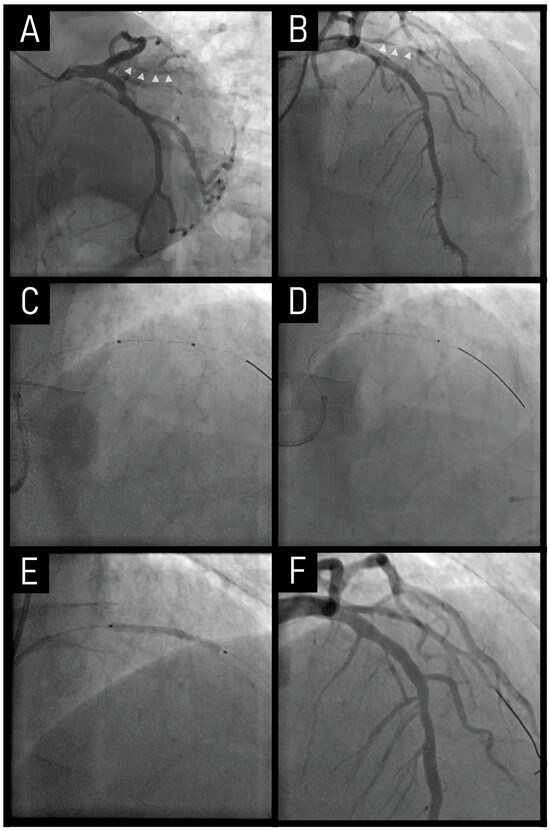
Figure 3.
Case of RA co-treated with a drug-coated balloon. The combination of RA with DCB therapy facilitates plaque modification and debulking, providing an alternative to stent implantation in small vessel disease. (A,B) Severe stenosis in the first diagonal branch (marked by small white triangles). (C) A 2.0 mm plain balloon failed to cross the lesion. (D) A 1.5 mm plain balloon was used. (E) A DCB angioplasty was performed. (F) The final angiogram showed a good result. RA, rotational atherectomy; DCB, drug-coated balloon.
To overcome this challenge, a 1.25 mm burr of RA was performed, followed by a DCB angioplasty, both of which were successfully completed in sequence (Figure 3E,F). A critical technical difficulty arose when the rota wire failed to advance to the distal vessel. To address this, the wire was gently maneuvered under Dynaglide mode, allowing for its correct positioning in the distal segment. This precise wire manipulation facilitated safe execution of RA, ensuring optimal lesion preparation without compromising vessel integrity.
This case highlights that, despite the inherent risks of RA in small vessels, it can be selectively utilized in refractory lesions where conventional approaches fail. Additionally, the combination of RA with DCB therapy may offer a promising strategy to improve long-term outcomes by facilitating plaque modification and debulking, providing an alternative to stent implantation, and reducing restenosis risk in such small vessel disease.
7.2. Clinical Evidences of Drug-Coated Balloon in De Novo Small Coronary Vessels
DCB has been shown to be safe and effective for use in de novo small coronary vessels [51]. To date, there have been several DCB studies compared with DES in de novo small vessel coronary lesions. In BELLO randomized controlled trial, DCB was associated with better angiographic outcomes and similar rates of adverse events as compared with first-generation DES [52]. After 3 years, a better clinical outcome emerges for DCB, in terms of MACE [53]. Two additional studies demonstrated non-inferiority of DCB compared with DES in terms of angiographic and clinical outcomes [54,55]. More recently, in the PICCOLETO randomized controlled trial, they showed the angiographic superiority in terms of late lumen loss of DCB over second-generation DES [56]. There have also been some studies that showed acceptable outcomes of DCBs in calcified coronary lesions [57,58]. However, it is unclear whether DCBs are effective in calcified coronary lesions.
7.3. Clinical Outcomes of RA Co-Treated with Drug-Coated Balloon in De Novo Small Vessel Coronary Lesions
Building on this background, clinical experiences of RA co-treated with DCB have been accumulated and translated in the literature as follows (Table 4). Nagai reported a single-center data of the acute and mid-term efficacy of DCB following RA. At mid-term follow-up duration (196 ± 37 days), there were two deaths (non-cardiovascular death), 16.4% of TLR, and 20.7% of TVR [59]. Dong et al. demonstrated safe and effective results of DCB-RA compared with DES-RA in selected severe coronary artery calcification [60]. There were similar incidence rates of major procedural complications and in-hospital events. DCB-RA group showed numerically lower incidence rates of TLR (13.8% vs. 7.0%) and MACCE (18.8% vs. 12.3%) compared with DES-RA group without statistical significance.

Table 4.
Clinical outcomes of DCB after RA.
8. Discussion and Future Direction
We reviewed and summarized clinical outcomes of RA treated with DES, with a particular focus on NG-DES. Clinical evidence consistently demonstrated the superior outcomes of NG-DES compared to EG-DES, even in severe CAC lesion treated with RA. The reported incidence rates of MACE (or TVF) ranged from 6.6% at 1 year to 21.1% at 1.5 years in NG-DES groups [21,22,23,61]. The observed improvements in clinical outcomes may be attributed to advancements in stent design, such as thinner strut platforms and more biocompatible polymers [62,63]. However, reviewed studies have inherent limitations that warrant careful interpretation. Notably, study periods for three of the five studies listed in Table 1 spanned approximately a decade, which may not fully reflect current clinical practices. Moreover, most of these studies were conducted before 2015, limiting their ability to incorporate recent innovations in medication strategies (including potent P2Y12 inhibitors, high-intensity statins, PCSK9 inhibitors, and four pillars of therapy for heart failure with reduced ejection fraction) and the concomitant use of intravascular imaging during procedures. Especially, intravascular imaging guidance is recommended by the European expert consensus on RA for ACS patients undergoing PCI with a Class IIA recommendation [64,65]. In CCS patients for complex lesions (such as heavily calcified ones), the most recent CCS guidelines assigned a Class I recommendation [66]. Intravascular imaging modalities, including IVUS and optical coherence tomography, offer complementary morphological and quantitative assessment of calcification and facilitate the optimal selection of techniques and devices during procedures [67]. However, as shown in our review, real-world data indicate that intravascular imaging is often underutilized (31.2–99.6%). Among these studies, a particularly noteworthy one is the recent study by Hachinohe et al. [23], in which intravascular imaging was used in 99.6% of patients. Interestingly, this study reported relatively better clinical outcomes compared to others. While the high utilization of intravascular imaging may have contributed to these results. Despite guideline recommendations, its utilization in real-world practice remains suboptimal, highlighting the need for improved implementation strategies.
Recently, the development of ultra-thin coronary stent (struts thickness < 70 μm) has emerged as a promising advancement in PCI. These stents, with their thinner struts, offer improved deliverability and reduced vessel injury, which may optimize outcomes, particularly in complex coronary lesions. Studies comparing ultra-thin stents with thicker-strut NG-DES have demonstrated non-inferiority in terms of TLF [68,69]. Consequently, ultra-thin coronary stents are now widely adopted in clinical practice due to their improved deliverability and reduced vessel injury, which may optimize outcomes in complex coronary lesions [70,71,72,73]. However, their performance in heavily calcified lesions remains insufficiently studied, highlighting the need for further research in this area.
In summary, limitations of the reviewed studies include prolonged study durations and the underrepresentation of ultra-thin coronary stents, underscoring the need for further research. Large-scale registries or randomized controlled trials are warranted to comprehensively evaluate the clinical outcomes of RA performed with ultra-thin coronary stents. These studies should integrate contemporary medication strategies as mentioned above as well as the mandatory use of intravascular imaging, to ensure that findings align with current clinical practices and provide robust evidence for optimal procedural strategies in heavily calcified lesions.
We also reviewed two studies comparing clinical outcomes based on polymer types, BP-DES and DP-DES. As summarized in Table 2, BP-DES showed a trend toward lower incidence rates of primary endpoint, although this did not reach statistical significance. Additionally, two studies reported conflicting results regarding TLR rates, highlighting potential variability in outcomes. However, in a subgroup analysis with small-stent groups (≤3.0 mm) of one study [27], BP-DES demonstrated better clinical outcomes than DP-DES, particularly in terms of cardiac death, MI, and TLR. These findings suggest that while BP-DES may offer certain advantages in subgroups with heavily calcified small vessel disease, the lack of consistent results underscores the need for further investigations. Further randomized controlled trials with larger cohorts and robust methodologies are urgently needed to validate these observations and provide practical insights into the optimal use of BP-DES versus DP-DES in heavily calcified coronary lesions. Specifically, future randomized controlled trials should address the limitations of prior research by incorporating stratification based on stent diameter (e.g., <3.0 mm vs. ≥3.0 mm), enabling a clearer understanding of the comparative performance of these stents in different lesion and vessel characteristics. Such an approach would not only address variability in outcomes but also refine procedural strategies for patients with heavily calcified small vessel disease.
We presented a case of CTO-PCI using RA, in a patient with poor co-axial and back-up support from the guiding catheter due to anatomical challenges. The procedure initially failed but was successfully performed using a 1.25 mm burr RA followed by balloon dilatation. According to current guideline [45], a single run with a 1.25 mm burr RA, as in our case, is generally sufficient to achieve good plaque modification in most cases. Given the clinical significance of RA in CTO-PCI, we also reviewed two studies investigating its clinical outcomes in this setting. One study demonstrated clinical efficacy of RA in CTO-PCI group compared to non-CTO-PCI group. As mentioned above, IVUS was more frequently used in CTO-PCI group than non-CTO-PCI group. Despite the inherent limitations of selection bias due to the small study population, these findings suggest that potential benefits of combining RA with IVUS in CTO-PCI to achieve acceptable and feasible clinical outcomes. However, in this study, patients who underwent RA were retrospectively compared between CTO-PCI and non-CTO-PCI groups. Because lesion characteristics were significantly different in terms of plaque burden, lesion length and the density of calcification, comparing two groups would not be appropriate. To overcome these limitations and accurately assess the efficacy of RA, it is crucial to design future studies with more refined methodologies. Specifically, future research should focus on conducting randomized controlled trials comparing combined RA + IVUS strategies with IVUS-only approaches in CTO-PCI procedures. This design would enable a direct evaluation of the additive benefits of RA. Such trials would provide reliable evidence to guide clinical decision-making and optimize procedural strategies for CTO-PCI.
We also explored the safety of RA in the case of dissection/re-entry during CTO-PCI. While some experts have suggested that subintimal RA may be a safe option in CTO-PCI, this remains a topic of ongoing debate. Although the literature we presented suggests the potential safety of RA, concerns about coronary dissection or perforation during RA in CTO-PCI remain significant. Thus, the decision to perform subintimal RA should be made cautiously and on a case-by-case basis by the operator, considering the individual patient’s risk profile and lesion characteristics. Expert consensus suggests that in heavily calcified CTOs where the lesion is crossed via subintimal tracking, balloon inflation pressures exceeding 14–16 atm and atherectomy are not advised [67].
In this paper, two studies of RA co-treated with DCB have been introduced. RA facilitates treatment by modifying calcified lesions, while DCB reduces the risk of restenosis through drug elution without the need for stent implantation. This combination presents a potential therapeutic option for the management of small vessel diseases. However, concerns remain regarding the risk of vascular injury caused by RA in small-caliber vessels and the uncertain efficacy of drug delivery from DCB in heavily calcified lesions.
Additionally, calcified nodules may increase the risk of adverse events or coronary perforation even after stent implantation [74]. In such cases, the combination of calcium modification techniques, such as atherectomy or intravascular lithotripsy, followed by DCB treatment, may serve as an optimal strategy for managing calcified nodule. However, evidence supporting the efficacy and safety of this strategy remains limited, and further research is needed to establish its clinical utility in this specific setting.
Nevertheless, in patients at a high bleeding risk, the ability to avoid stent implantation and minimize the duration of dual antiplatelet therapy while achieving effective revascularization suggests that this approach could be both practical and valuable when safely applied to selected patient populations. Further research is needed to validate the efficacy and safety of this strategy and to establish its potential for broader clinical applications.
Limitations
One of the key challenges in interpreting the available literature from our study is the heterogeneity observed across studies. This heterogeneity arises from differences in study design (prospectively enrolled registry-based studies vs. retrospective studies), study population, lesion characteristics, and procedural strategies. Additionally, variations in clinical endpoints, definitions of MACE, and follow-up durations further contribute to the difficulty in making direct comparisons between studies.
As a result, the reported clinical outcomes of RA in contemporary practice show considerable variation, making it difficult to derive definitive conclusions regarding its efficacy and safety outcomes. While randomized trials provide robust data, real-world registries offer valuable insights into clinical practice patterns. However, the inherent limitations of observational studies, including selection bias and the presence of unmeasured confounders, must be considered.
Future studies should aim to standardize study population and definitions of outcome to facilitate more meaningful comparisons. Furthermore, subgroup analyses focusing on lesion subsets, the role of intravascular imaging, and post-procedural pharmacotherapy may provide deeper insights into optimizing RA strategies in contemporary clinical practice.
9. Conclusions
RA has demonstrated favorable clinical outcomes, particularly when used with NG-DES. Clinical evidence consistently demonstrated the superior outcomes of NG-DES compared to EG-DES, even in severe calcified lesions treated with RA.
However, several critical gaps in knowledge remain, highlighting the need for future investigations. First, while ultra-thin coronary stents have shown promising results, their performance in heavily calcified lesions remains insufficiently studied. Large-scale registries and randomized controlled trials (RCTs) are needed to assess their efficacy in this subset. Second, the comparative effectiveness of BP-DES versus DP-DES in heavily calcified small vessel disease remains unclear. Further RCTs incorporating stratified analyses based on stent diameter could provide clearer insights into their clinical benefits.
Additionally, the role of RA in CTO-PCI warrants further investigation. Future studies should evaluate whether the addition of RA to intravascular imaging improves procedural and long-term outcomes compared to IVUS-guided PCI alone. Finally, while DCBs following RA offer a potential strategy to reduce restenosis in calcified nodules and small vessel disease, particularly in patients who are unsuitable for DES implantation.
Author Contributions
Conceptualization, K.L. and S.-H.H.; methodology, K.L. and S.-H.H.; resources, Y.K.; data curation, Y.K.; writing—original draft preparation, K.L. and Y.K.; writing—review and editing, Y.K. and K.L.; visualization, K.L.; supervision, S.-H.H.; funding acquisition, S.-H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| CAD | coronary artery disease |
| DES | drug-eluting stent(s) |
| MACE | major adverse cardiac event |
| MI | myocardial infarction |
| PCI | percutaneous coronary intervention |
| RA | rotational atherectomy |
| TVF | target-vessel failure |
| TVR | target-vessel revascularization |
References
- Grüntzig, A.R.; Senning, A.; Siegenthaler, W.E. Nonoperative dilatation of coronary-artery stenosis: Percutaneous transluminal coronary angioplasty. N. Engl. J. Med. 1979, 301, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Gertz, S.D.; Malekzadeh, S.; Dollar, A.L.; Kragel, A.H.; Roberts, W.C. Composition of atherosclerotic plaques in the four major epicardial coronary arteries in patients greater than or equal to 90 years of age. Am. J. Cardiol. 1991, 67, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Waller, B.F.; Roberts, W.C. Cardiovascular disease in the very elderly. Analysis of 40 necropsy patients aged 90 years or over. Am. J. Cardiol. 1983, 51, 403–421. [Google Scholar] [CrossRef]
- Guedeney, P.; Claessen, B.E.; Mehran, R.; Mintz, G.S.; Liu, M.; Sorrentino, S.; Giustino, G.; Farhan, S.; Leon, M.B.; Serruys, P.W.; et al. Coronary Calcification and Long-Term Outcomes According to Drug-Eluting Stent Generation. JACC Cardiovasc. Interv. 2020, 13, 1417–1428. [Google Scholar] [CrossRef]
- Okai, I.; Dohi, T.; Okazaki, S.; Jujo, K.; Nakashima, M.; Otsuki, H.; Tanaka, K.; Arashi, H.; Okabe, R.; Nagura, F.; et al. Clinical Characteristics and Long-Term Outcomes of Rotational Atherectomy-J2T Multicenter Registry. Circ. J. 2018, 82, 369–375. [Google Scholar] [CrossRef]
- Allali, A.; Abdel-Wahab, M.; Elbasha, K.; Mankerious, N.; Traboulsi, H.; Kastrati, A.; El-Mawardy, M.; Hemetsberger, R.; Sulimov, D.S.; Neumann, F.J.; et al. Rotational atherectomy of calcified coronary lesions: Current practice and insights from two randomized trials. Clin. Res. Cardiol. 2023, 112, 1143–1163. [Google Scholar] [CrossRef]
- Pagnotta, P.; Briguori, C.; Mango, R.; Visconti, G.; Focaccio, A.; Belli, G.; Presbitero, P. Rotational atherectomy in resistant chronic total occlusions. Catheter. Cardiovasc. Interv. 2010, 76, 366–371. [Google Scholar] [CrossRef]
- Huang, W.C.; Teng, H.I.; Chan, W.L.; Lu, T.M. Short-term and long-term clinical outcomes of rotational atherectomy in resistant chronic total occlusion. J. Interv. Cardiol. 2018, 31, 458–464. [Google Scholar] [CrossRef]
- Généreux, P.; Madhavan, M.V.; Mintz, G.S.; Maehara, A.; Palmerini, T.; Lasalle, L.; Xu, K.; McAndrew, T.; Kirtane, A.; Lansky, A.J.; et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) TRIALS. J. Am. Coll. Cardiol. 2014, 63, 1845–1854. [Google Scholar] [CrossRef]
- Madhavan, M.V.; Tarigopula, M.; Mintz, G.S.; Maehara, A.; Stone, G.W.; Généreux, P. Coronary artery calcification: Pathogenesis and prognostic implications. J. Am. Coll. Cardiol. 2014, 63, 1703–1714. [Google Scholar] [CrossRef]
- Vliegenthart, R.; Oudkerk, M.; Hofman, A.; Oei, H.H.; van Dijck, W.; van Rooij, F.J.; Witteman, J.C. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation 2005, 112, 572–577. [Google Scholar] [CrossRef]
- Hemetsberger, R.; Abdelghani, M.; Toelg, R.; Mankerious, N.; Allali, A.; Garcia-Garcia, H.M.; Windecker, S.; Lefèvre, T.; Saito, S.; Slagboom, T.; et al. Impact of Coronary Calcification on Clinical Outcomes After Implantation of Newer-Generation Drug-Eluting Stents. J. Am. Heart Assoc. 2021, 10, e019815. [Google Scholar] [CrossRef]
- Sangiorgi, G.; Rumberger, J.A.; Severson, A.; Edwards, W.D.; Gregoire, J.; Fitzpatrick, L.A.; Schwartz, R.S. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: A histologic study of 723 coronary artery segments using nondecalcifying methodology. J. Am. Coll. Cardiol. 1998, 31, 126–133. [Google Scholar] [CrossRef]
- Kelly-Arnold, A.; Maldonado, N.; Laudier, D.; Aikawa, E.; Cardoso, L.; Weinbaum, S. Revised microcalcification hypothesis for fibrous cap rupture in human coronary arteries. Proc. Natl. Acad. Sci. USA 2013, 110, 10741–10746. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arter. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Wiemer, M.; Butz, T.; Schmidt, W.; Schmitz, K.P.; Horstkotte, D.; Langer, C. Scanning electron microscopic analysis of different drug eluting stents after failed implantation: From nearly undamaged to major damaged polymers. Catheter. Cardiovasc. Interv. 2010, 75, 905–911. [Google Scholar] [CrossRef]
- Barath, P.; Fishbein, M.C.; Vari, S.; Forrester, J.S. Cutting balloon: A novel approach to percutaneous angioplasty. Am. J. Cardiol. 1991, 68, 1249–1252. [Google Scholar] [CrossRef]
- Kawaguchi, R.; Tsurugaya, H.; Hoshizaki, H.; Toyama, T.; Oshima, S.; Taniguchi, K. Impact of lesion calcification on clinical and angiographic outcome after sirolimus-eluting stent implantation in real-world patients. Cardiovasc. Revasc. Med. 2008, 9, 2–8. [Google Scholar] [CrossRef]
- Bourantas, C.V.; Zhang, Y.J.; Garg, S.; Iqbal, J.; Valgimigli, M.; Windecker, S.; Mohr, F.W.; Silber, S.; Vries, T.; Onuma, Y.; et al. Prognostic implications of coronary calcification in patients with obstructive coronary artery disease treated by percutaneous coronary intervention: A patient-level pooled analysis of 7 contemporary stent trials. Heart 2014, 100, 1158–1164. [Google Scholar] [CrossRef]
- Choi, K.H.; Song, Y.B.; Lee, J.M.; Lee, S.Y.; Park, T.K.; Yang, J.H.; Choi, J.H.; Choi, S.H.; Gwon, H.C.; Hahn, J.Y. Impact of Intravascular Ultrasound-Guided Percutaneous Coronary Intervention on Long-Term Clinical Outcomes in Patients Undergoing Complex Procedures. JACC Cardiovasc. Interv. 2019, 12, 607–620. [Google Scholar] [CrossRef]
- Lee, K.; Jung, J.H.; Lee, M.; Kim, D.W.; Park, M.W.; Choi, I.J.; Lee, J.H.; Lee, J.H.; Lee, S.R.; Lee, P.H.; et al. Clinical Outcome of Rotational Atherectomy in Calcified Lesions in Korea-ROCK Registry. Medicina 2021, 57, 694. [Google Scholar] [CrossRef]
- Jinnouchi, H.; Kuramitsu, S.; Shinozaki, T.; Kobayashi, Y.; Hiromasa, T.; Morinaga, T.; Mazaki, T.; Sakakura, K.; Soga, Y.; Hyodo, M.; et al. Two-Year Clinical Outcomes of Newer-Generation Drug-Eluting Stent Implantation Following Rotational Atherectomy for Heavily Calcified Lesions. Circ. J. 2015, 79, 1938–1943. [Google Scholar] [CrossRef]
- Hachinohe, D.; Kashima, Y.; Kanno, D.; Kobayashi, K.; Sugie, T.; Kaneko, U.; Tadano, Y.; Watanabe, T.; Shitan, H.; Fujita, T. Rotational atherectomy and new-generation drug-eluting stent implantation. Catheter. Cardiovasc. Interv. 2018, 91, 1026–1034. [Google Scholar] [CrossRef]
- Kawamoto, H.; Latib, A.; Ruparelia, N.; Ielasi, A.; D’Ascenzo, F.; Pennacchi, M.; Sardella, G.; Garbo, R.; Meliga, E.; Moretti, C.; et al. In-hospital and midterm clinical outcomes of rotational atherectomy followed by stent implantation: The ROTATE multicentre registry. EuroIntervention 2016, 12, 1448–1456. [Google Scholar] [CrossRef]
- Allali, A.; Holy, E.W.; Sulimov, D.S.; Toelg, R.; Richardt, G.; Abdel-Wahab, M. Long-Term Clinical Outcome of Early Generation Versus New-Generation Drug-Eluting Stents in 481 Patients Undergoing Rotational Atherectomy: A Retrospective Analysis. Cardiol. Ther. 2018, 7, 89–99. [Google Scholar] [CrossRef]
- Buiten, R.A.; Ploumen, E.H.; Zocca, P.; Doggen, C.J.M.; van Houwelingen, K.G.; Danse, P.W.; Schotborgh, C.E.; Stoel, M.G.; Scholte, M.; Linssen, G.C.M.; et al. Three contemporary thin-strut drug-eluting stents implanted in severely calcified coronary lesions of participants in a randomized all-comers trial. Catheter. Cardiovasc. Interv. 2020, 96, E508–E515. [Google Scholar] [CrossRef]
- Mankerious, N.; Hemetsberger, R.; Traboulsi, H.; Toelg, R.; Abdel-Wahab, M.; Richardt, G.; Allali, A. Outcomes of patients treated with a biodegradable-polymer sirolimus-eluting stent versus durable-polymer everolimus-eluting stents after rotational atherectomy. Clin. Res. Cardiol. 2021, 110, 1574–1585. [Google Scholar] [CrossRef]
- Kim, K.A.; Her, S.H.; Lee, K.; Choi, I.J.; Lee, J.H.; Lee, J.H.; Lee, S.R.; Lee, P.H.; Lee, S.W.; Yoo, K.D.; et al. Clinical Outcomes of Biodegradable versus Durable Polymer Drug Eluting Stents in Rotational Atherectomy: Results from ROCK Registry. J. Clin. Med. 2022, 11, 6251. [Google Scholar] [CrossRef]
- Sakakura, K.; Nakano, M.; Otsuka, F.; Yahagi, K.; Kutys, R.; Ladich, E.; Finn, A.V.; Kolodgie, F.D.; Virmani, R. Comparison of pathology of chronic total occlusion with and without coronary artery bypass graft. Eur. Heart J. 2014, 35, 1683–1693. [Google Scholar] [CrossRef]
- Guo, J.; Maehara, A.; Mintz, G.S.; Ashida, K.; Pu, J.; Shang, Y.; Leon, M.B.; Stone, G.W.; Moses, J.W.; Ochiai, M. A virtual histology intravascular ultrasound analysis of coronary chronic total occlusions. Catheter. Cardiovasc. Interv. 2013, 81, 464–470. [Google Scholar] [CrossRef]
- Karacsonyi, J.; Karmpaliotis, D.; Alaswad, K.; Jaffer, F.A.; Yeh, R.W.; Patel, M.; Mahmud, E.; Lombardi, W.; Wyman, M.R.; Doing, A.; et al. Impact of Calcium on Chronic Total Occlusion Percutaneous Coronary Interventions. Am. J. Cardiol. 2017, 120, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Danek, B.A.; Karatasakis, A.; Karmpaliotis, D.; Alaswad, K.; Yeh, R.W.; Jaffer, F.A.; Patel, M.P.; Mahmud, E.; Lombardi, W.L.; Wyman, M.R.; et al. Development and Validation of a Scoring System for Predicting Periprocedural Complications During Percutaneous Coronary Interventions of Chronic Total Occlusions: The Prospective Global Registry for the Study of Chronic Total Occlusion Intervention (PROGRESS CTO) Complications Score. J. Am. Heart Assoc. 2016, 5, e004272. [Google Scholar] [CrossRef] [PubMed]
- Okamura, A.; Yamane, M.; Muto, M.; Matsubara, T.; Igarashi, Y.; Nakamura, S.; Muramatsu, T.; Fujita, T.; Oida, A.; Tsuchikane, E. Complications during retrograde approach for chronic coronary total occlusion: Sub-analysis of Japanese multicenter registry. Catheter. Cardiovasc. Interv. 2016, 88, 7–14. [Google Scholar] [CrossRef]
- Morino, Y.; Abe, M.; Morimoto, T.; Kimura, T.; Hayashi, Y.; Muramatsu, T.; Ochiai, M.; Noguchi, Y.; Kato, K.; Shibata, Y.; et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: The J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc. Interv. 2011, 4, 213–221. [Google Scholar] [CrossRef]
- Szijgyarto, Z.; Rampat, R.; Werner, G.S.; Ho, C.; Reifart, N.; Lefevre, T.; Louvard, Y.; Avran, A.; Kambis, M.; Buettner, H.J.; et al. Derivation and Validation of a Chronic Total Coronary Occlusion Intervention Procedural Success Score From the 20,000-Patient EuroCTO Registry: The EuroCTO (CASTLE) Score. JACC Cardiovasc. Interv. 2019, 12, 335–342. [Google Scholar] [CrossRef]
- Cosgrove, C.; Mahadevan, K.; Spratt, J.C.; McEntegart, M. The Impact of Calcium on Chronic Total Occlusion Management. Interv. Cardiol. 2021, 16, e30. [Google Scholar] [CrossRef]
- Fujii, K.; Carlier, S.G.; Mintz, G.S.; Yang, Y.M.; Moussa, I.; Weisz, G.; Dangas, G.; Mehran, R.; Lansky, A.J.; Kreps, E.M.; et al. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: An intravascular ultrasound study. J. Am. Coll. Cardiol. 2005, 45, 995–998. [Google Scholar] [CrossRef]
- Doi, H.; Maehara, A.; Mintz, G.S.; Yu, A.; Wang, H.; Mandinov, L.; Popma, J.J.; Ellis, S.G.; Grube, E.; Dawkins, K.D.; et al. Impact of post-intervention minimal stent area on 9-month follow-up patency of paclitaxel-eluting stents: An integrated intravascular ultrasound analysis from the TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, Long Lesion, and Direct Stent Trials. JACC Cardiovasc. Interv. 2009, 2, 1269–1275. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011, 124, e574–e651. [Google Scholar] [CrossRef]
- Moussa, I.; Di Mario, C.; Moses, J.; Reimers, B.; Di Francesco, L.; Martini, G.; Tobis, J.; Colombo, A. Coronary stenting after rotational atherectomy in calcified and complex lesions. Angiogr. Clin. Follow-Up Results. Circ. 1997, 96, 128–136. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Richardt, G.; Joachim Büttner, H.; Toelg, R.; Geist, V.; Meinertz, T.; Schofer, J.; King, L.; Neumann, F.J.; Khattab, A.A. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: The randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc. Interv. 2013, 6, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, M.; Toelg, R.; Byrne, R.A.; Geist, V.; El-Mawardy, M.; Allali, A.; Rheude, T.; Robinson, D.R.; Abdelghani, M.; Sulimov, D.S.; et al. High-Speed Rotational Atherectomy Versus Modified Balloons Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Lesions. Circ. Cardiovasc. Interv. 2018, 11, e007415. [Google Scholar] [CrossRef] [PubMed]
- Gruberg, L.; Mehran, R.; Dangas, G.; Hong, M.K.; Mintz, G.S.; Kornowski, R.; Lansky, A.J.; Kent, K.M.; Pichard, A.D.; Satler, L.F.; et al. Effect of plaque debulking and stenting on short- and long-term outcomes after revascularization of chronic total occlusions. J. Am. Coll. Cardiol. 2000, 35, 151–156. [Google Scholar] [CrossRef]
- Moliterno, D.J. Rotational atherectomy for resistant chronic total occlusions: Another spin for tough old problems. Catheter. Cardiovasc. Interv. 2010, 76, 372–373. [Google Scholar] [CrossRef]
- Barbato, E.; Carrié, D.; Dardas, P.; Fajadet, J.; Gaul, G.; Haude, M.; Khashaba, A.; Koch, K.; Meyer-Gessner, M.; Palazuelos, J.; et al. European expert consensus on rotational atherectomy. EuroIntervention 2015, 11, 30–36. [Google Scholar] [CrossRef]
- Lee, S.N.; Her, S.H.; Jang, W.Y.; Moon, D.; Moon, K.W.; Yoo, K.D.; Lee, K.; Choi, I.J.; Lee, J.H.; Lee, J.H.; et al. Impact of chronic total occlusion lesions on clinical outcomes in patients receiving rotational atherectomy: Results from the ROCK registry. Heart Vessel. 2021, 36, 1617–1625. [Google Scholar] [CrossRef]
- Ayoub, M.; Corpataux, N.; Behnes, M.; Schupp, T.; Forner, J.; Akin, I.; Neumann, F.J.; Westermann, D.; Rudolph, V.; Mashayekhi, K. Safety and Efficiency of Rotational Atherectomy in Chronic Total Coronary Occlusion-One-Year Clinical Outcomes of an Observational Registry. J. Clin. Med. 2023, 12, 3510. [Google Scholar] [CrossRef]
- Azzalini, L.; Dautov, R.; Ojeda, S.; Serra, A.; Benincasa, S.; Bellini, B.; Giannini, F.; Chavarría, J.; Gheorghe, L.L.; Pan, M.; et al. Long-term outcomes of rotational atherectomy for the percutaneous treatment of chronic total occlusions. Catheter. Cardiovasc. Interv. 2017, 89, 820–828. [Google Scholar] [CrossRef]
- Sandoval, Y.; Brilakis, E.S. The role of rotational atherectomy in contemporary chronic total occlusion percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 2017, 89, 829–831. [Google Scholar] [CrossRef]
- Abdelaziz, A.; Elsayed, H.; Hamdaalah, A.; Atta, K.; Mechi, A.; Kadhim, H.; Aboutaleb, A.M.; Elaraby, A.; Ellabban, M.H.; Rzk, F.M.; et al. Safety and feasibility of rotational atherectomy (RA) versus conventional stenting in patients with chronic total occlusion (CTO) lesions: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2024, 24, 4. [Google Scholar] [CrossRef]
- Jeger, R.V.; Farah, A.; Ohlow, M.A.; Mangner, N.; Möbius-Winkler, S.; Weilenmann, D.; Wöhrle, J.; Stachel, G.; Markovic, S.; Leibundgut, G.; et al. Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (BASKET-SMALL 2): 3-year follow-up of a randomised, non-inferiority trial. Lancet 2020, 396, 1504–1510. [Google Scholar] [CrossRef]
- Latib, A.; Colombo, A.; Castriota, F.; Micari, A.; Cremonesi, A.; De Felice, F.; Marchese, A.; Tespili, M.; Presbitero, P.; Sgueglia, G.A.; et al. A randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels: The BELLO (Balloon Elution and Late Loss Optimization) study. J. Am. Coll. Cardiol. 2012, 60, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Latib, A.; Ruparelia, N.; Menozzi, A.; Castriota, F.; Micari, A.; Cremonesi, A.; De Felice, F.; Marchese, A.; Tespili, M.; Presbitero, P.; et al. 3-Year Follow-Up of the Balloon Elution and Late Loss Optimization Study (BELLO). JACC Cardiovasc. Interv. 2015, 8, 1132–1134. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Qiao, S.; Su, X.; Chen, Y.; Jin, Z.; Chen, H.; Xu, B.; Kong, X.; Pang, W.; Liu, Y.; et al. Drug-Coated Balloon Versus Drug-Eluting Stent for Small-Vessel Disease: The RESTORE SVD China Randomized Trial. JACC Cardiovasc. Interv. 2018, 11, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Jeger, R.V.; Farah, A.; Ohlow, M.A.; Mangner, N.; Möbius-Winkler, S.; Leibundgut, G.; Weilenmann, D.; Wöhrle, J.; Richter, S.; Schreiber, M.; et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): An open-label randomised non-inferiority trial. Lancet 2018, 392, 849–856. [Google Scholar] [CrossRef]
- Cortese, B.; Di Palma, G.; Guimaraes, M.G.; Piraino, D.; Orrego, P.S.; Buccheri, D.; Rivero, F.; Perotto, A.; Zambelli, G.; Alfonso, F. Drug-Coated Balloon Versus Drug-Eluting Stent for Small Coronary Vessel Disease: PICCOLETO II Randomized Clinical Trial. JACC Cardiovasc. Interv. 2020, 13, 2840–2849. [Google Scholar] [CrossRef]
- Mitsui, K.; Lee, T.; Miyazaki, R.; Hara, N.; Nagamine, S.; Nakamura, T.; Terui, M.; Okata, S.; Nagase, M.; Nitta, G.; et al. Drug-coated balloon strategy following orbital atherectomy for calcified coronary artery compared with drug-eluting stent: One-year outcomes and optical coherence tomography assessment. Catheter. Cardiovasc. Interv. 2023, 102, 11–17. [Google Scholar] [CrossRef]
- Ito, R.; Ueno, K.; Yoshida, T.; Takahashi, H.; Tatsumi, T.; Hashimoto, Y.; Kojima, Y.; Kitamura, T.; Morita, N. Outcomes after drug-coated balloon treatment for patients with calcified coronary lesions. J. Interv. Cardiol. 2018, 31, 436–441. [Google Scholar] [CrossRef]
- Nagai, T.; Mizobuchi, M.; Funatsu, A.; Kobayashi, T.; Nakamura, S. Acute and mid-term outcomes of drug-coated balloon following rotational atherectomy. Cardiovasc. Interv. Ther. 2020, 35, 242–249. [Google Scholar] [CrossRef]
- Dong, H.; Shan, Y.; Gong, S.; Li, R.; Li, Y.; Lu, X.; Sun, G. Clinical research of drug-coated balloon after rotational atherectomy for severe coronary artery calcification. BMC Cardiovasc. Disord. 2023, 23, 40. [Google Scholar] [CrossRef]
- Kawashima, H.; Kyono, H.; Nakashima, M.; Okai, I.; Jujo, K.; Dohi, T.; Otsuki, H.; Tanaka, K.; Nagura, F.; Okazaki, S.; et al. Prognostic Impact of Scoring Balloon Angioplasty After Rotational Atherectomy in Heavily Calcified Lesions Using Second-Generation Drug-Eluting Stents: A Multicenter Registry-Based Study. Cardiovasc. Revasc Med. 2020, 21, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Torii, S.; Jinnouchi, H.; Sakamoto, A.; Mori, H.; Park, J.; Amoa, F.C.; Sawan, M.; Sato, Y.; Cornelissen, A.; Kuntz, S.H.; et al. Vascular responses to coronary calcification following implantation of newer-generation drug-eluting stents in humans: Impact on healing. Eur. Heart J. 2020, 41, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Vorpahl, M.; Nakano, M.; Foerst, J.; Newell, J.B.; Sakakura, K.; Kutys, R.; Ladich, E.; Finn, A.V.; Kolodgie, F.D.; et al. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation 2014, 129, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Milzi, A.; Simonetto, F.; Landi, A. Percutaneous Revascularization of Thrombotic and Calcified Coronary Lesions. J. Clin. Med. 2025, 14, 692. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Barbato, E.; Gallinoro, E.; Abdel-Wahab, M.; Andreini, D.; Carrié, D.; Di Mario, C.; Dudek, D.; Escaned, J.; Fajadet, J.; Guagliumi, G.; et al. Management strategies for heavily calcified coronary stenoses: An EAPCI clinical consensus statement in collaboration with the EURO4C-PCR group. Eur. Heart J. 2023, 44, 4340–4356. [Google Scholar] [CrossRef]
- Bangalore, S.; Toklu, B.; Patel, N.; Feit, F.; Stone, G.W. Newer-Generation Ultrathin Strut Drug-Eluting Stents Versus Older Second-Generation Thicker Strut Drug-Eluting Stents for Coronary Artery Disease. Circulation 2018, 138, 2216–2226. [Google Scholar] [CrossRef]
- Iglesias, J.F.; Degrauwe, S.; Cimci, M.; Chatelain, Q.; Roffi, M.; Windecker, S.; Pilgrim, T. Differential Effects of Newer-Generation Ultrathin-Strut Versus Thicker-Strut Drug-Eluting Stents in Chronic and Acute Coronary Syndromes. JACC Cardiovasc. Interv. 2021, 14, 2461–2473. [Google Scholar] [CrossRef]
- Pilgrim, T.; Heg, D.; Roffi, M.; Tüller, D.; Muller, O.; Vuilliomenet, A.; Cook, S.; Weilenmann, D.; Kaiser, C.; Jamshidi, P.; et al. Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): A randomised, single-blind, non-inferiority trial. Lancet 2014, 384, 2111–2122. [Google Scholar] [CrossRef]
- von Birgelen, C.; Zocca, P.; Buiten, R.A.; Jessurun, G.A.J.; Schotborgh, C.E.; Roguin, A.; Danse, P.W.; Benit, E.; Aminian, A.; van Houwelingen, K.G.; et al. Thin composite wire strut, durable polymer-coated (Resolute Onyx) versus ultrathin cobalt-chromium strut, bioresorbable polymer-coated (Orsiro) drug-eluting stents in allcomers with coronary artery disease (BIONYX): An international, single-blind, randomised non-inferiority trial. Lancet 2018, 392, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Kandzari, D.E.; Mauri, L.; Koolen, J.J.; Massaro, J.M.; Doros, G.; Garcia-Garcia, H.M.; Bennett, J.; Roguin, A.; Gharib, E.G.; Cutlip, D.E.; et al. Ultrathin, bioresorbable polymer sirolimus-eluting stents versus thin, durable polymer everolimus-eluting stents in patients undergoing coronary revascularisation (BIOFLOW V): A randomised trial. Lancet 2017, 390, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.O.; Thayssen, P.; Maeng, M.; Ravkilde, J.; Krusell, L.R.; Raungaard, B.; Junker, A.; Terkelsen, C.J.; Veien, K.T.; Villadsen, A.B.; et al. Randomized Comparison of a Biodegradable Polymer Ultrathin Strut Sirolimus-Eluting Stent With a Biodegradable Polymer Biolimus-Eluting Stent in Patients Treated With Percutaneous Coronary Intervention: The SORT OUT VII Trial. Circ. Cardiovasc. Interv. 2016, 9, e003610. [Google Scholar] [CrossRef]
- Morofuji, T.; Kuramitsu, S.; Shinozaki, T.; Jinnouchi, H.; Sonoda, S.; Domei, T.; Hyodo, M.; Shirai, S.; Ando, K. Clinical impact of calcified nodule in patients with heavily calcified lesions requiring rotational atherectomy. Catheter. Cardiovasc. Interv. 2021, 97, 10–19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).