Abstract
Background/Objectives: Maternal thyroid function plays a crucial role in fetal brain development, yet the potential impact of maternal hypothyroidism and thyroid autoimmunity on fetal intracranial structures remains inadequately explored. To investigate the impact of maternal hypothyroidism and thyroid autoimmunity on fetal intracranial structures, focusing on potential alterations in critical brain parameters during mid-gestation. Methods: This prospective case-control study included pregnant women between 18 and 24 weeks of gestation. Participants were divided into three groups: hypothyroidism and antibodies (Ab) group, hypothyroidism and Ab(–) group, and the control group. Ultrasonographic measurements of fetal intracranial structures such as the posterior lateral ventricle (PLV), cavum septum pellucidi (CSP), cisterna magna (CM), thalamus, and transcerebellar diameter (TCD) were recorded and compared. Results: A total of 153 pregnant women were evaluated (n = 52 in the hypothyroidism and Ab(+) group, n = 51 in the hypothyroidism and Ab(−) group, and n = 50 in the control group). Although most of the biometric parameters were similar across the groups, the hypothyroidism and Ab(+) group exhibited significantly lower PLV and thalamus measurements compared to the control group (p < 0.05). Additionally, there was a notable difference in the BMI among the groups, with hypothyroid participants (with or without antibodies) showing higher rates of being overweight or obese. Conclusions: Maternal hypothyroidism and the presence of thyroid autoantibodies may be associated with subtle changes in fetal brain structures during the mid-gestation period, particularly in the thalamus and PLV.
1. Introduction
Thyroid dysfunction is one of the most common endocrine disorders among women of reproductive age [1]. Because the fetus only begins producing thyroid hormones between the 16th and 20th weeks of gestation, the thyroid hormones necessary for the optimal growth and development of various organ systems, particularly the fetal brain, are predominantly supplied by transplacental transfer from the mother, starting as early as the first trimester [2]. Hashimoto’s thyroiditis, an autoimmune disease, is the most common cause of hypothyroidism and has a prevalence of approximately 8–10% among women of reproductive age [3]. Characterized by the presence of anti-thyroid antibodies, this condition can lead to the transplacental passage of thyroid autoantibodies, potentially suppressing neonatal thyroid function. However, such suppression is typically transient, and there is insufficient evidence of any long-term adverse effects on the infant [4,5]. Thyroid autoimmunity may alter the course and outcome of pregnancy [6,7,8]. It is thought that anti-thyroid-antibody positivity, even in the presence of a euthyroid state, reflects a broader autoimmune imbalance that can give rise to increased pregnancy complications [9].
The adverse effects of hypothyroidism during pregnancy are well documented and include associated fetal and neonatal complications such as preterm birth, low birth weight, intrauterine fetal death, increased incidence of neonatal respiratory distress, and neurodevelopmental dysfunction in the infant [5,10,11,12,13,14,15,16]. Maternal hypothyroidism during pregnancy is defined as an elevated thyroid-stimulating hormone (TSH) concentration exceeding the upper limit of the gestation-specific reference range [17]. Previous research also suggests that thyroid peroxidase antibodies (TPOAb) may negatively affect pregnancy outcomes in euthyroid women, although the precise mechanism remains a subject of debate.
While various fetal and maternal complications have been documented in pregnancies complicated by thyroid autoantibodies or hypothyroidism, no study to date has focused specifically on fetal intracranial development and related changes. Therefore, in this study, we aimed to evaluate the fetal intracranial structures sonographically in hypothyroid pregnant women, considering both the presence of autoantibodies and the need for treatment.
2. Materials and Methods
This prospective case-control study was conducted between June and December 2024 at the Perinatology Clinic of İzmir City Hospital. Ethical approval for the study was obtained from the ethics committee of Izmir City Hospital (approval no: 2024/51). All of the pregnant women participating in the study were informed about the research, and detailed informed consent was obtained from each participant.
Patients presenting to our clinic between 18 and 24 weeks of gestation for fetal anomaly screening were included in the study. Participants were divided into three groups, namely hypothyroidism and antibodies (Ab)[+]): pregnant women with hypothyroidism secondary to Hashimoto’s thyroiditis diagnosed prior to pregnancy, receiving levothyroxine therapy, and testing positive for TPOAb and thyroglobulin antibodies (TGAb); hypothyroidism and Ab[−]: pregnant women without a history of hypothyroidism, who had negative TPOAb and TGAb levels but had TSH > 2.5 mIU/L in the first trimester and subsequently received levothyroxine therapy; and the control group: pregnant women with no hypothyroidism or thyroid autoantibodies.
The presence of TPOAb and TGAb was determined using enzyme-linked immunosorbent assay (ELISA; ORGENTEC, Mainz, Germany) according to the manufacturer’s instructions. The cutoff value for TPOAb positivity was >34 IU/mL, and for TGAb positivity was >40 IU/mL.
Exclusion criteria were multiple gestations; pregnancies complicated by fetal malformations or chromosomal anomalies; a history of thyroid surgery, thyroid nodules, or radioactive iodine therapy; and the presence of additional systemic diseases.
Fetal biometric parameters and fetal intracranial structures were assessed via ultrasonography. The following fetal intracranial measurements were included: posterior lateral ventricle (PLV), cavum septi pellucidum (CSP), thalamus, cisterna magna (CM), and transverse cerebellar diameter (TCD). Prenatal ultrasonographic examinations were performed using a Voluson E8 Expert system (GE Healthcare, Tiefenbach, Austria) equipped with a 2–9 MHz 2D curvilinear transducer. All ultrasound assessments were conducted once by the same perinatologist, following the guidelines of the International Society of Ultrasound in Obstetrics and Gynecology and the World Association of Perinatal Medicine [18,19]. The PLV was measured on an axial transventricular plane that displays the anterior and posterior horns of the lateral ventricles, at the level of the atrium, perpendicular to the long axis of the ventricle. Calipers were placed on the inner (echogenic) edges of the ventricle walls at the widest point (inner-to-inner measurement). The thalamus, cerebellum, and CM were visualized on a transcerebellar plane. The transverse thalamic diameter was measured between the widest points of the thalami. For the TCD, calipers were placed on the outer edges of the cerebellum. CM was measured as the greatest distance between the posterior edge of the cerebellar vermis and the inner surface of the occipital bone. CSP was assessed on both the transventricular and transcerebellar planes, with the measurement taken on the transventricular plane. CSP width was measured from inner edge to inner edge, perpendicular to the midline (Figure 1 and Figure 2).
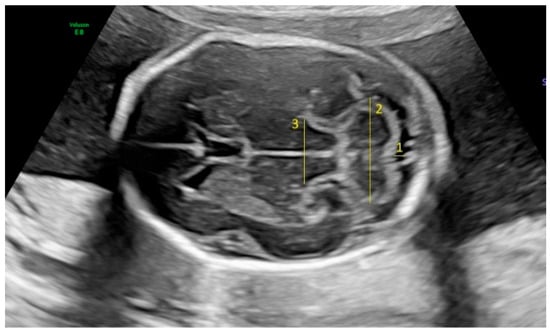
Figure 1.
Fetal intracranial structures in the transcerebellar plane (1: cisterna magna; 2: cerebellum; 3: thalamus).
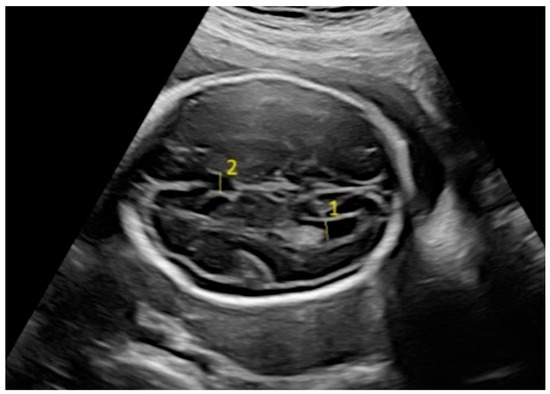
Figure 2.
Fetal intracranial structures in the transventricular plane (1: posterior lateral ventricle; 2: cavum septum pellucidum).
Statistical Analysis
All statistical analyses were performed using the IBM® SPSS® Statistics 26 software package (IBM Corp., Armonk, NY, USA). Descriptive statistics are presented as the mean ± standard deviation, frequency, and percentage. Pearson’s chi-square test was used to analyze categorical variables. A one-way ANOVA was employed for comparisons among groups, and when significant differences were identified, post hoc Bonferroni tests were used for pairwise comparisons. A p-value less than 0.05 was considered statistically significant.
3. Results
A total of 52 pregnant women [hypothyroidism and Ab(+)] with hypothyroidism due to Hashimoto’s thyroiditis diagnosed before pregnancy who were receiving levothyroxine therapy and tested positive for TPOAb and TGAb, 51 pregnant women [hypothyroidism and Ab(−)] with no prior history of hypothyroidism but with negative TPOAb and TGAb levels and a first-trimester TSH > 2.5 mIU/L who received levothyroxine therapy during pregnancy, and 50 pregnant women [control] in the control group were included in the study.
According to the comparison among the study groups (Table 1), age, gestational age, gravidity, and parity were similar across the three groups (p > 0.05 for all). However, a significant difference was found in the body mass index (BMI) parameter (p = 0.012). A post hoc Bonferroni analysis was performed to determine which groups differed, indicating that both the hypothyroidism and Ab(−) and hypothyroidism and Ab(+) groups differed from the control group (p = 0.031 and p = 0.028, respectively).

Table 1.
Comparing parameters among groups.
Table 2 shows the results of the comparison of obstetric parameters among the groups. No statistically significant differences were observed in biparietal diameter (BPD), head circumference (HC), abdominal circumference (AC), femur length (FL), CSP, cerebellum, or CM measurements across the groups. Significant differences were observed in the PLV and thalamus parameters. For the PLV measurements, a significant difference was found only between the control group (6.48 ± 0.84 mm) and the hypothyroidism and Ab(+) group (5.92 ± 1.03 mm), with lower values in the hypothyroidism and Ab(+) group (p = 0.012). Similarly, for the thalamus measurements, a significant difference was noted only between the control group (18.20 ± 3.24 mm) and the hypothyroidism and Ab(+) group (16.71 ± 2.86 mm), again indicating lower values in the hypothyroidism and Ab(+) group (p = 0.027). These findings suggest that maternal hypothyroidism accompanied by thyroid autoantibodies may be associated with subtle alterations in specific fetal brain structures, particularly the PLV and thalamus, during mid-gestation.

Table 2.
Comparing sonographic parameters among groups.
BMI groups were subsequently formed based on the World Health Organization anthropometric scale, and comparisons were made among the study groups. A significant difference in the prevalence of being overweight or obese was noted between the hypothyroidism and Ab(+) group and the control group (67.3% vs. 48.0%, p = 0.002). These findings suggest that there may be an association between maternal hypothyroidism and an increased BMI, especially in the presence of thyroid autoantibodies. Comparison of BMI groups among the study groups is presented in Table 3.

Table 3.
Comparing BMI groups among research groups.
Table 4 presents comparisons restricted to the overweight and obese BMI groups; no significant differences were found among the study groups in the BPD, HC, FL, PLV, CSP, cerebellum, or CM. However, significant differences were detected in the AC and thalamus measurements (p = 0.034 and p = 0.024, respectively). Post hoc analyses indicated that for the AC, there was a significant difference between the control and hypothyroidism and Ab(−) groups (p = 0.029). Regarding the thalamus parameter, significant differences were found between the control and hypothyroidism and Ab(+) groups, and also between the hypothyroidism and Ab(−) and hypothyroidism and Ab(+) groups (p = 0.022).

Table 4.
Comparing obstetric parameters among groups (only overweight and obese BMI).
Finally, Table 5 shows comparisons restricted to the underweight and normal BMI groups; no significant differences were observed among the study groups in terms of the BPD, HC, AC, FL, CSP, thalamus, cerebellum, or CM. A significant difference was identified only in the PLV measurements (p = 0.044). Post hoc analysis revealed that the difference was between the control group and the hypothyroidism and Ab(+) group, with lower values detected in the hypothyroidism and Ab(+) group (p = 0.043).

Table 5.
Comparing obstetric parameters among groups (only underweight and normal BMI).
4. Discussion
The CSP, PLV, CM, TCD, and thalamus are parameters that are often evaluated in fetal neurosonography. In this study, we found significant differences in fetal PLV and thalamus measurements among pregnant women who tested positive for TPOAb and TGAb and received levothyroxine therapy for hypothyroidism secondary to Hashimoto’s thyroiditis (hypothyroidism and Ab(+)). Compared with the control group, the hypothyroidism and Ab(+) group exhibited significantly lower PLV values (p = 0.012). Similarly, thalamus measurements were significantly lower in the hypothyroidism and Ab(+) group than in the control group (p = 0.027). These findings suggest that hypothyroidism and the presence of thyroid autoantibodies may lead to certain alterations in fetal brain structures. In particular, changes in critical brain regions such as the thalamus and PLV indicate that hypothyroidism may exert adverse effects on fetal neurological development.
The thalamus, located in the central part of the brain above the cerebral hemispheres, plays a key role in the processing and relay of sensory and motor signals. It has extensive functions, including sensory integration, motor coordination, cognitive processes, regulation of sleep and wakefulness, and emotion processing through its connections with the limbic system [20,21]. Any damage or dysfunction in the thalamus may have wide-ranging effects on sensory processing, motor control, and cognitive functions.
Because the fetal thyroid gland becomes functionally mature only after the 20th week of gestation, thyroid hormones transferred transplacentally from the mother before and after the onset of fetal thyroid function are crucial [22,23]. During the second trimester when neuronal proliferation, migration, and structural organization occur, fetal brain development largely depends on maternally derived thyroid hormones. In later stages of fetal brain development (glial cell proliferation, migration, and myelination) from the third trimester onward, the primary source of thyroid hormones is the fetal thyroid gland itself. At birth, approximately 30% of the serum thyroxine (T4) measured in the cord blood is known to originate from the mother [24]. This observation strongly suggests that maternal T4 transfer via the placenta persists until birth. In this context, severe maternal hypothyroidism that emerges in the second trimester may lead to irreversible neurological defects, whereas hypothyroidism diagnosed later in pregnancy tends to be less severe and partially reversible in terms of fetal brain injury [25].
In our study, we measured fetal intracranial structures in pregnancies between 18- and 24-weeks’ gestation, during the period in which the fetus is largely dependent on maternal thyroid hormones to evaluate fetal brain development. Assessing maternal antibody status is important because subclinical hypothyroidism and positive TPOAb in pregnant women are associated with an increased risk of adverse pregnancy outcomes including miscarriage, preterm birth, perinatal death, and postpartum dysfunction, and these outcomes can occur at lower TSH values compared to in TPOAb-negative women [26,27]. In the American College of Obstetricians and Gynecologists (ACOG) guidelines on thyroid disease in pregnancy, women who were TPOAb-positive with TSH > 2.5 mU/L had a clearly elevated risk of pregnancy-related complications, whereas TPOAb-negative women did not consistently exhibit such risks until TSH values exceeded 5–10 mU/L [28].
Several studies have reported a link between maternal thyroid autoimmunity and impaired neurodevelopment in offspring [16,29,30,31,32,33]. Thyroid autoantibodies may have significant implications during pregnancy for both the mother and the fetus. Their potential influence on fetal brain development includes delayed neurological maturation, intellectual disability, and motor or behavioral issues. In addition, they may affect placental function and thereby influence fetal neurodevelopment [11,25,34,35]. A study by Haddow and colleagues highlighted that mild or subclinical maternal thyroid dysfunction during pregnancy may be associated with disturbances in the normal brain development of the child [11], illustrating the critical role of thyroid hormones in neurological maturation. Impaired fetal brain development may arise from a combination of factors linked to perinatal hypothyroidism, including low thyroid hormone levels that directly affect the developing brain, as well as indirect effects such as alterations in placental function. Wasserman and colleagues evaluated TPOAb levels in the third trimester of pregnancy and found that children of TPOAb-positive mothers had lower IQ scores compared with those of TPOAb-negative mothers [33]. Previous research, which reported a significant association between a high maternal TPOAb concentration in the third trimester and sensorineural hearing loss in children (prevalence OR 7.5, 95% CI 2.4–23.3), suggested that the lower IQ scores might be mediated by sensorineural hearing loss [16]. Additionally, a study by Wilson et al., investigating the association between maternal TPOAb status and neonatal brain weight, found that infants of TPOAb-positive mothers had smaller head circumferences and reduced brain weights (β = −407; standard error [SE] = 0.200; p < 0.05 and β = −10.307; SE = 5.001; p < 0.05, respectively) [36]. A commonly accepted normal limit for the PLV measurement is less than 10 mm. Values exceeding this threshold can suggest the possibility of hydrocephalus, chromosomal anomalies, intracranial hemorrhage, infections, or other causes of fetal brain abnormalities [37,38]. In our study, the hypothyroidism and Ab(+) group showed lower PLV measurements compared with the control group, although they were still within normal ranges. This decrease is not associated with any known poor prognosis and does not carry clinical management implications. Although the long-term neurodevelopmental negative outcomes of maternal hypothyroidism and thyroid autoantibody positivity are well known, sonographic findings that can predict these negative outcomes in the intrauterine period have not yet been reported. In our study, we found smaller PLV and thalamus sizes in hypothyroid and autoantibody-positive cases, but our data only included mid-trimester examinations, so no evidence could be provided showing the relationship between these findings and long-term outcomes.
We found a statistically significant difference in the BMI between the control group and both hypothyroid groups—those with negative antibodies (hypothyroidism and Ab(−)) and those with positive antibodies (hypothyroidism and Ab(+)) (p = 0.012). These results suggest that hypothyroidism and antibody positivity may affect the body mass index in pregnancy, possibly through metabolic alterations. Such findings emphasize the importance of considering these factors in clinical practice, especially during pregnancy management and follow-up of thyroid function. When examining only the overweight and obese BMI subgroups, the hypothyroidism and Ab(+) group again exhibited significantly lower thalamus values than the hypothyroidism and Ab(−) and control groups, suggesting that this difference in thalamus measurement is not driven by BMI status.
Overall, these results underscore the importance of closely monitoring and adequately treating hypothyroidism during pregnancy. Tailored interventions and follow-up strategies may be necessary to support fetal neurological development in women with thyroid dysfunction during pregnancy. Such findings could have practical implications in perinatology, particularly in the monitoring and management protocols for fetal health among pregnant women with thyroid disorders. Previous studies investigating the neurological development of children born to mothers with hypothyroidism and thyroid autoimmunity have mainly focused on the postnatal and childhood periods. To our knowledge, no study to date has specifically examined fetal neurodevelopment and intracranial changes in this population. Hence, our research serves as a valuable initial contribution to this field.
The primary limitation of our study is that the evaluations were limited to mid-gestation; therefore, it remains unknown as to what changes may occur in the third trimester and postpartum period. Moreover, we did not assess fetal cortical structures, and our study was conducted at a single center with a limited number of participants. Future multicenter studies with different populations to assess fetal brain structures, including cortical structures, and to examine the relationship of findings to long-term postnatal outcomes would be useful to better understand the clinical impact of these findings.
In conclusion, this study was conducted to better understand the effects of hypothyroidism and thyroid autoimmunity on pregnancy. Our findings contribute to the current body of knowledge and highlight the need for future research aimed at refining management strategies for thyroid disorders in pregnancy.
Author Contributions
Conceptualization, R.T. and H.G.; methodology, R.T.; software, R.T.; validation, R.T., C.S. and Z.E.C.; formal analysis, S.T.C.; investigation, R.T.; resources, I.G.; data curation, H.A.A.; writing—original draft preparation, I.T. and Z.E.C.; writing—review and editing, R.T. and H.G.; visualization, R.T.; supervision, H.G. and A.E.; project administration, M.S.; funding acquisition, R.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Research involving human subjects complied with all the relevant national regulations and institutional policies, was in accordance with the tenets of the Helsinki Declaration (as revised in 2013), and was approved by the authors. Izmir City Hospital ethic committee 2024/51 (5 June 2024).
Informed Consent Statement
Informed consent was obtained from all the individuals included in this study.
Data Availability Statement
The datasets analyzed in this study are available from the corresponding author upon reasonable request and with permission of the local Ethics Committee.
Conflicts of Interest
The authors state no conflicts of interest.
References
- Nader, S. Thyroid disease and other endocrine disorders in pregnancy. Obs. Gynecol. Clin. N. Am. 2004, 31, 257–285. [Google Scholar] [CrossRef]
- Thorpe-Beeston, J.G.; Nicolaides, K.H.; Felton, C.V.; Butler, J.; McGregor, A.M. Maturation of the secretion of thyroid hormone and thyroid-stimulating hormone in the fetus. N. Engl. J. Med. 1991, 324, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Hollowell, J.G.; Staehling, N.W.; Flanders, W.D.; Hannon, W.H.; Gunter, E.W.; Spencer, C.A.; Braverman, L.E. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 2002, 87, 489–499. [Google Scholar] [CrossRef]
- Chen, X.; Jin, B.; Xia, J.; Tao, X.; Huang, X.; Sun, L.; Yuan, Q. Effects of Thyroid Peroxidase Antibody on Maternal and Neonatal Outcomes in Pregnant Women in an Iodine-Sufficient Area in China. Int. J. Endocrinol. 2016, 2016, 6461380. [Google Scholar] [CrossRef]
- Nazarpour, S.; Ramezani Tehrani, F.; Simbar, M.; Azizi, F. Thyroid dysfunction and pregnancy outcomes. Iran J. Reprod. Med. 2015, 13, 387–396. [Google Scholar]
- Iijima, T.; Tada, H.; Hidaka, Y.; Mitsuda, N.; Murata, Y.; Amino, N. Effects of autoantibodies on the course of pregnancy and fetal growth. Obstet. Gynecol. 1997, 90, 364–369. [Google Scholar] [CrossRef]
- Dendrinos, S.; Papasteriades, C.; Tarassi, K.; Christodoulakos, G.; Prasinos, G.; Creatsas, G. Thyroid autoimmunity in patients with recurrent spontaneous miscarriages. Gynecol. Endocrinol. 2000, 14, 270–274. [Google Scholar] [CrossRef]
- Bagis, T.; Gokcel, A.; Saygili, E.S. Autoimmune thyroid disease in pregnancy and the postpartum period: Relationship to spontaneous abortion. Thyroid 2001, 11, 1049–1053. [Google Scholar] [CrossRef]
- Meena, M.; Chopra, S.; Jain, V.; Aggarwal, N. The Effect of Anti-Thyroid Peroxidase Antibodies on Pregnancy Outcomes in Euthyroid Women. J. Clin. Diagn. Res. 2016, 10, QC04–QC07. [Google Scholar] [CrossRef]
- Allan, W.C.; Haddow, J.E.; Palomaki, G.E.; Williams, J.R.; Mitchell, M.L.; Hermos, R.J.; Faix, J.D.; Klein, R.Z. Maternal thyroid deficiency and pregnancy complications: Implications for population screening. J. Med. Screen. 2000, 7, 127–130. [Google Scholar] [CrossRef]
- Haddow, J.E.; Palomaki, G.E.; Allan, W.C.; Williams, J.R.; Knight, G.J.; Gagnon, J.; O’Heir, C.E.; Mitchell, M.L.; Hermos, R.J.; Waisbren, S.E.; et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N. Engl. J. Med. 1999, 341, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Glinoer, D.; Soto, M.F.; Bourdoux, P.; Lejeune, B.; Delange, F.; Lemone, M.; Kinthaert, J.; Robijn, C.; Grun, J.P.; de Nayer, P. Pregnancy in patients with mild thyroid abnormalities: Maternal and neonatal repercussions. J. Clin. Endocrinol. Metab. 1991, 73, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Stagnaro-Green, A. Thyroid antibodies and fetal loss: An evolving story. Thyroid 2001, 11, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.H. Thyroid disorders associated with pregnancy: Etiology, diagnosis, and management. Treat Endocrinol. 2005, 4, 31–41. [Google Scholar] [CrossRef]
- Prummel, M.F.; Wiersinga, W.M. Thyroid peroxidase autoantibodies in euthyroid subjects. Best Pr. Res. Clin. Endocrinol. Metab. 2005, 19, 1–15. [Google Scholar] [CrossRef]
- Wasserman, E.E.; Nelson, K.; Rose, N.R.; Eaton, W.; Pillion, J.P.; Seaberg, E.; Talor, M.V.; Burek, L.; Duggan, A.; Yolken, R.H. Maternal thyroid autoantibodies during the third trimester and hearing deficits in children: An epidemiologic assessment. Am. J. Epidemiol. 2008, 167, 701–710. [Google Scholar] [CrossRef]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389, Erratum in Thyroid 2017, 27, 1212. [Google Scholar] [CrossRef]
- De Robertis, V.; Sen, C.; Timor-Tritsch, I.; Chaoui, R.; Volpe, P.; Galindo, A.; Achiron, R.; Pooh, R.; Khalil, A.; Volpe, N.; et al. WAPM-World Association of Perinatal Medicine Practice Guidelines: Fetal central nervous system examination. J. Perinat. Med. 2021, 49, 1033–1041. [Google Scholar] [CrossRef]
- Malinger, G.; Paladini, D.; Haratz, K.K.; Monteagudo, A.; Pilu, G.L.; Timor-Tritsch, I.E. ISUOG Practice Guidelines (updated): Sonographic examination of the fetal central nervous system. Part 1: Performance of screening examination and indications for targeted neurosonography. Ultrasound Obstet. Gynecol. 2020, 56, 476–484, Erratum in Ultrasound Obstet. Gynecol. 2022, 60, 591. [Google Scholar] [CrossRef]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M.; Siegelbaum, S.; Hudspeth, A.J.; Mack, S. (Eds.) Principles of Neural Science; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Hall, W.; LaMantia, A.S.; White, L. Neuroscience; De Boeck Supérieur: Paris, France, 2019. [Google Scholar]
- Contempré, B.; Jauniaux, E.; Calvo, R.; Jurkovic, D.; Campbell, S.; de Escobar, G.M. Detection of thyroid hormones in human embryonic cavities during the first trimester of pregnancy. J. Clin. Endocrinol. Metab. 1993, 77, 1719–1722. [Google Scholar] [CrossRef]
- Calvo, R.M.; Jauniaux, E.; Gulbis, B.; Asunción, M.; Gervy, C.; Contempré, B.; Morreale de Escobar, G. Fetal tissues are exposed to biologically relevant free thyroxine concentrations during early phases of development. J. Clin. Endocrinol. Metab. 2002, 87, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Vulsma, T.; Gons, M.H.; de Vijlder, J.J. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N. Engl. J. Med. 1989, 321, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Poppe, K.; Glinoer, D. Thyroid autoimmunity and hypothyroidism before and during pregnancy. Hum. Reprod. Update 2003, 9, 149–161. [Google Scholar] [CrossRef]
- Liu, H.; Shan, Z.; Li, C.; Mao, J.; Xie, X.; Wang, W.; Fan, C.; Wang, H.; Zhang, H.; Han, C.; et al. Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: A prospective cohort study. Thyroid 2014, 24, 1642–1649. [Google Scholar] [CrossRef]
- Stagnaro-Green, A.; Roman, S.H.; Cobin, R.H.; El-Harazy, E.; Alvarez-Marfany, M.; Davies, T.F. Detection of at-risk pregnancy by means of highly sensitive assays for thyroid autoantibodies. JAMA 1990, 264, 1422–1425. [Google Scholar]
- American College of Obstetricians and Gynecologists. Thyroid Disease in Pregnancy: ACOG Practice Bulletin, Number 223. Obs. Gynecol. 2020, 135, e261–e274. [Google Scholar] [CrossRef]
- Li, Y.; Shan, Z.; Teng, W.; Yu, X.; Li, Y.; Fan, C.; Teng, X.; Guo, R.; Wang, H.; Li, J.; et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clin. Endocrinol. 2010, 72, 825–829. [Google Scholar] [CrossRef]
- Pop, V.J.; de Vries, E.; van Baar, A.L.; Waelkens, J.J.; de Rooy, H.A.; Horsten, M.; Donkers, M.M.; Komproe, I.H.; van Son, M.M.; Vader, H.L. Maternal thyroid peroxidase antibodies during pregnancy: A marker of impaired child development? J. Clin. Endocrinol. Metab. 1995, 80, 3561–3566. [Google Scholar] [CrossRef]
- Williams, F.L.; Watson, J.; Ogston, S.A.; Visser, T.J.; Hume, R.; Willatts, P. Maternal and umbilical cord levels of T4, FT4, TSH, TPOAb, and TgAb in term infants and neurodevelopmental outcome at 5.5 years. J. Clin. Endocrinol. Metab. 2013, 98, 829–838. [Google Scholar] [CrossRef]
- Ghassabian, A.; Bongers-Schokking, J.J.; de Rijke, Y.B.; van Mil, N.; Jaddoe, V.W.; de Muinck Keizer-Schrama, S.M.; Hooijkaas, H.; Hofman, A.; Visser, W.; Roman, G.C.; et al. Maternal thyroid autoimmunity during pregnancy and the risk of attention deficit/hyperactivity problems in children: The Generation R Study. Thyroid 2012, 22, 178–186. [Google Scholar] [CrossRef]
- Wasserman, E.E.; Pillion, J.P.; Duggan, A.; Nelson, K.; Rohde, C.; Seaberg, E.C.; Talor, M.V.; Yolken, R.H.; Rose, N.R. Childhood IQ, hearing loss, and maternal thyroid autoimmunity in the Baltimore Collaborative Perinatal Project. Pediatr. Res. 2012, 72, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Berbel, P.; Mestre, J.L.; Santamaría, A.; Palazón, I.; Franco, A.; Graells, M.; González-Torga, A.; de Escobar, G.M. Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: The importance of early iodine supplementation. Thyroid 2009, 19, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Tosun, G.; Kose, S.; İşbilen Başok, B.; Altunyurt, S. First-trimester placental function in levothyroxine-using pregnant women: A case-control study. Gynecol. Endocrinol. 2020, 36, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.E.; Salihu, H.M.; Groer, M.W.; Dagne, G.; O’Rourke, K.; Mbah, A.K. Impact of maternal thyroperoxidase status on fetal body and brain size. J. Thyroid Res. 2014, 2014, 872410. [Google Scholar] [CrossRef]
- Mehlhorn, A.J.; Morin, C.E.; Wong-You-Cheong, J.J.; Contag, S.A. Mild fetal cerebral ventriculomegaly: Prevalence, characteristics, and utility of ancillary testing in cases presenting to a tertiary referral center. Prenat. Diagn. 2017, 37, 647–657. [Google Scholar] [CrossRef]
- Pisapia, J.M.; Sinha, S.; Zarnow, D.M.; Johnson, M.P.; Heuer, G.G. Fetal ventriculomegaly: Diagnosis, treatment, and future directions. Childs Nerv. Syst. 2017, 33, 1113–1123. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).