The Role of Palmar Cutaneous Branch Release in Enhancing Surgical Outcomes for Severe Carpal Tunnel Syndrome
Abstract
1. Introduction
2. Materials and Methods
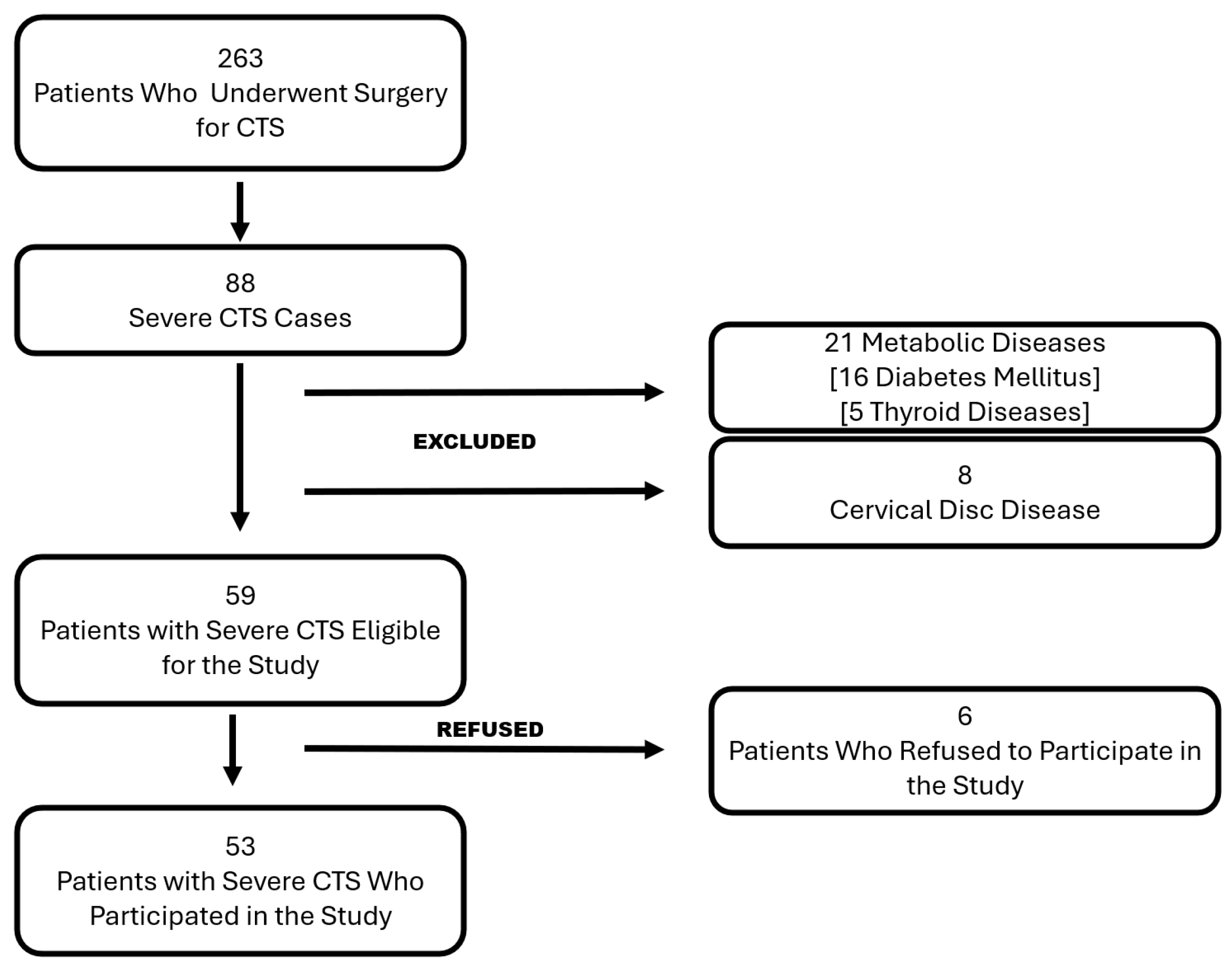
2.1. Patient Selection
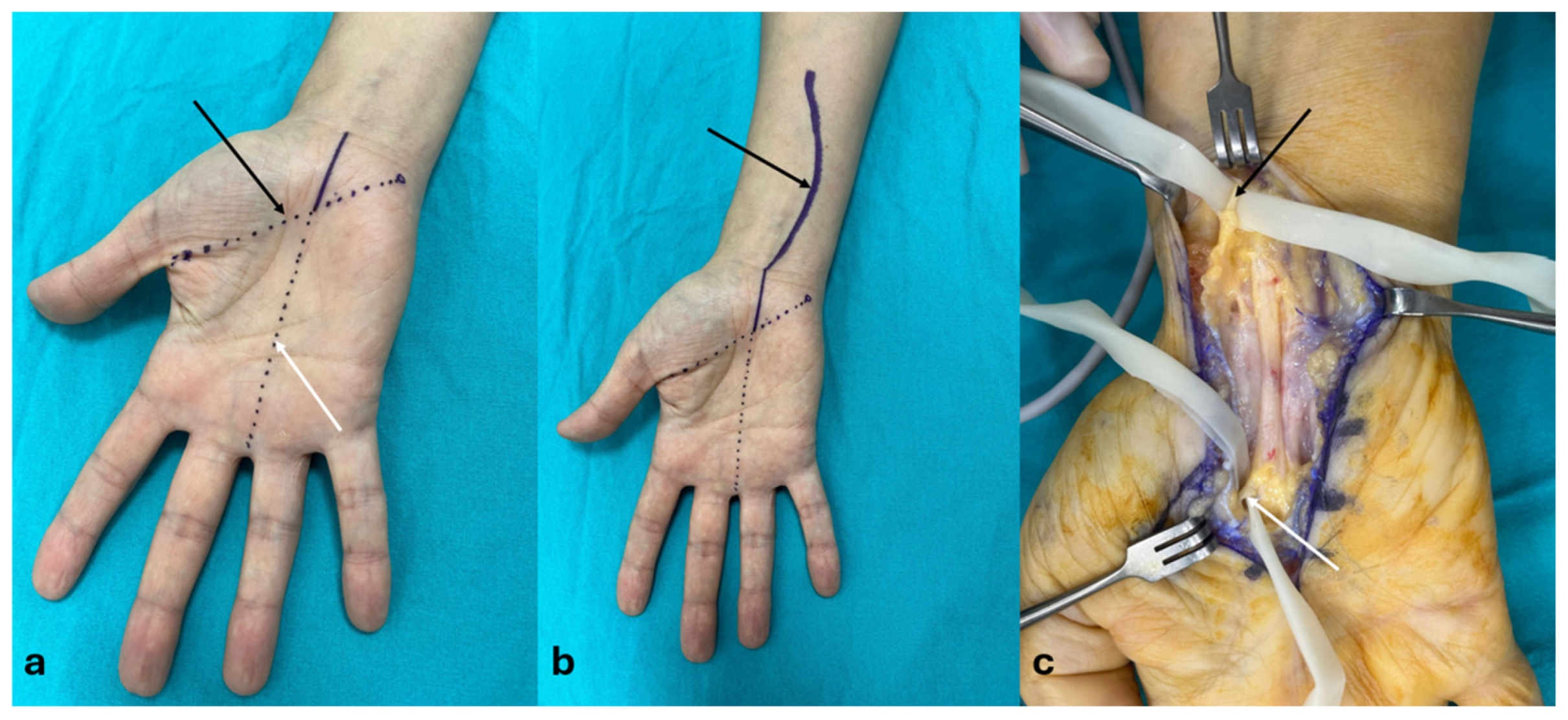
2.2. Surgical Techniques
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTS | Carpal tunnel syndrome |
| CTR | Carpal tunnel release |
| OCTR | Open carpal tunnel release |
| ECTR | Endoscopic carpal tunnel release |
| RMB | Recurrent motor branch |
| PCBm | Palmar cutaneous branch of the median nerve |
| EOCTR | Extended open carpal tunnel release |
| AANEM | American Association of Neuromuscular and Electrodiagnostic Medicine |
| TCL | Transverse carpal ligament |
| KCL | Kaplan’s cardinal line |
| DASH | Disabilities of the Arm, Shoulder, and Hand Questionnaire |
| BCTQ | Boston Carpal Tunnel Syndrome Questionnaire |
| SSS | Symptom severity scale |
| FS | Functional scale |
| VAS | Visual Analog Scale |
| mOCTR | Mini-open carpal tunnel release |
References
- Fajardo, M.; Kim, S.H.; Szabo, R.M. Incidence of carpal tunnel release: Trends and implications within the United States ambulatory care setting. J. Hand Surg. Am. 2012, 37, 1599–1605. [Google Scholar] [PubMed]
- Osiak, K.; Elnazir, P.; Walocha, J.A.; Pasternak, A. Carpal tunnel syndrome: State-of-the-art review. Folia Morphol. 2022, 81, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Padua, L.; Coraci, D.; Erra, C.; Pazzaglia, C.; Paolasso, I.; Loreti, C.; Caliandro, P.; Hobson-Webb, L.D. Carpal tunnel syndrome: Clinical features, diagnosis, and management. Lancet Neurol. 2016, 15, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Binsaleem, S. Median nerve entrapment neuropathy: A review on the pronator syndrome. JSES Rev. Rep. Tech. 2025, 5, 70–78. [Google Scholar] [CrossRef]
- Kim, P.T.; Lee, H.J.; Kim, T.G.; Jeon, I.H. Current approaches for carpal tunnel syndrome. Clin. Orthop. Surg. 2014, 6, 253–257. [Google Scholar] [CrossRef]
- Wu, P.T.; Chern, T.C.; Wu, T.T.; Shao, C.J.; Wu, K.C.; Kuo, L.C.; Jou, I.M. Safe Zones for Percutaneous Carpal Tunnel Release. Hand Clin. 2022, 38, 83–90. [Google Scholar] [CrossRef]
- Sayegh, E.T.; Strauch, R.J. Open versus endoscopic carpal tunnel release: A meta-analysis of randomized controlled trials. Clin. Orthop. Relat. Res. 2015, 473, 1120–1132. [Google Scholar] [CrossRef]
- Shin, E.K. Endoscopic Versus Open Carpal Tunnel Release. Curr. Rev. Musculoskelet. Med. 2019, 12, 509–514. [Google Scholar] [CrossRef]
- Shimizu, K.; Iwasaki, R.; Hoshikawa, H.; Yamamuro, T. Entrapment neuropathy of the palmar cutaneous branch of the median nerve by the fascia of flexor digitorum superficialis. J. Hand Surg. Am. 1988, 13, 581–583. [Google Scholar] [CrossRef]
- Vieira de Pádua Maia, M.; Rosifini Alves Rezende, L.G. Isolated Compression of the Recurrent Motor Branch of the Median Nerve: A Case Report. Hand 2022, 17, Np1–Np5. [Google Scholar] [CrossRef]
- Albano, D.; Jengojan, S.A. Multiparametric Ultrasound Assessment of Carpal Tunnel Syndrome: Beyond Nerve Cross-sectional Area. In Seminars in Musculoskeletal Radiology; Thieme Medical Publishers, Inc.: New York, NY, USA, 2024; Volume 28, pp. 661–671. [Google Scholar]
- Stevens, J.C. AAEM minimonograph #26: The electrodiagnosis of carpal tunnel syndrome. American Association of Electrodiagnostic Medicine. Muscle Nerve 1997, 20, 1477–1486. [Google Scholar] [PubMed]
- Düger, T.; Yakut, E.; Öksüz, Ç.; Yörükan, S.; Bilgutay, B.; Ayhan, Ç.; Leblebicioglu, G.; Kayihan, H.; Kirdi, N.; Yakut, Y.; et al. Reliability and validity of the Turkish version of the Disabilities of the Arm, Shoulder and Hand (DASH) Questionnaire. Turk. J. Physiother. Rehabil. 2006, 17, 3. [Google Scholar]
- İlhan, D.; Toker, S.; Kılıncıoğlu, V.; Gülcan, E. Assessment of the Boston questionnaire in diagnosis of idiopathic carpal tunnel syndrome: Comparing scores with clinical and neurophysiological findings. Duzce Med. J. 2008, 10, 4–9. [Google Scholar]
- Hattori, Y.; Doi, K.; Koide, S.; Sakamoto, S. Endoscopic release for severe carpal tunnel syndrome in octogenarians. J. Hand Surg. Am. 2014, 39, 2448–2453. [Google Scholar] [CrossRef]
- Boya, H.; Özcan, Ö.; Özteki, N.H. Long-term complications of open carpal tunnel release. Muscle Nerve 2008, 38, 1443–1446. [Google Scholar]
- Akkurt, M.O.; Düzgün, S.; Ateş, A.; Yaradılmış, Y.U. Comparison of two approaches for carpal tunnel release: Extended versus mini-open technique. Jt. Dis. Relat. Surg. 2020, 31, 50–55. [Google Scholar]
- Murthy, P.G.; Goljan, P.; Mendez, G.; Jacoby, S.M.; Shin, E.K.; Osterman, A.L. Mini-open versus extended open release for severe carpal tunnel syndrome. Hand 2015, 10, 34–39. [Google Scholar]
- Uluc, K.; Aktas, I.; Sunter, G.; Kahraman Koytak, P.; Akyuz, G.; İsak, B.; Tanridag, T.; Us, O. Palmar cutaneous nerve conduction in patients with carpal tunnel syndrome. Int. J. Neurosci. 2015, 125, 817–822. [Google Scholar]
- Jeong, H.M.; Jeong, Y.H.; Yoon, J.S. Is Palmar Cutaneous Branch of the Median Nerve More Swollen in Carpal Tunnel Syndrome? Ann. Rehabil. Med. 2021, 45, 325–330. [Google Scholar] [CrossRef]
- Hobbs, R.A.; Magnussen, P.A.; Tonkin, M.A. Palmar cutaneous branch of the median nerve. J. Hand Surg. Am. 1990, 15, 38–43. [Google Scholar]
- Rathakrishnan, R.; Therimadasamy, A.K.; Chan, Y.H.; Wilder-Smith, E.P. The median palmar cutaneous nerve in normal subjects and CTS. Clin. Neurophysiol. 2007, 118, 776–780. [Google Scholar] [PubMed]
- Wada, T.; Imai, T.; Ishii, S. Entrapment neuropathy of the palmar cutaneous branch of the median nerve concomitant with carpal tunnel syndrome: A case report. J. Hand Surg. Br. 2002, 27, 583–585. [Google Scholar] [PubMed]
- Vasiliadis, H.S.; Georgoulas, P.; Shrier, I.; Salanti, G.; Scholten, R.J. Endoscopic release for carpal tunnel syndrome. Cochrane Database Syst. Rev. 2014, 2014, Cd008265. [Google Scholar]
- Orhurhu, V.; Orman, S.; Peck, J.; Urits, I.; Orhurhu, M.S.; Jones, M.R.; Manchikanti, L.; Kaye, A.D.; Odonkor, C.; Hirji, S.; et al. Carpal Tunnel Release Surgery- A Systematic Review of Open and Endoscopic Approaches. Anesth. Pain Med. 2020, 10, e112291. [Google Scholar]
- Aslani, H.R.; Alizadeh, K.; Eajazi, A.; Karimi, A.; Karimi, M.H.; Zaferani, Z.; Khameneh, S.M.H. Comparison of carpal tunnel release with three different techniques. Clin. Neurol. Neurosurg. 2012, 114, 965–968. [Google Scholar]
- Yücetaş, S.C.; Yildirim, A. Comparative results of standard open and mini open, KnifeLight instrument-assisted carpal tunnel release. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2013, 74, 393–399. [Google Scholar]
- Palmer, A.K. Complications of endoscopic and open carpal tunnel release. J. Hand Surg. Am. 2000, 25, 185. [Google Scholar]
- Karl, J.W.; Gancarczyk, S.M.; Strauch, R.J. Complications of Carpal Tunnel Release. Orthop. Clin. N. Am. 2016, 47, 425–433. [Google Scholar] [CrossRef]
- Kretschmer, T.; Antoniadis, G.; Richter, H.P.; König, R.W. Avoiding iatrogenic nerve injury in endoscopic carpal tunnel release. Neurosurg. Clin. N. Am. 2009, 20, 65–71. [Google Scholar]
- Henry, B.M.; Zwinczewska, H.; Roy, J.; Vikse, J.; Ramakrishnan, P.K.; Walocha, J.A.; Tomaszewski, K.A. The Prevalence of Anatomical Variations of the Median Nerve in the Carpal Tunnel: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0136477. [Google Scholar]
| Variable | Entire Study Population (n = 53) | Group EOCTR (n = 24) | Group OCTR (n = 29) | p |
|---|---|---|---|---|
| Age, year (mean ± SD) | 50.4 ± 7.7 | 50.3 ± 7.3 | 50.4 ± 8.1 | 0.94 |
| Gender, n (%) | ||||
| Female | 45 (85) | 21 (88) | 24 (83) | 0.71 |
| Male | 8 (15) | 3 (12) | 5 (17) | |
| Side, n (%) | ||||
| Right | 45 (85) | 19 (79) | 26 (90) | 0.44 |
| Left | 8 (15) | 5 (21) | 3 (10) | |
| Dominance status, n (%) | ||||
| Dominant | 41 (77) | 18 (75) | 23 (79) | 0.75 |
| Non-dominant | 12 (23) | 6 (25) | 6 (22) | |
| BMI (mean ± SD) | 26.5 ± 2.7 | 27.2 ± 2.6 | 26 ± 2.7 | 0.12 |
| Follow-up, months (mean ± SD) | 22 ± 5.0 | 22 ± 5.4 | 21 ± 4.7 | 0.42 |
| Parameter | Group EOCR (Mean ± SD) | Group OCR (Mean ± SD) | p-Value |
|---|---|---|---|
| Preoperative VAS | 7.5 ± 0.4 | 7.8 ± 0.6 | 0.076 |
| Postoperative VAS | 3.3 ± 0.3 | 3.8 ± 0.2 | <0.001 |
| Preoperative DASH | 47.2 ± 2.9 | 47.7 ± 4.1 | 0.802 |
| Postoperative DASH | 16.5 ± 1.6 | 20.7 ± 2.7 | <0.001 |
| Preoperative BCTQ-SSS | 3.9 ± 0.2 | 3.9 ± 0.2 | 0.878 |
| Postoperative BCTQ-SSS | 1.9 ± 0.1 | 2 ± 0.1 | <0.001 |
| Preoperative BCTQ-FS | 3.9 ± 0.3 | 3.9 ± 0.2 | 0.971 |
| Postoperative BCTQ-FS | 2 ± 0.1 | 2.1 ± 0.1 | 0.058 |
| Grip Strength (kg) | 22.7 ± 1.4 | 22.4 ± 1.6 | 0.520 |
| Pinch Strength (kg)—Tip-to-Tip | 2.4 ± 0.3 | 2.3 ± 0.2 | 0.540 |
| Pinch Strength (kg)—Lateral | 3.1 ± 0.4 | 3 ± 0.2 | 0.061 |
| Pinch Strength (kg)—3-Point | 3 ± 0.3 | 2.9 ± 0.1 | 0.170 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayer, G.; Gunsoy, Z.; Golgelioglu, F.; Bayrakcioglu, O.F.; Kizkapan, T.B.; Ozboluk, S.; Dinc, M.; Oguzkaya, S. The Role of Palmar Cutaneous Branch Release in Enhancing Surgical Outcomes for Severe Carpal Tunnel Syndrome. J. Clin. Med. 2025, 14, 2196. https://doi.org/10.3390/jcm14072196
Sayer G, Gunsoy Z, Golgelioglu F, Bayrakcioglu OF, Kizkapan TB, Ozboluk S, Dinc M, Oguzkaya S. The Role of Palmar Cutaneous Branch Release in Enhancing Surgical Outcomes for Severe Carpal Tunnel Syndrome. Journal of Clinical Medicine. 2025; 14(7):2196. https://doi.org/10.3390/jcm14072196
Chicago/Turabian StyleSayer, Gokhan, Zeki Gunsoy, Fatih Golgelioglu, Omer Faruk Bayrakcioglu, Turan Bilge Kizkapan, Sener Ozboluk, Mustafa Dinc, and Sinan Oguzkaya. 2025. "The Role of Palmar Cutaneous Branch Release in Enhancing Surgical Outcomes for Severe Carpal Tunnel Syndrome" Journal of Clinical Medicine 14, no. 7: 2196. https://doi.org/10.3390/jcm14072196
APA StyleSayer, G., Gunsoy, Z., Golgelioglu, F., Bayrakcioglu, O. F., Kizkapan, T. B., Ozboluk, S., Dinc, M., & Oguzkaya, S. (2025). The Role of Palmar Cutaneous Branch Release in Enhancing Surgical Outcomes for Severe Carpal Tunnel Syndrome. Journal of Clinical Medicine, 14(7), 2196. https://doi.org/10.3390/jcm14072196






