The Utility of Nuclear Imaging in Hypertrophic Cardiomyopathy: A Narrative Review
Abstract
1. Introduction
2. Background
2.1. Genetic Basis of Disease
2.2. The Pathophysiology of HCM
2.3. Principles of Diagnosis and Stratification of Risk
2.4. Management of HCM
3. Methods
4. Results
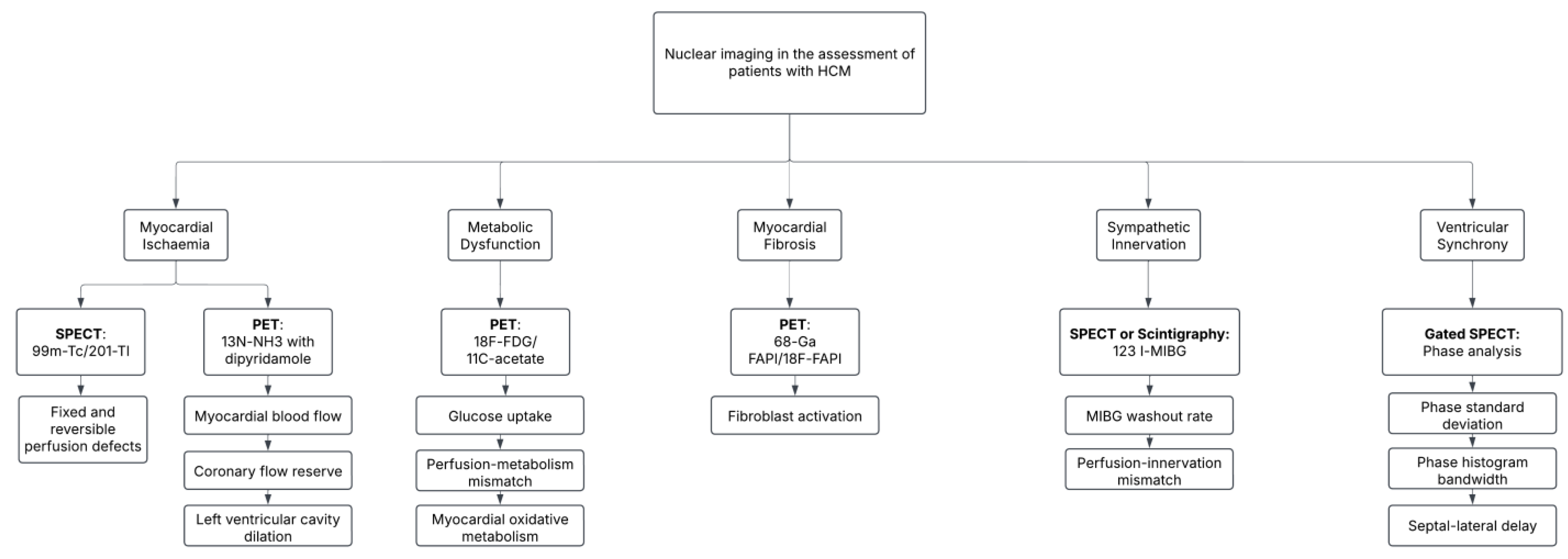
4.1. Nuclear Imaging Modalities in HCM
4.2. Characterizing Myocardial Ischemia
4.2.1. Myocardial Perfusion Imaging with SPECT
4.2.2. PET and Myocardial Blood Flow
4.3. Metabolic and Functional Imaging
4.4. Early Detection of Myocardial Fibrosis
4.5. Evaluation of Ventricular Synchronization
4.6. Imaging of Abnormalities in Sympathetic Innervation
4.7. Therapeutic and Post-Interventional Imaging
4.8. Myocardial Energetic Efficiency
5. Discussion
5.1. Summary of Key Findings
5.2. Comparison to Conventional Imaging Modalities
5.2.1. Echocardiography vs. Nuclear Imaging
5.2.2. Cardiac MRI vs. Nuclear Imaging
5.2.3. Nuclear Imaging as a Complementary Tool
5.3. Role of Nuclear Imaging in Guiding Treatment and Risk Stratification
5.3.1. Risk Stratification Models
5.3.2. Guiding Clinical Decision-Making
5.4. Recent Advancements in Nuclear Imaging Methods
5.5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FDG | Fluorodeoxyglucose |
| FAPI | Fibroblast Activation Protein Inhibitor |
| HCM | Hypertrophic Cardiomyopathy |
| H/M Ratio | Heart-to-Mediastinum Ratio |
| LVCD | Left Ventricular Cavity Dilation |
| MBF | Myocardial Blood Flow |
| MIBG | Metaiodobenzylguanidine |
| MPI | Myocardial Perfusion Imaging |
| MRI | Magnetic Resonance Imaging |
| MYBPC3 | Myosin-Binding Protein C |
| MYH7 | Beta-Myosin Heavy Chain |
| PET | Positron Emission Tomography |
| PHB | Phase Histogram Bandwidth |
| PSD | Phase Standard Deviation |
| SCD | Sudden Cardiac Death |
| SPECT | Single-Photon Emission Computed Tomography |
| Tl | Thallium |
| TNNI3 | Troponin I Type 3 |
| TNNT2 | Troponin T Type 2 |
| TPM1 | Tropomyosin 1 |
References
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the european society of Cardiology working group on myocardial and pericardial diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Shiwani, H.; Davies, R.H.; Topriceanu, C.C.; Ditaranto, R.; Owens, A.; Raman, B.; Augusto, J.; Hughes, R.K.; Torlasco, C.; Dowsing, B.; et al. Demographic-Based Personalized Left Ventricular Hypertrophy Thresholds for Hypertrophic Cardiomyopathy Diagnosis. J. Am. Coll. Cardiol. 2025, 85, 685–695. [Google Scholar] [CrossRef]
- Malhotra, A.; Sharma, S. Hypertrophic Cardiomyopathy in Athletes. Eur. Cardiol. Rev. 2017, 12, 80. [Google Scholar] [CrossRef]
- Lopes, L.R.; Ho, C.Y.; Elliott, P.M. Genetics of hypertrophic cardiomyopathy: Established and emerging implications for clinical practice. Eur. Heart J. 2024, 45, 2727–2734. [Google Scholar] [CrossRef]
- Tudurachi, B.S.; Zăvoi, A.; Leonte, A.; Țăpoi, L.; Ureche, C.; Bîrgoan, S.G.; Chiuariu, T.; Anghel, L.; Radu, R.; Sascău, R.A.; et al. An Update on MYBPC3 Gene Mutation in Hypertrophic Cardiomyopathy. Int. J. Mol. Sci. 2023, 24, 10510. [Google Scholar] [CrossRef]
- Richard, P.; Charron, P.; Carrier, L.; Ledeuil, C.; Cheav, T.; Pichereau, C.; Benaiche, A.; Isnard, R.; Dubourg, O.; Burban, M.; et al. Hypertrophic Cardiomyopathy. Circulation 2003, 107, 2227–2232. [Google Scholar] [CrossRef]
- Robinson, P.; Liu, X.; Sparrow, A.; Patel, S.; Zhang, Y.H.; Casadei, B.; Watkins, H.; Redwood, C. Hypertrophic cardiomyopathy mutations increase myofilament Ca2+ buffering, alter intracellular Ca2+ handling, and stimulate Ca2+-dependent signaling. J. Biol. Chem. 2018, 293, 10487. [Google Scholar] [CrossRef]
- Sequeira, V.; Waddingham, M.T.; Tsuchimochi, H.; Maack, C.; Pearson, J.T. Mechano-energetic uncoupling in hypertrophic cardiomyopathy: Pathophysiological mechanisms and therapeutic opportunities. J. Mol. Cell. Cardiol. Plus 2023, 4, 100036. [Google Scholar] [CrossRef]
- Gersh, B.J.; Maron, B.J.; Bonow, R.O.; Dearani, J.A.; Fifer, M.A.; Link, M.S.; Naidu, S.S.; Nishimura, R.A.; Ommen, S.R.; Rakowski, H.; et al. ACCF/AHA Guideline 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines e783. Circulation 2011, 124, 783–831. [Google Scholar] [CrossRef]
- Maron, M.S.; Olivotto, I.; Maron, B.J.; Prasad, S.K.; Cecchi, F.; Udelson, J.E.; Camici, P.G. The Case for Myocardial Ischemia in Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2009, 54, 866–875. [Google Scholar] [CrossRef]
- Cannon, R.O.; Schenke, W.H.; Maron, B.J.; Tracy, C.M.; Leon, M.B.; Brush, J.E.; Rosing, D.R.; Epstein, S.E. Differences in coronary flow and myocardial metabolism at rest and during pacing between patients with obstructive and patients with nonobstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 1987, 10, 53–62. [Google Scholar] [CrossRef]
- Lee, W.W. Recent Advances in Nuclear Cardiology. Nucl. Med. Mol. Imaging 2016, 50, 196. [Google Scholar] [CrossRef]
- Amano, Y.; Kitamura, M.; Takano, H.; Yanagisawa, F.; Tachi, M.; Suzuki, Y.; Kumita, S.; Takayama, M. Cardiac MR Imaging of Hypertrophic Cardiomyopathy: Techniques, Findings, and Clinical Relevance. Magn. Reson. Med. Sci. 2018, 17, 120. [Google Scholar] [CrossRef]
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Diagnosis and Evaluation of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 372–389. [Google Scholar] [CrossRef]
- Mestres, C.A.; Bartel, T.; Sorgente, A.; Müller, S.; Gruner, C.; Dearani, J.; Quintana, E. Hypertrophic obstructive cardiomyopathy: What, when, why, for whom? Eur. J. Cardio-Thorac. Surg. 2018, 53, 700–707. [Google Scholar] [CrossRef]
- Lebowitz, S.; Kowalewski, M.; Raffa, G.M.; Chu, D.; Greco, M.; Gandolfo, C.; Mignosa, C.; Lorusso, R.; Suwalski, P.; Pilato, M. Review of Contemporary Invasive Treatment Approaches and Critical Appraisal of Guidelines on Hypertrophic Obstructive Cardiomyopathy: State-of-the-Art Review. J. Clin. Med. 2022, 11, 3405. [Google Scholar] [CrossRef]
- Sanchez, D.J.; Lozano, I.F. Implantable cardioverter–defibrillator in hypertrophic cardiomyopathy. Glob. Cardiol. Sci. Pr. 2018, 2018, 31. [Google Scholar] [CrossRef]
- Keng, F.Y.J.; Chang, S.M.; Cwajg, E.; He, Z.X.; Lakkis, N.M.; Nagueh, S.F.; Spencer, W.H., 3rd; Verani, M.S. Gated SPECT in patients with hypertrophic obstructive cardiomyopathy undergoing transcoronary ethanol septal ablation. J. Nucl. Cardiol. 2002, 9, 594–600. [Google Scholar] [CrossRef]
- Shimizu, M.; Ino, H.; Yamaguchi, M.; Terai, H.; Hayashi, K.; Nakajima, K.; Taki, J.; Mabuchi, H. Heterogeneity of cardiac sympathetic nerve activity and systolic dysfunction in patients with hypertrophic cardiomyopathy. J. Nucl. Med. 2002, 43, 15–20. [Google Scholar]
- Sipola, P.; Vanninen, E.; Aronen, H.J.; Lauerma, K.; Simula, S.; Jääskeläinen, P.; Laakso, M.; Peuhkurinen, K.; Kuusisto, J.; Kuikka, J.T. Cardiac adrenergic activity is associated with left ventricular hypertrophy in genetically homogeneous subjects with hypertrophic cardiomyopathy. J. Nucl. Med. 2003, 44, 487–493. [Google Scholar]
- Parker Ward, R.; Hemlata, M.D.; Pokharna, K.; Lang, R.M.; Williams, K.A. Resting “Solar Polar” map pattern and reduced apical flow reserve: Characteristics of apical hypertrophic cardiomyopathy on SPECT myocardial perfusion imaging. J. Nucl. Cardiol. 2003, 10, 506–512. [Google Scholar] [CrossRef]
- Romero-Farina, G.; Candell-Riera, J.; Galve, E.; Armadans, L.; Ramos, F.; Castell, J.; Aguadé, S.; Nogales, J.M.; Soler-Soler, J. Do myocardial perfusion SPECT and radionuclide angiography studies in adult patients with hypertrophic cardiomyopathy have prognostic implications? J. Nucl. Cardiol. 2004, 11, 578–586. [Google Scholar] [CrossRef]
- Sorajja, P.; Chareonthaitawee, P.; Ommen, S.R.; Miller, T.D.; Hodge, D.O.; Gibbons, R.J. Prognostic utility of single-photon emission computed tomography in adult patients with hypertrophic cardiomyopathy. Am. Heart J. 2006, 151, 426–435. [Google Scholar] [CrossRef]
- Kawasaki, T.; Akakabe, Y.; Yamano, M.; Miki, S.; Kamitani, T.; Kuribayashi, T.; Sugihara, H. Gated single-photon emission computed tomography detects subendocardial ischemia in hypertrophic cardiomyopathy. Circ. J. 2007, 71, 256–260. [Google Scholar]
- Cianciulli, T.F.; Saccheri, M.C.; Masoli, O.H.; Redruello, M.F.; Lax, J.A.; Morita, L.A.; Gagliardi, J.A.; Dorelle, A.N.; Prezioso, H.A.; Vidal, L.A. Myocardial perfusion SPECT in the diagnosis of apical hypertrophic cardiomyopathy. J. Nucl. Cardiol. 2009, 16, 391–395. [Google Scholar] [CrossRef]
- Baba, Y.; Kubo, T.; Okawa, M.; Yamasaki, N.; Matsumura, Y.; Kitaoka, H.; Doi, Y. 201-Thallium SPECT Could Predict the Prognosis of Dilated Phase of Hypertrophic Cardiomyopathy. J. Card. Fail. 2010, 16, S151–S152. [Google Scholar] [CrossRef]
- Chen, J.; Nagaraj, H.; Bhambhani, P.; Kliner, D.E.; Soman, P.; Garcia, E.V.; Heo, J.; Iskandrian, A.E. Effect of alcohol septal ablation in patients with hypertrophic cardiomyopathy on left-ventricular mechanical dyssynchrony as assessed by phase analysis of gated SPECT myocardial perfusion imaging. Int. J. Cardiovasc. Imaging 2012, 28, 1375–1384. [Google Scholar] [CrossRef]
- Cocker, M.S.; Dwivedi, G.; Marvin, B.; Poirier, M.; Dennie, C.; Wells, G.; Ascah, K.; Roberts, R.; Dick, A.; Ruddy, T.D. Abstract 19661: Integrin Imaging for the Detection of Diffuse Myocardial Fibrosis in Patients with Hypertrophic Cardiomyopathy: Direct Comparison Between Single-Photon Emission Computer Tomography and Cardiovascular Magnetic Resonance The SCAR Study. Circulation 2012, 126, A19661. [Google Scholar] [CrossRef]
- Utanohara, Y.; Iguchi, N.; Takayama, M.; Umemura, J.; Sumiyoshi, T.; Tomoike, H. Prognostic Utility of Myocardial Single Photon Emission Computed Tomography (SPECT) with Iodine-123-Beta-Methyl Iodophenyl-Pentadecanoic Acid (BMIPP) in Risk Stratification of Hypertrophic Cardiomyopathy. Circulation 2012, 126 (suppl. 21), A13134. [Google Scholar] [CrossRef]
- Hashimura, H.; Kiso, K.; Yamada, N.; Kono, A.; Morita, Y.; Fukushima, K.; Higashi, M.; Noguchi, T.; Ishibashi-Ueda, H.; Naito, H. Myocardial Impairment Detected by Late Gadolinium Enhancement in Hypertrophic Cardiomyopathy: Comparison with 99mTc-MIBI/Tetrofosmin and 123I-BMIPP SPECT. Kobe J. Med. Sci. 2013, 59, 81–92. [Google Scholar]
- Isobe, S.; Okumura, T.; Unno, K.; Ohshima, S.; Hayashi, D.; Cheng, X.W.; Hirashiki, A.; Murohara, T. The relationship between 99mTc-sestamibi washout and mitochondrial morphological changes in cardiomyopathy patients. Eur. Heart J. 2014, 35, 1133. [Google Scholar]
- Zhang, L.; Liu, R.; Qiao, S.B.; Sun, X.X.; He, Z.X.; Tian, Y.Q. Evaluation of left ventricular myocardial perfusion and function using gated SPECT in patients with hypertrophic obstruction cardiomyopathy following percutaneous transluminal septal myocardial ablation. Nucl. Med. Commun. 2014, 35, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Goto, M.; Aita, M.; Date, A.; Sugiyama, E.; Minoshima, A.; Sakamoto, N.; Tanabe, Y.; Sato, N.; Hasebe, N. Acombinationof 123I-bmipp spect and stress 201TL myocardial perfusion spect as a useful prognostic marker of asymptomatic patients with non-obstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, A1287. [Google Scholar] [CrossRef]
- Utanohara, Y.; Iguchi, N.; Tamura, H.; Takayama, M.; Umemura, J.; Sumiyoshi, T.; Tomoike, H. Abstract 16793: Association of Impairment of Myocardial Metabolism and Ventricular Arrhythmic Events in Patients With Hypertrophic Cardiomyopathy by Single Photon Emission Computed Tomography With A Solid-state Dedicated Cardiac Camera. Circulation 2015, 132, A16793. [Google Scholar] [CrossRef]
- Ramalho, A.V. Impact of left ventricle mechanical systolic dyssynchrony on hypertrophic cardiomyopathy patients. Rev. Port. Cardiol. 2016, 35, 203. [Google Scholar]
- Tsai, S.Y.; Wang, S.Y.; Shiau, Y.C.; Wu, Y.W. Mechanical dyssynchrony diastolic dysfunction are common in LVH: A pilot correlation study using Doppler echocardiography and CZT gated-SPECT MPI. Sci. Rep. 2018, 8, 4182. [Google Scholar] [CrossRef] [PubMed]
- Yuki, H.; Utsunomiya, D.; Shiraishi, S.; Takashio, S.; Sakamoto, F.; Tsuda, N.; Oda, S.; Kidoh, M.; Nakaura, T.; Tsujita, K. Correlation of left ventricular dyssynchrony on gated myocardial perfusion SPECT analysis with extent of late gadolinium enhancement on cardiac magnetic resonance imaging in hypertrophic cardiomyopathy. Heart Vessel. 2018, 33, 623–629. [Google Scholar] [CrossRef]
- Ferreira, G.; Alves, V.; Martins, E.; Pereira, J. P286 Left ventricular dyssynchrony according to phase analysis from myocardial perfusion imaging in patients with hypertrophic cardiomyopathy. Eur. Heart J.-Cardiovasc. Imaging 2019, 20, jez148-015. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, D.; Tang, H.; Xu, Y.; Wang, C.; Jiang, Z.; Xu, F.; Zhao, Z.; Li, C.; Tang, S. Development and validation of a new method to diagnose apical hypertrophic cardiomyopathy by gated single-photon emission computed tomography myocardial perfusion imaging. Nucl. Med. Commun. 2019, 40, 206–211. [Google Scholar] [CrossRef]
- Li, S.T.; Tack, C.J.; Fananapazir, L.; Goldstein, D.S. Myocardial perfusion and sympathetic innervation in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2000, 35, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Tadamura, E.; Yoshibayashi, M.; Yonemura, T.; Kudoh, T.; Kubo, S.; Motooka, M.; Nohara, R.; Matsumori, A.; Sasayama, S.; Matsuda, T. Significant regional heterogeneity of coronary flow reserve in paediatric hypertrophic cardiomyopathy. Eur. J. Nucl. Med. 2000, 27, 1340–1348. [Google Scholar] [CrossRef]
- Cecchi, F.; Olivotto, I.; Gistri, R.; Lorenzoni, R.; Chiriatti, G.; Camici, P.G. Coronary Microvascular Dysfunction and Prognosis in Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2003, 349, 1027–1035. [Google Scholar] [CrossRef]
- Jörg-Ciopor, M.; Namdar, M.; Turina, J.; Jenni, R.; Schwitter, J.; Turina, M.; Hess, O.M.; Kaufmann, P.A. Regional myocardial ischemia in hypertrophic cardiomyopathy: Impact of myectomy. J. Thorac. Cardiovasc. Surg. 2004, 128, 163–169. [Google Scholar] [CrossRef]
- Knaapen, P.; van Dockum, W.G.; Götte, M.J.W.; Broeze, K.A.; Kuijer, J.P.A.; Zwanenburg, J.J.M.; Marcus, J.T.; Kok, W.E.; van Rossum, A.C.; Lammertsma, A.A. Regional heterogeneity of resting perfusion in hypertrophic cardiomyopathy is related to delayed contrast enhancement but not to systolic function: A PET and MRI study. J. Nucl. Cardiol. 2006, 13, 660–667. [Google Scholar] [CrossRef]
- Olivotto, I.; Cecchi, F.; Gistri, R.; Lorenzoni, R.; Chiriatti, G.; Girolami, F.; Torricelli, F.; Camici, P.G. Relevance of coronary microvascular flow impairment to long-term remodeling and systolic dysfunction in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2006, 47, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Sciagrà, R.; Sotgia, B.; Olivotto, I.; Cecchi, F.; Nistri, S.; Camici, P.G.; Pupi, A. Relationship between atrial fibrillation and blunted hyperemic myocardial blood flow in patients with hypertrophic cardiomyopathy. J. Nucl. Cardiol. 2009, 16, 92–96. [Google Scholar] [CrossRef]
- Gaemperli, O.; Gebhard, C.; O’Hanlon, R.; Ismail, T.; Wage, R.; Clark, S.; Camici, P.; Prasad, S.; Rimoldi, O. Impact of fibrosis and sympathetic activity on coronary flow reserve in hypertrophiccardiomyopathy. J. Cardiovasc. Magn. Reson. 2011, 13, P265. [Google Scholar] [CrossRef]
- Olivotto, I.; Girolami, F.; Sciagr, R.; Ackerman, M.J.; Sotgia, B.; Bos, J.M.; Nistri, S.; Sgalambro, A.; Grifoni, C.; Torricelli, F. Microvascular function is selectively impaired in patients with hypertrophic cardiomyopathy and sarcomere myofilament gene mutations. J. Am. Coll. Cardiol. 2011, 58, 839–848. [Google Scholar] [CrossRef]
- Timmer, S.A.J.; Knaapen, P.; Germans, T.; Dijkmans, P.A.; Lubberink, M.; ten Berg, J.M.; Ten Cate, F.J.; Rüssel, I.K.; Götte, M.J.; Lammertsma, A.A. Effects of alcohol septal ablation on coronary microvascular function and myocardial energetics in hypertrophic obstructive cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, 129–137. [Google Scholar] [CrossRef]
- Timmer, S.A.J.; Knaapen, P.; Germans, T.; Lubberink, M.; Dijkmans, P.A.; Vonk-Noordegraaf, A.; Ten Berg, J.M.; Ten Cate, F.J.; Lammertsma, A.A.; van Rossum, A.C. Right Ventricular Energetics in Patients with Hypertrophic Cardiomyopathy and the Effect of Alcohol Septal Ablation. J. Card. Fail. 2011, 17, 827–831. [Google Scholar] [CrossRef]
- Timmer, S.A.J.; Germans, T.; Gtte, M.J.W.; Rssel, I.K.; Lubberink, M.; Ten Berg, J.M.; Ten Cate, F.J.; Lammertsma, A.A.; Knaapen, P.; van Rossum, A.C. Relation of Coronary Microvascular Dysfunction in Hypertrophic Cardiomyopathy to Contractile Dysfunction Independent from Myocardial Injury. Am. J. Cardiol. 2011, 107, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Bravo, P.E.; Pinheiro, A.; Higuchi, T.; Rischpler, C.; Merrill, J.; Santaularia-Tomas, M.; Abraham, M.R.; Wahl, R.L.; Abraham, T.P.; Bengel, F.M. PET/CT assessment of symptomatic individuals with obstructive and nonobstructive hypertrophic cardiomyopathy. J. Nucl. Med. 2012, 53, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Guclu, A.; Knaapen, P.; Harms, H.J.; Vermeer, A.M.C.; Wilde, A.A.M.; Lammertsma, A.A.; Van Rossum, A.C.; Germans, T.; Van der Welden, J. Myocardial energetic impairment differs in pre-hypertrophic carriers with mutations in MYH7 and MYBPC3-a PET and MRI study. Eur. Heart J. 2013, 34, P3885. [Google Scholar] [CrossRef]
- Bravo, P.E.; Zimmerman, S.L.; Luo, H.C.; Pozios, I.; Rajaram, M.; Pinheiro, A.; Steenbergen, C.; Kamel, I.R.; Wahl, R.L.; Bluemke, D.A.; et al. Relationship of delayed enhancement by magnetic resonance to myocardial perfusion by positron emission tomography in hypertrophic cardiomyopathy. Circ. Cardiovasc. Imaging 2013, 6, 210–217. [Google Scholar] [CrossRef]
- Witjas-Paalberends, E.R.; Güçlü, A.; Germans, T.; Knaapen, P.; Harms, H.J.; Vermeer, A.M.; Christiaans, I.; Wilde, A.A.; Dos Remedios, C.; Lammertsma, A.A.; et al. Gene-specific increase in the energetic cost of contraction in hypertrophic cardiomyopathy caused by thick filament mutations. Cardiovasc. Res. 2014, 103, 248–257. [Google Scholar] [CrossRef]
- Guclu, A.; Knaapen, P.; Harms, H.; Klein, P.; Michels, M.; Schinkel, A.; Lammertsma, A.; van Rossum, A.; Germans, T.; Van der Welden, J. Septal myectomy does not improve septal myocardial efficiency in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, A1281. [Google Scholar] [CrossRef]
- Bravo, P.E.; Tahari, A.; Pozios, I.; Luo, H.C.; Bengel, F.M.; Wahl, R.L.; Abraham, M.R.; Abraham, T.P. Apparent left ventricular cavity dilatation during PET/CT in hypertrophic cardiomyopathy: Clinical predictors and potential mechanisms. J. Nucl. Cardiol. 2016, 23, 1304–1314. [Google Scholar] [CrossRef]
- Castagnoli, H.; Ferrantini, C.; Coppini, R.; Passeri, A.; Baldini, K.; Berti, V.; Cecchi, F.; Olivotto, I.; Sciagrà, R. Role of quantitative myocardial positron emission tomography for risk stratification in patients with hypertrophic cardiomyopathy: A 2016 reappraisal. Eur. J. Nucl. Med. Mol Imaging 2016, 43, 2413–2422. [Google Scholar] [CrossRef]
- Yalçin, H.; Valenta, I.; Yalçin, F.; Corona-Villalobos, C.; Vasquez, N.; Ra, J.; Kucukler, N.; Tahari, A.; Pozios, I.; Zhou, Y. Effect of Diffuse Subendocardial Hypoperfusion on Left Ventricular Cavity Size by 13N-Ammonia Perfusion PET in Patients With Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2016, 118, 1908–1915. [Google Scholar] [CrossRef]
- Aoyama, R.; Takano, H.; Kobayashi, Y.; Kitamura, M.; Asai, K.; Amano, Y.; Kumita, S.I.; Shimizu, W. Evaluation of myocardial glucose metabolism in hypertrophic cardiomyopathy using 18F-fluorodeoxyglucose positron emission tomography. PLoS ONE 2017, 12, e0188479. [Google Scholar] [CrossRef] [PubMed]
- Güçlü, A.; Knaapen, P.; Harms, H.J.; Parbhudayal, R.Y.; Michels, M.; Lammertsma, A.A.; van Rossum, A.C.; Germans, T.; van der Velden, J. Disease stage-dependent changes in cardiac contractile performance and oxygen utilization underlie reduced myocardial efficiency in human inherited hypertrophic cardiomyopathy. Circ. Cardiovasc. Imaging 2017, 10, e005604. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, M.; Nakahara, T.; Murata, M.; Ogata, Y.; Matsusaka, Y.; Iwabuchi, Y.; Yamada, Y.; Fukuda, K.; Jinzaki, M. Incidental spade-shaped FDG uptake in the left ventricular apex suggests apical hypertrophic cardiomyopathy. Ann. Nucl. Med. 2017, 31, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Sciagrà, R.; Calabretta, R.; Cipollini, F.; Passeri, A.; Castello, A.; Cecchi, F.; Olivotto, I.; Pupi, A. Myocardial blood flow and left ventricular functional reserve in hypertrophic cardiomyopathy: A 13NH3 gated PET study. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 866–875. [Google Scholar] [CrossRef]
- Valenta, I.; Szabo, Z.; Mathews, W.; Dannals, R.; Pomper, M.; Abraham, T.; Schindler, T. Abnormal regional increase of myocardial angiotensin II type 1 receptors in hypertrophic obstructive cardiomyopathy patients as determined with 11C-KR31173 and PET/CT. J. Nucl. Med. 2017, 58, 439. [Google Scholar]
- Lu, D.Y.; Yalçin, H.; Yalçin, F.; Zhao, M.; Sivalokanathan, S.; Valenta, I.; Tahari, A.; Pomper, M.G.; Abraham, T.P.; Schindler, T.H.; et al. Stress Myocardial Blood Flow Heterogeneity Is a Positron Emission Tomography Biomarker of Ventricular Arrhythmias in Patients With Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2018, 121, 1081–1089. [Google Scholar] [CrossRef]
- Zhao, M.; YALCIN, H.; Liu, M.; Pomper, M.; Valenta, I.; Tsui, B.; Schindler, T.; Wong, D.; Leal, J.; Abraham, T. Impaired Myocardial Flow Reserve Is Associated with Pulmonary Hypertension in Patients with Hypertrophic Cardiomyopathy. J. Nucl. Med. 2018, 59, 1535. [Google Scholar]
- Zhao, M.; Liu, M.; Leal, J.P.; Tsui Id, B.M.W.; Wong, D.F.; Pomper, M.G.; Zhou, Y. Association of PET-measured myocardial flow reserve with echocardiography-estimated pulmonary artery systolic pressure in patients with hypertrophic cardiomyopathy. PLoS ONE 2019, 14, e0212573. [Google Scholar] [CrossRef]
- Lu, D.Y.; Yalçin, H.; Sivalokanathan, S.; Greenland, G.V.; Vasquez, N.; Yalçin, F.; Zhao, M.; Valenta, I.; Ganz, P.; Pampaloni, M.H.; et al. Higher incidence of vasodilator-induced left ventricular cavity dilation by PET when compared to treadmill exercise-ECHO in hypertrophic cardiomyopathy. J. Nucl. Cardiol. 2020, 27, 2031–2043. [Google Scholar] [CrossRef]
- Magnusson, P.; Nordström, J.; Harms, H.J.; Lubberink, M.; Gadler, F.; Sörensen, J.; Mörner, S. Positron emission tomography (15O-water, 11C-acetate, 11C-HED) risk markers and nonsustained ventricular tachycardia in hypertrophic cardiomyopathy. IJC Heart Vasc. 2020, 26, 100452. [Google Scholar] [CrossRef]
- Parbhudayal, R.Y.; Harms, H.J.; Michels, M.; van Rossum, A.C.; Germans, T.; van der Velden, J. Increased Myocardial Oxygen Consumption Precedes Contractile Dysfunction in Hypertrophic Cardiomyopathy Caused by Pathogenic TNNT2 Gene Variants. J. Am. Heart Assoc. 2020, 9, e015316. [Google Scholar] [CrossRef] [PubMed]
- Calabretta, R.; Kokomani, A.; Fumagalli, C.; Olivotto, I.; Camici, P.G.; Hacker, M.; Sciagrà, R. Evaluation of stress myocardial blood flow patterns in patients with apical hypertrophic cardiomyopathy. J. Nucl. Cardiol. 2022, 29, 1946–1951. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Su, Y.; Wang, J.; Xiao, M.; Xi, X.-Y.; Chen, B.-X.; Zhang, Y.; Zhao, S. Activation of cardiac fibroblasts identifies high-risk hypertrophic cardiomyopathy. J. Nucl. Med. 2022, 63, 2295. [Google Scholar]
- Cho, S.-G.; Kim, H.Y.; Park, K.S.; Kim, J.; Moon, J.B.; Song, H.-C. Impaired myocardial blood flow and oxidative metabolism in hypertrophic cardiomyopathy and severe aortic stenosis: A prospective C-11 acetate PET study. J. Nucl. Med. 2023, 64, P80. [Google Scholar]
- Svanstroem, P.; Nordstrom, J.; Lubberink, M.; Harms, H.J.; Magnusson, P.; Sigfridsson, J.; Sorensen, J. A unique perfusion pattern with 15O-water PET as a risk marker for arrhythmias in hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2023, 24, jead119-390. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Wang, J.; Xiao, M.; Xi, X.Y.; Chen, B.X.; Su, Y.; Zhang, Y.; Xie, B.; Dong, Z.; et al. Myocardial Activity at 18F-FAPI PET/CT and Risk for Sudden Cardiac Death in Hypertrophic Cardiomyopathy. Radiology 2023, 306, e221052. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, Z.; Wang, L.; Wang, Y.L.; Chen, B.X.; Su, Y.; Zhao, S.; Yang, M.F. Functional significance of myocardial activity at 18F-FAPI PET/CT in hypertrophic cardiomyopathy identified by cardiac magnetic resonance feature-tracking strain analysis. Eur. J. Nucl. Med. Mol. Imaging 2023, 51, 110–122. [Google Scholar] [CrossRef]
- Alves, P.; Fernandes, C.R.; Silva, R.; Gomes, A.; Abrunhosa, A.; Castelo-Branco, M.; Goncalves, L.; Ferreira, M.J. Left atrium wall metabolism by PET-CT in patients with hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2024, 25, jeae142-074. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, H.; Chen, X.; Wang, H.; Wang, W.; You, Z.; Gao, L.; Zhang, Q.; Zhao, J. Enhanced detection of damaged myocardium and risk stratification in hypertrophic cardiomyopathy using integrated [68Ga]Ga-FAPI-04 PET/CMR imaging. Eur. J. Nucl. Med. Mol. Imaging 2024, 52, 98–108. [Google Scholar] [CrossRef]
- Wasfy, M.M.; Gustus, S.; Kijewski, M.; Perillo, A.; Boyd, K.; Tower-Rader, A.; Fifer, M.A.; Di Carli, M.F. Myocardial metabolic efficiency in healthy athletes versus patients with nonobstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2024, 83, 2335. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Marques-Alves, P.; Silva, R.; Gomes, A.; Abrunhosa, A.; Castelo-Branco, M.; Jaber, W.; Gonçalves, L. Molecular imaging in hypertrophic cardiomyopathy: An exploratory study with 2-[18F]FDG and [13N]NH3. EJNMMI Res. 2025, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, L.; Rigolin, V.H.; Bonow, R.O. Integrated Imaging in Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2017, 119, 328–339. [Google Scholar] [CrossRef] [PubMed]
- O’Gara, P.T.; Bonow, R.O.; Maron, B.J.; Damske, B.A.; Van Lingen, A.; Bacharach, S.L.; Larson, S.M.; Epstein, S.E. Myocardial perfusion abnormalities in patients with hypertrophic cardiomyopathy: Assessment with thallium-201 emission computed tomography. Circulation 1987, 76, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, H.; Taniguchi, Y.; Kinoshita, N.; Nakamura, T.; Hirasaki, S.; Azuma, A.; Ushijima, Y.; Okuyama, C.; Nakagawa, M.; Maeda, T. Reverse redistribution of Tc-99m-tetrofosmin in exercise myocardial SPECT in patients with hypertrophic cardiomyopathy. Ann. Nucl. Med. 1998, 12, 287–292. [Google Scholar] [CrossRef]
- Chiribiri, A.; Leuzzi, S.; Conte, M.R.; Bongioanni, S.; Bratis, K.; Olivotti, L.; De Rosa, C.; Lardone, E.; Di Donna, P.; Villa, A.D.; et al. Rest perfusion abnormalities in hypertrophic cardiomyopathy: Correlation with myocardial fibrosis and risk factors for sudden cardiac death. Clin. Radiol. 2015, 70, 495. [Google Scholar] [CrossRef]
- Schwenck, J.; Sonanini, D.; Cotton, J.M.; Rammensee, H.G.; la Fougère, C.; Zender, L.; Pichler, B.J. Advances in PET imaging of cancer. Nat. Rev. Cancer 2023, 23, 474–490. [Google Scholar] [CrossRef]
- Funabashi, N.; Nakagawa, K.; Komuro, I. Partial myocardial fibrosis in hypertrophic cardiomyopathy demonstrated by 18F-fluoro-deoxyglucose positron emission tomography and multislice computed tomography. Int. J. Cardiol. 2006, 107, 284–286. [Google Scholar] [CrossRef]
- Zaret, B.L.; Battler, A.; Berger, H.J.; Bodenheimer, M.M.; Borer, J.S.; Brochier, M.; Hugenholtz, P.G.; Neufeld, H.N.; Pfisterer, M.E. Report of the joint international society and federation of cardiology/world health organization task force on nuclear cardiology. Eur. Heart J. 1984, 5, 850–863. [Google Scholar] [CrossRef]
- Visser, F.C. Imaging of cardiac metabolism using radiolabelled glucose, fatty acids and acetate. Coron. Artery Dis. 2001, 12, S12–S18. [Google Scholar]
- Bittencourt, M.I.; Cader, S.A.; Araújo, D.V.; Salles, A.L.F.; de Albuquerque, F.N.; Spineti, P.P.d.M.; Albuquerque, D.C.; Mourilhe-Rocha, R. Role of Myocardial Fibrosis in Hypertrophic Cardiomyopathy: A Systematic Review and Updated Meta-Analysis of Risk Markers for Sudden Death. Arq. Bras. Cardiol. 2019, 112, 281–289. [Google Scholar] [CrossRef]
- Mavrogeni, S.; Petrou, E.; Kolovou, G.; Theodorakis, G.; Iliodromitis, E. Prediction of ventricular arrhythmias using cardiovascular magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Toms, J.; Kogler, J.; Maschauer, S.; Daniel, C.; Schmidkonz, C.; Kuwert, T.; Prante, O. Targeting Fibroblast Activation Protein: Radiosynthesis and Preclinical Evaluation of an 18F-Labeled FAP Inhibitor. J. Nucl. Med. 2020, 61, 1806–1813. [Google Scholar] [CrossRef]
- Modin, D.; Biering-Sørensen, S.R.; Møgelvang, R.; Jensen, J.S.; Biering-Sørensen, T. Prognostic Importance of Left Ventricular Mechanical Dyssynchrony in Predicting Cardiovascular Death in the General Population. Circ. Cardiovasc. Imaging 2018, 11, e007528. [Google Scholar] [CrossRef]
- Clements, I.P.; Sinak, L.J.; Gibbons, R.J.; Brown, M.L.; O’Connor, M.K. Determination of Diastolic Function by Radionuclide Ventriculography. Mayo Clin. Proc. 1990, 65, 1007–1019. [Google Scholar] [CrossRef]
- Verma, P.; Chanadana; Hephzibah, J.; Shanthly, N.; Oommen, R. Iodine-131MIBG SPECT/CT in neuroendocrine tumours: An institutional experience. Indian J. Nucl. Med. 2012, 27, 246. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Yamada, T.; Tamaki, S.; Watanabe, T.; Morita, T.; Furukawa, Y.; Kawasaki, M.; Kikuchi, A.; Kawai, T.; Nakamura, J.; et al. Prognostic Significance of Cardiac 123I-MIBG SPECT Imaging in Heart Failure Patients With Preserved Ejection Fraction. JACC Cardiovasc. Imaging 2022, 15, 655–668. [Google Scholar] [CrossRef]
- Nakajima, K.; Bunko, H.; Taki, J.; Shimizu, M.; Muramori, A.; Hisada, K. Quantitative analysis of 123I-metaiodobenzylguanidine (MIBG) uptake in hypertrophic cardiomyopathy. Am. Heart J. 1990, 119, 1329–1337. [Google Scholar] [CrossRef]
- Isobe, S.; Izawa, H.; Iwase, M.; Nanasato, M.; Nonokawa, M.; Ando, A.; Ohshima, S.; Nagata, K.; Kato, K.; Nishizawa, T.; et al. Cardiac 123I-MIBG Reflects Left Ventricular Functional Reserve in Patients with Nonobstructive Hypertrophic Cardiomyopathy. J. Nucl. Med. 2005, 46, 909–916. [Google Scholar] [PubMed]
- Dong, T.; Gilliland, Y.; Kramer, C.M.; Theodore, A.; Desai, M. Multimodality imaging of hypertrophic cardiomyopathy. Prog. Cardiovasc. Dis. 2023, 80, 14–24. [Google Scholar] [CrossRef]
- Delgado, V.; Bax, J.J. Clinical topic: Nuclear imaging in hypertrophic cardiomyopathy. J. Nucl. Cardiol. 2015, 22, 408–418. [Google Scholar] [CrossRef]
- Mandeş, L.; Roşca, M.; Ciupercă, D.; Popescu, B.A. The role of echocardiography for diagnosis and prognostic stratification in hypertrophic cardiomyopathy. J. Echocardiogr. 2020, 18, 137. [Google Scholar] [CrossRef]
- Crean, A.M.; Small, G.S.; Chow, B.J.W.; Ruddy, T.D.; Beanlands, R.S.B.; deKemp, R.A. High-resolution PET demonstrates extensive ischemia/fibrosis mismatch in a patient with hypertrophic cardiomyopathy: The power of multimodality image fusion. J. Nucl. Cardiol. 2023, 30, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Valenta, I.; Szabo, Z.; Mathews, W.; Dannals, R.; Abraham, T.; Pomper, M.; Abraham, R.; Schindler, T. PET/CT Imaging of Cardiac Angiotensin II Type 1 Receptors in Patients with Hypertrophic Obstructive Cardiomyopathy—Initial Results. J. Nucl. Med. 2016, 57, 11. [Google Scholar]
- Valenta, I.; Szabo, Z.; Mathews, W.B.; Abraham, T.P.; Abraham, M.R.; Schindler, T.H. PET/CT Imaging of Cardiac Angiotensin II Type 1 Receptors in Nonobstructive Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2019, 12, 1895–1896. [Google Scholar] [CrossRef] [PubMed]
- Stack, J.P.; Fries, R.C.; Kruckman, L.; Kadotani, S.; Wallace, G. Galectin-3 as a novel biomarker in cats with hypertrophic cardiomyopathy. J. Vet. Cardiol. 2023, 48, 54–62. [Google Scholar] [CrossRef]
- Uz Zaman, M.; Fatima, N.; Zaman, U. Artificial Intelligence (AI) in Nuclear Medicine: Is a Friend Not Foe. World J. Nucl. Med. 2024, 23, 1. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| Author [Ref] | Year | Outcomes | No. of Patients (Controls) | Nuclear Imaging Type | Results |
|---|---|---|---|---|---|
| Keng et al. [19] | 2002 | Myocardial perfusion post-ethanol septal ablation | 30 | Thallium 201 SPECT (for patients <200 lb)/Technetium 99 m tetrofosmin (for patients > 200 lb) |
|
| Shimizu et al. [20] | 2002 | Sympathetic activity | 25 (10) | 123I-metaiodobenzylguanidine (MIBG) SPECT |
|
| Sipola et al. [21] | 2003 | Sympathetic activity | 21 (9) | (123)I-metaiodobenzylguanidine (MIBG) SPECT |
|
| Ward et al. [22] | 2003 | Apical HCM diagnosis | 11 (14) | Dual-isotope rest (thallium 201) and exercise or adenosine stress (technetium 99m tetrofosmin) myocardial perfusion SPECT |
|
| Romero-Farina et al. [23] | 2004 | Myocardial perfusion | 101 | Myocardial perfusion SPECT |
|
| Sorajja et al. [24] | 2006 | Stress MPI | 158 | Thallium 201 SPECT |
|
| Kawasaki et al. [25] | 2007 | Stress myocardial perfusion | 26 | 99mTc-tetrofosmin Gated SPECT |
|
| Cianciulli et al. [26] | 2009 | Diagnosis of apical HCM | 20 | Tc-99m sestamibi SPECT with dipyridamole stress |
|
| Baba et al. [27] | 2010 | Myocardial perfusion | 16 | Thallium-201 SPECT |
|
| Chen et al. [28] | 2012 | Septal activation and left ventricular dyssynchrony after alcohol septal ablation | 32 (28) | Technetium-99m sestamibi SPECT |
|
| Cocker et al. [29] | 2012 | Myocardial fibrosis | 5 | SPECT 99mTc-NC100692 |
|
| Utanohara et al. [30] | 2012 | Myocardial metabolism | 146 | 123I-BMIPP and 201-thallium SPECT |
|
| Hashimura et al. [31] | 2013 | Myocardial fibrosis | 20 | Technetium-99m MIBI tetrofosmin SPECT, Iodine-123 BMIPP SPECT and Cardiac MRI |
|
| Isobe et al. [32] | 2014 | Mitochondrial dysfunction | 20 | 99mTc-sestamibi SPECT |
|
| Zhang et al. [33] | 2014 | Left ventricular myocardial perfusion after alcohol septal ablation | 35 | Gated Technetium-99m MIBI SPECT |
|
| Takeuchi et al. [34] | 2015 | Myocardial metabolism and perfusion | 35 | 123I-BMIPP and stress 201-thallium SPECT |
|
| Utanohara et al. [35] | 2015 | Myocardial metabolism and perfusion | 125 | Dual BMIPP and technetium-99m sestamibi or thallium-201 SPECT |
|
| Ramalho et al. [36] | 2016 | Left ventricular dyssynchrony | 24 | Gated MPI SPECT |
|
| Tsai et al. [37] | 2018 | Left ventricular dyssynchrony | 50 | Gated SPECT MPI with thallium-201 and cadmium-zinc-telluride |
|
| Yuki et al. [38] | 2018 | Left ventricular dyssynchrony | 20 | 111 MBq 201-Thallium gated myocardial perfusion SPECT |
|
| Ferreira et al. [39] | 2019 | Left ventricular dyssynchrony | 35 | Gated-SPECT myocardial perfusion imaging |
|
| Zhou et al. [40] | 2019 | Diagnosis of apical HCM | 22 | Gated-SPECT myocardial perfusion imaging |
|
| Author [Ref] | Year | Outcomes | No. of Patients (Controls) | Nuclear Imaging Type | Results |
|---|---|---|---|---|---|
| Li et al. [41] | 2000 | Myocardial perfusion and sympathetic innervation | 8 (15) | 13N-ammonia (13NH3) and 6-[18F]-fluorodopamine (18F-FDA) PET |
|
| Tadamura et al. [42] | 2000 | Microvascular dysfunction | 12 (6) | 13N-NH3 PET with dipyradimole |
|
| Cecchi et al. [43] | 2003 | Microvascular dysfunction | 51 (12) | 13N-NH3 PET with dipyradimole |
|
| Jörg-Ciopor et al. [44] | 2004 | Microvascular dysfunction post-myectomy | 22 (15) | 13N-NH3 PET with dipyradimole |
|
| Knaapen et al. [45] | 2006 | Microvascular dysfunction | 14 | Oxygen 15-labeled water PET |
|
| Olivotto et al. [46] | 2006 | Microvascular dysfunction | 51 (12) | 13N-NH3 PET with dipyradimole |
|
| Sciagra et al. [47] | 2009 | Microvascular dysfunction | 95 | 13N-NH3 PET with dipyradimole |
|
| Gaemperli et al. [48] | 2011 | Sympathetic activity and microvascular dysfunction | 13 (12) | 11C-hydroxyephedrine and 15O-labeled water PET |
|
| Olivotto et al. [49] | 2011 | Microvascular dysfunction | 61 | 13N-NH3 PET with dipyradimole |
|
| Timmer et al. [50] | 2011 | Post-septal ablation myocardial energetics | 15 | 15O-water PET |
|
| Timmer et al. [51] | 2011 | Myocardial energetics | 21 (11) | 11C-acetate PET |
|
| Timmer et al. [52] | 2011 | Microvascular dysfunction | 19 (11) | Oxygen-15 water PET with adenosine stress |
|
| Bravo et al. [53] | 2012 | Microvascular dysfunction and outflow tract obstruction | 33 | 13N-NH3 PET-CT with dipyradimole |
|
| Guclu et al. [54] | 2013 | Myocardial energetics | 23 asymptomatic carriers of HCM genes (14) | 11C-acetate PET |
|
| Bravo et al. [55] | 2013 | Microvascular dysfunction | 47 | 13N-NH3 PET-CT with dipyradimole |
|
| Witjas-Paalberends et al. [56] | 2014 | Myocardial external efficiency | 28 (14) | 11C-acetate PET |
|
| Guclu et al. [57] | 2015 | Myocardial external efficiency post-myectomy | 8 | 11C-acetate PET |
|
| Bravo et al. [58] | 2016 | Left ventricular cavity dilatation | 61 | 13N-NH3 PET-CT |
|
| Castagnoli et al. [59] | 2016 | Microvascular dysfunction | 100 | 13N-NH3 PET-CT with dipyridamole |
|
| Yalcin et al. [60] | 2016 | Microvascular dysfunction with dipyridamole stress | 104 | 13N-NH3 PET-CT with dipyridamole |
|
| Aoyama et al. [61] | 2017 | Myocardial glucose metabolism | 30 | 18F- FDG PET-CT |
|
| Güçlü et al. [62] | 2017 | Myocardial efficiency | 10 asymptomatic mutation carriers, 10 patients with HOCM (14) | 11C-acetate PET-CT |
|
| Katagiri et al. [63] | 2017 | Apical HCM diagnosis | 34 | 18F- FDG PET-CT |
|
| Sciagra et al. [64] | 2017 | Microvascular dysfunction with dipyridamole stress | 34 (18 preserved LVEF, 16 abnormal LVEF) | 13N-NH3 PET with dipyradimole |
|
| Valenta et al. [65] | 2017 | Myocardial angiotensin II type 1 receptors | 4 (4) | 11C-KR31173 PET-CT |
|
| Lu et al. [66] | 2018 | Microvascular dysfunction | 133 | 13N-NH3 PET-CT |
|
| Zhao et al. [67] | 2018 | Microvascular dysfunction | 89 | 13N-NH3 PET |
|
| Zhao et al. [68] | 2019 | Microvascular dysfunction | 89 | 13N-NH3 PET-CT |
|
| Lu et al. [69] | 2020 | Left ventricular cavity dilatation | 108 | 13N-NH3 PET-CT with dipyradimole |
|
| Magnusson et al. [70] | 2020 | Microvascular Dysfunction | 25 | 15O-water, 11C-acetate and 11C-HED PET-CT |
|
| Parbhudayal et al. [71] | 2020 | Myocardial energetics | 14 (14) | 11C-acetate PET |
|
| Calabretta et al. [72] | 2022 | Microvascular dysfunction | 12 | 13N-NH3 PET-CT |
|
| Wang et al. [73] | 2022 | Myocardial fibrosis | 44 | 18F-labeled FAPI PET-CT |
|
| Cho et al. [74] | 2023 | Microvascular dysfunction | 50 (20) | C-11 acetate PET |
|
| Svanstroem et al. [75] | 2023 | Microvascular dysfunction | 24 | 15O-water PET |
|
| Wang et al. [76] | 2023 | Myocardial fibrosis | 50 (22) | 18F-labeled FAPI PET/CT |
|
| Zhang et al. [77] | 2023 | Myocardial fibrosis | 49 | 18F–labeled FAPI PET/CT |
|
| Alves et al. [78] | 2024 | Myocardial glucose metabolism and diastolic dysfunction | 30 | 2-[18F]-FDG PET-CT |
|
| Ding et al. [79] | 2024 | Myocardial fibrosis | 20 (11) | [68Ga]Ga-FAPI-04 PET/CMR |
|
| Wasfy et al. [80] | 2024 | Myocardial metabolic efficiency | 15 (15) | 13C-acetate PET |
|
| Ferreira et al. [81] | 2025 | Myocardial perfusion and metabolism | 30 | 13N-NH3 and 2-[18F]-FDG PET-CT |
|
| Feature | Potential Risk Markers | Imaging Modality |
|---|---|---|
| Myocardial Ischemia | Reduced MBF, impaired CFR, LVCD, subendocardial ischemia | PET (13N-NH3, Rubidium-82), SPECT (99m-Tc) |
| Metabolic Dysfunction | Increased FDG uptake, reduced acetate clearance, metabolic–perfusion mismatch | PET (18F-FDG, 11C-Acetate) |
| Myocardial Fibrosis | LGE > 15% LV mass, increased FAPI uptake, early fibroblast activation | PET (68Ga/18F-FAPI), CMR (LGE) |
| Ventricular Dyssynchrony | Increased PHB, PSD, septal–lateral delay > 100 ms | SPECT (Phase Analysis) |
| Sympathetic Innervation | Reduced H/M ratio, increased MIBG washout, perfusion–innervation mismatch | SPECT (MIBG Scintigraphy) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradeep Kundur, S.; Malik, A.; Sivalokanathan, S. The Utility of Nuclear Imaging in Hypertrophic Cardiomyopathy: A Narrative Review. J. Clin. Med. 2025, 14, 2183. https://doi.org/10.3390/jcm14072183
Pradeep Kundur S, Malik A, Sivalokanathan S. The Utility of Nuclear Imaging in Hypertrophic Cardiomyopathy: A Narrative Review. Journal of Clinical Medicine. 2025; 14(7):2183. https://doi.org/10.3390/jcm14072183
Chicago/Turabian StylePradeep Kundur, Sukruth, Ali Malik, and Sanjay Sivalokanathan. 2025. "The Utility of Nuclear Imaging in Hypertrophic Cardiomyopathy: A Narrative Review" Journal of Clinical Medicine 14, no. 7: 2183. https://doi.org/10.3390/jcm14072183
APA StylePradeep Kundur, S., Malik, A., & Sivalokanathan, S. (2025). The Utility of Nuclear Imaging in Hypertrophic Cardiomyopathy: A Narrative Review. Journal of Clinical Medicine, 14(7), 2183. https://doi.org/10.3390/jcm14072183







