Acute Hemodynamic Changes Induced by Cardiac Contractility Modulation Evaluated Using the NICaS® System: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Poulation
2.2. CCM Implantation
2.3. NICaS® Measurements
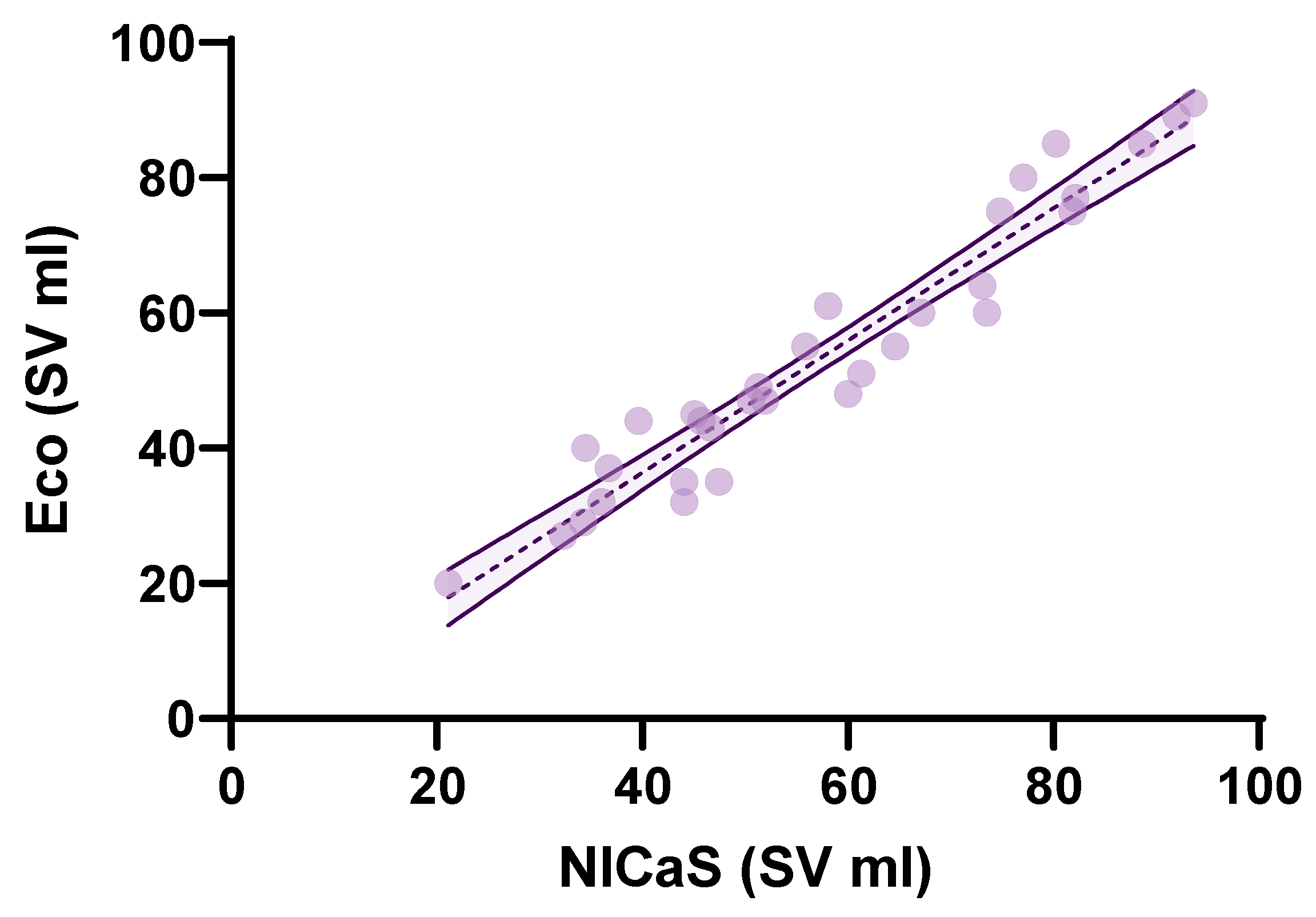
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HF | Heart Failure |
| NYHA | New York Heart Association |
| CRT | Cardiac Resynchronization Therapy |
| LV | Left Ventricular |
| ICD | Implantable Cardioverter Defibrillator |
| ESC | European Society of Cardiology |
| CCM | Cardiac Contractility Modulation |
| SV | Stroke Volume |
| TPRI | Total Peripheral Resistance Index |
| CO | Cardiac Output |
| SVR | Systemic Vascular Resistance |
| BNP | B-type natriuretic peptide |
| LVEF | Left Ventricular Ejection Fraction |
| OMT | Optical Medical Therapy |
References
- Tomasoni, D.; Pagnesi, M.; Colombo, G.; Chiarito, M.; Stolfo, D.; Baldetti, L.; Lombardi, C.M.; Adamo, M.; Maggi, G.; Inciardi, R.M.; et al. Guideline-directed medical therapy in severe heart failure with reduced ejection fraction: An analysis from the HELP-HF registry. Eur. J. Heart Fail. 2024, 26, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Bazoukis, G.; Saplaouras, A.; Efthymiou, P.; Yiannikourides, A.; Liu, T.; Efremidis, M.; Lampropoulos, K.; Xydonas, S.; Tse, G.; Armoundas, A.A. Cardiac contractility modulation in patients with heart failure—A review of the literature. Heart Fail. Rev. 2024, 29, 689–705. [Google Scholar] [CrossRef] [PubMed]
- Masarone, D.; Kittleson, M.M.; D’onofrio, A.; Falco, L.; Fumarulo, I.; Massetti, M.; Crea, F.; Aspromonte, N.; Pacileo, G. Basic science of cardiac contractility modulation therapy: Molecular and electrophysiological mechanisms. Heart Rhythm 2024, 21, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Rao, I.V.; Burkhoff, D. Cardiac contractility modulation for the treatment of moderate to severe HF. Expert Rev. Med. Devices 2021, 18, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, N.; Borlaug, B.A.; Bailey, A.L.; Eckman, P.M.; Guglin, M.; Hall, S.; Montgomery, M.; Ramani, G.; Khazanie, P. Practical guidance for hemodynamic assessment by right heart catheterization in management of heart failure. JACC Heart Fail. 2024, 12, 1141–1156. [Google Scholar] [CrossRef] [PubMed]
- Tanino, Y.; Shite, J.; Paredes, O.L.; Shinke, T.; Ogasawara, D.; Sawada, T.; Kawamori, H.; Miyoshi, N.; Kato, H.; Yoshino, N.; et al. Whole body bioimpedance monitoring for outpatient chronic heart failure follow up. Circ. J. 2009, 73, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Lavie, A.; Ram, M.; Lev, S.; Blecher, Y.; Amikam, U.; Shulman, Y.; Avnon, T.; Weiner, E.; Many, A. Maternal cardiovascular hemodynamics in normotensive versus preeclamptic pregnancies: A prospective longitudinal study using a noninvasive cardiac system (NICaS™). BMC Pregnancy Childbirth 2018, 18, 229. [Google Scholar] [CrossRef]
- Imhoff, M.; Lehner, J.H.; Löhlein, D. Noninvasive whole-body electrical bioimpedance cardiac output and invasive thermodilution cardiac output in high-risk surgical patients. Crit. Care Med. 2000, 28, 2812–2818. [Google Scholar] [CrossRef] [PubMed]
- Goedje, O.; Hoeke, K.; Lichtwarck-Aschoff, M.; Faltchauser, A.; Lamm, P.; Reichart, B. Continuous cardiac output by femoral arterial thermodilution calibrated pulse contour analysis: Comparison with pulmonary arterial thermodilution. Crit. Care Med. 1999, 27, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Contaldi, C.; De Vivo, S.; Martucci, M.L.; D’onofrio, A.; Ammendola, E.; Nigro, G.; Errigo, V.; Pacileo, G.; Masarone, D. Effects of cardiac contractility modulation therapy on right ventricular function: An echocardiographic study. Appl. Sci. 2022, 12, 7917. [Google Scholar] [CrossRef]
- Kuschyk, J.; Falk, P.; Demming, T.; Marx, O.; Morley, D.; Rao, I.; Burkhoff, D. Long-term clinical experience with cardiac contractility modulation therapy delivered by the Optimizer Smart system. Eur. J. Heart Fail. 2021, 23, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Mohri, S.; Shimizu, J.; Mika, Y.; Shemer, I.; Wang, J.; Ben-Haim, S.; Burkhoff, D. Electric currents applied during refractory period enhance contractility and systolic calcium in the ferret heart. Am. J. Physiol. Circ. Physiol. 2003, 284, H1119–H1123. [Google Scholar] [CrossRef] [PubMed]
- Giallauria, F.; Cuomo, G.; Parlato, A.; Raval, N.Y.; Kuschyk, J.; Coats, A.J.S. A comprehensive individual patient data meta-analysis of the effects of cardiac contractility modulation on functional capacity and heart failure-related quality of life. ESC Heart Fail. 2020, 7, 2922–2932. [Google Scholar] [CrossRef] [PubMed]
- De Donno, F.; Dell’elce, G.; Ottaviani, A.; Faustino, M.; Gallina, S.; Renda, G. Effects of Cardiac Contractility Modulation in a Patient With Heart Failure and High Arrhythmic Burden. JACC Case Rep. 2025, 30, 102997. [Google Scholar] [CrossRef] [PubMed]
- Callans, D.J.; Fuchs, S.; Mika, D.Y.; Shemer, I.; Aviv, D.R.; Haddad, W.; Darvish, N.; Ben-Haim, S.A.; Kornowski, R. Global improvement in left ventricular performance observed with cardiac contractility modulation is the result of changes in regional contractility. Heart Fail. Rev. 2001, 6, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Pappone, C.; Augello, G.; Rosanio, S.; Vicedomini, G.; Santinelli, V.; Romano, M.; Agricola, E.; Maggi, F.; Buchmayr, G.; Moretti, G.; et al. First human chronic experience with cardiac contractility modulation by nonexcitatory electrical currents for treating systolic heart failure: Mid-term safety and efficacy results from a multicenter study. J. Cardiovasc. Electrophysiol. 2004, 15, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Tschöpe, C.; Van Linthout, S.; Spillmann, F.; Klein, O.; Biewener, S.; Remppis, A.; Gutterman, D.; Linke, W.A.; Pieske, B.; Hamdani, N.; et al. Cardiac contractility modulation signals improve exercise intolerance and maladaptive regulation of cardiac key proteins for systolic and diastolic function in HFpEF. Int. J. Cardiol. 2016, 203, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Masarone, D.; Kittleson, M.M.; De Vivo, S.; D’onofrio, A.; Ammendola, E.; Nigro, G.; Contaldi, C.; Martucci, M.L.; Errigo, V.; Pacileo, G. The effects of device-based cardiac contractility modulation therapy on left ventricle global longitudinal strain and myocardial mechano-energetic efficiency in patients with heart failure with reduced ejection fraction. J. Clin. Med. 2022, 11, 5866. [Google Scholar] [CrossRef] [PubMed]
- Linde, C.; Grabowski, M.; Ponikowski, P.; Rao, I.; Stagg, A.; Tschöpe, C. Cardiac contractility modulation therapy improves health status in patients with heart failure with preserved ejection fraction: A pilot study (CCM-HFpEF). Eur. J. Heart Fail. 2022, 24, 2275–2284. [Google Scholar] [CrossRef] [PubMed]


| LVEF | Sex | Cardiomyopathy | Comorbidities | Therapy |
|---|---|---|---|---|
| 28 | Male | Dilatative | Chronic kidney disease Pulmonary nodule under follow-up | Omeprazole Aspirin (Low dose) Carvedilol Furosemide |
| 45 | Male | Dilatative | Permanent Atrial Fibrillation Type 2 Diabetes Mellitus Obesity Obstructive Sleep Apnea syndrome (OSAS) | Pantoprazole Rivaroxaban Sacubitril-Valsartan Bisoprolol Furosemide Spironolactone Empagliflozin |
| 43 | Male | Dilatative | Type 2 Diabetes Mellitus | Sacubitril-Valsartan Bisoprolol Furosemide Spironolactone Empagljflozin |
| 33 | Female | Dilatative | Atrial Fibrillation Hyperhomocysteinemia Chronic Obstructive Pulmonary Disease (COPD) Anxiety Depressive Disorder Bronchopneumonia focus | Pantoprazole Sacubitril-Valsartan Apixaban Clopidogrel Metoprolol Ivabradine Furosemide Spironolactone Rosuvastatin Ezetimibe Folic Acid Fluticasone/Vilanterol Empaglifozin |
| 40 | Female | Dilatative | Diabetes Obesity Hyperuricemia | Pantoprazole Sacubitril-Valsartan Apixaban Clopidogrel Metoprolol Ivabradine Furosemide Spironolactone Empaglifozin |
| 31 | Male | Ischemic | Arterial Hypertension Dyslipidemia Diabetes Mellitus Chronic Kidney Disease | Pantoprazole Spironolactone Bisoprolol Clopidogrel Allopurilnol Empaglifozin Ivabradine Ezetimibe/Atorvastatin Furosemide Sacubitril-Valsartan Linagliptin Dapaglifozin Ibandonatre (Neodidro) |
| 36 | Male | Ischemic | Normochromic-normocytic Anemia Diabetes Mellitus Hyperuricemia Previous Transient Ischemic Attack Carotid Atheromatosis | Pantoprazole Clopidogrel/Aspirin Furosemide Spironolactone Sacutril/Valsartan Carvedilol Ranolazine Allopurinol Ezetimibe/Atorvastatin Empaglifozin |
| 33 | Male | Ischemic | Chronic Kidney Disease Aneurysm treated with Endovascular Aneurysm Repair Arterial Hypertension Dyslipidemia | Omeoprazol Metoprolol Amiodarone Furosemide Clopidogrel Aspirin Febuxostat Valsartan Atorvastatin Empaglifozin |
| Comparison | Mann–Whitney | Kolmogorov–Smirnov | ||
|---|---|---|---|---|
| U | p-Value | D | p-Value | |
| One Week–Baseline | 1377 | 0.002 | 0.2969 | 0.0071 |
| One Month–Baseline | 1014 | <0.001 | 0.5 | <0.0001 |
| Three Months–Baseline | 752 | <0.001 | 0.5625 | <0.0001 |
| One Month–One Week | 1384 | 0.0014 | 0.3750 | 0.02 |
| Three Months–One Week | 999 | 0.0014 | 0.4844 | <0.0001 |
| Three Months–One Month | 1377 | 0.0013 | 0.3281 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madeo, A.; De Bonis, S.; Cavaliere, A.L.; Bisignani, G. Acute Hemodynamic Changes Induced by Cardiac Contractility Modulation Evaluated Using the NICaS® System: A Pilot Study. J. Clin. Med. 2025, 14, 2172. https://doi.org/10.3390/jcm14072172
Madeo A, De Bonis S, Cavaliere AL, Bisignani G. Acute Hemodynamic Changes Induced by Cardiac Contractility Modulation Evaluated Using the NICaS® System: A Pilot Study. Journal of Clinical Medicine. 2025; 14(7):2172. https://doi.org/10.3390/jcm14072172
Chicago/Turabian StyleMadeo, Andrea, Silvana De Bonis, Anna Lucia Cavaliere, and Giovanni Bisignani. 2025. "Acute Hemodynamic Changes Induced by Cardiac Contractility Modulation Evaluated Using the NICaS® System: A Pilot Study" Journal of Clinical Medicine 14, no. 7: 2172. https://doi.org/10.3390/jcm14072172
APA StyleMadeo, A., De Bonis, S., Cavaliere, A. L., & Bisignani, G. (2025). Acute Hemodynamic Changes Induced by Cardiac Contractility Modulation Evaluated Using the NICaS® System: A Pilot Study. Journal of Clinical Medicine, 14(7), 2172. https://doi.org/10.3390/jcm14072172





