Cognitive and Psychomotor Performance of Patients After Ischemic Stroke Undergoing Early and Late Rehabilitation
Abstract
1. Introduction
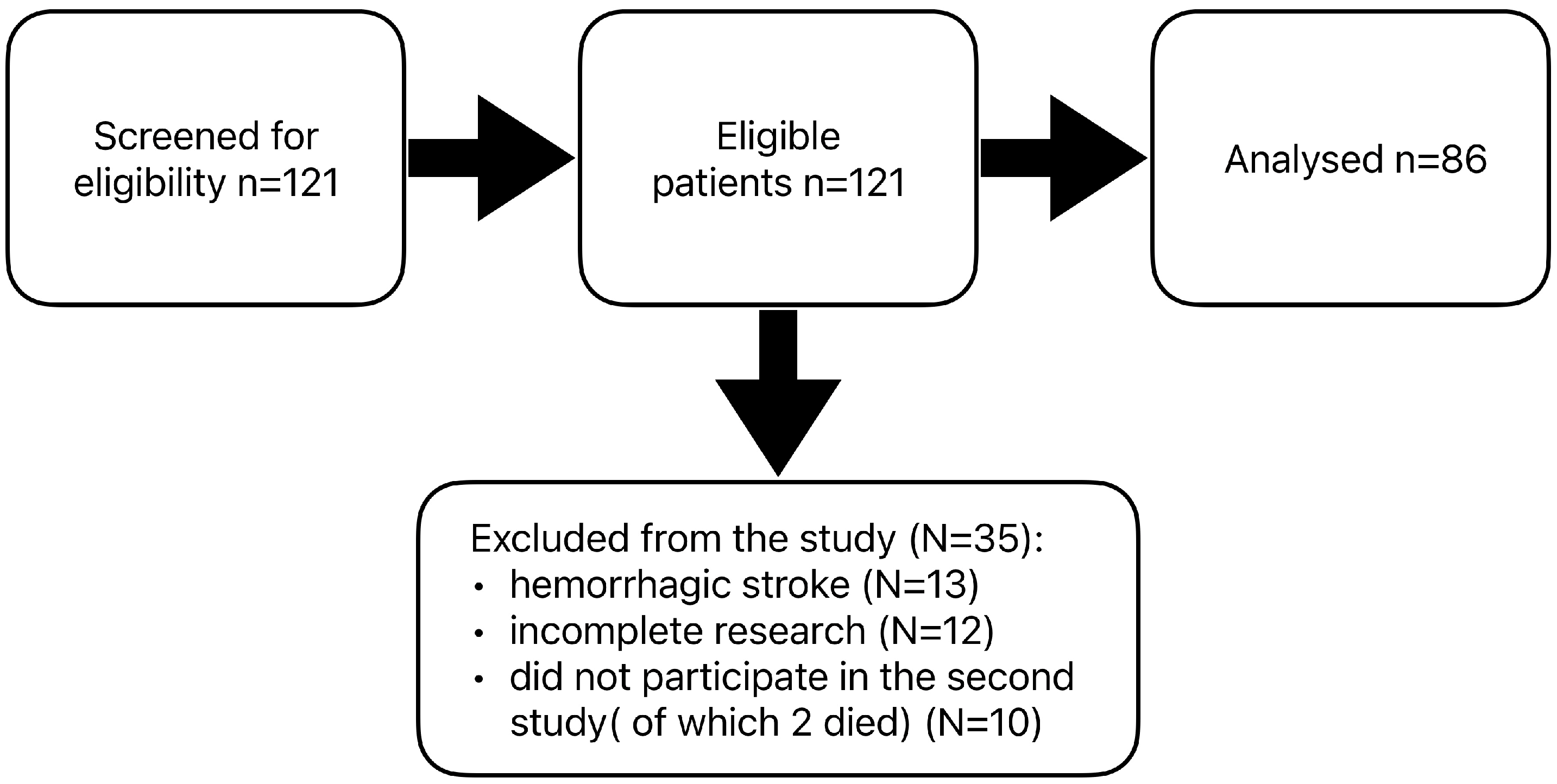
2. Materials and Methods
- -
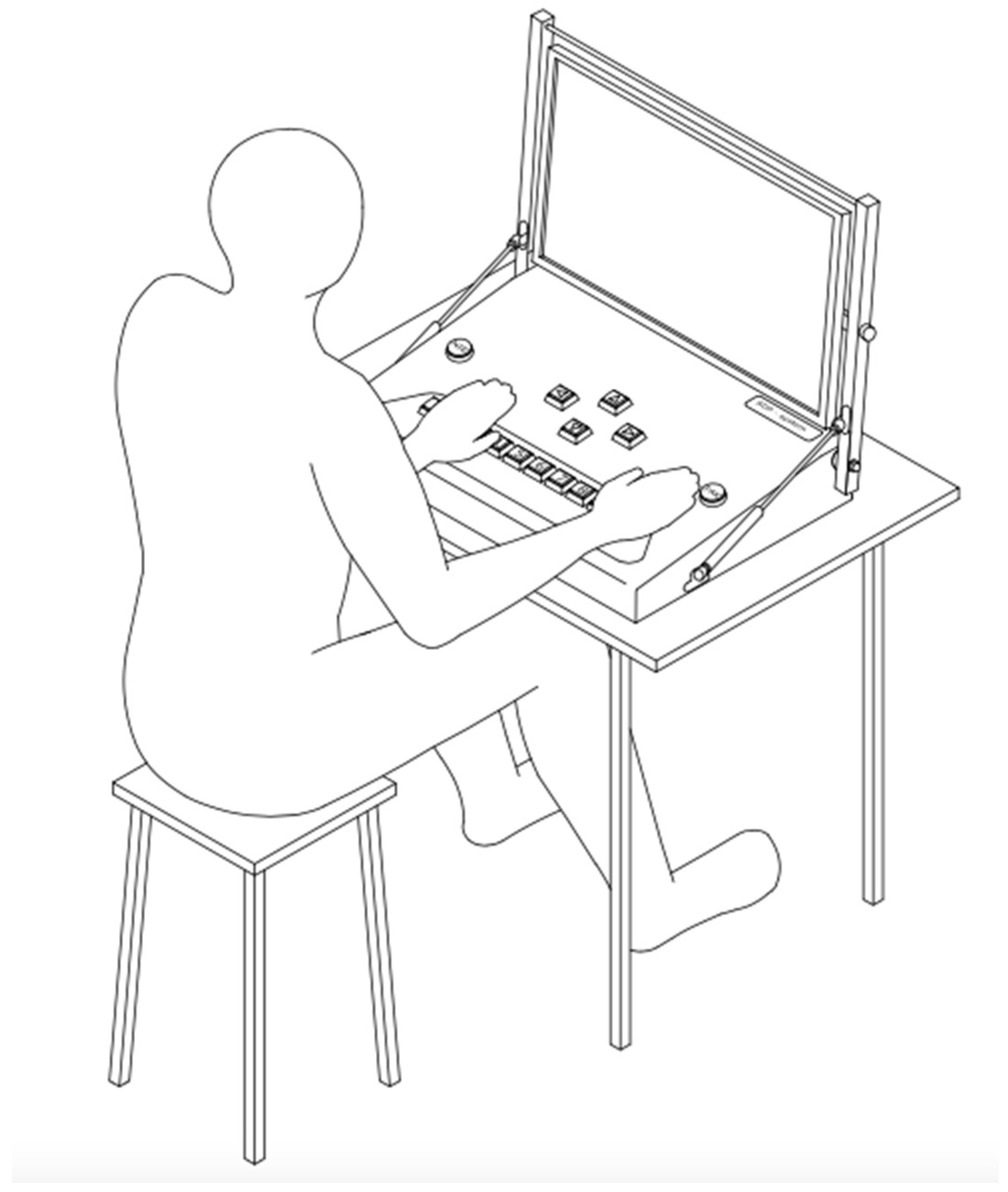
- The Addition Test is based on a set of positive natural numbers from 0 to 9. As a result, it requires no special mathematical skills. The task absorbs attention in terms of concentration and the ability to perform simple logical operations.
- -
- Number Test—The task involves remembering the numbers on the first board and then finding them among the set of numbers on the second board. Working memory and perceptiveness play an important role in this task.
- -
- The Line Test is used as a method to measure visual receptor performance. It allows the ‘selectivity’ of perception to be determined by focusing the eye on the ‘detail’ of the image presented.
- -
- The Simple Coordination Test is a modified version of a method popular among psychologists for testing eye–hand coordination and precision of movement.
- -
- The Complex Coordination Test is an extended method of measuring psychomotor performance with the addition of a thinking component.
Statistical Analyses
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EUSI | According to the European Stroke Initiative |
| ESO | European Stroke Organisation |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| CTD | Clock Drawing Test (CDT |
| CNS | Central nervous system |
References
- Sabiniewicz, M.; Niwald, M.; Machnia, M.; Włodarczyk, L.; Miller, E. Selected Cognitive Dysfunctions after Brain Stroke—Clinical Characteristics and Diagnosis. Aktual. Neurol. 2015, 15, 35–40. [Google Scholar] [CrossRef]
- Ejma, M.; Madetko, N.; Brzecka, A.; Alster, P.; Budrewicz, S.; Koszewicz, M.; Misiuk-Hojło, M.; Tomilova, I.K.; Somasundaram, S.G.; Kirkland, C.E. The Role of Stem Cells in the Therapy of Stroke. Curr. Neuropharmacol. 2022, 20, 630–647. [Google Scholar] [CrossRef]
- Seniów, J. Poudarowe Ogniskowe Zespoły Poznawcze w Kontekście Rehabilitacji. Piel. Zdr. Publ. 2003, 20, 141–152. [Google Scholar]
- Kot-Brycko, K.; Pietraszkiewicz, F. Psychologia w Medycynie. Część 2-Rehabilitacja Neuropsychologiczna Po Udarze Mózgu. Med. Ogólna I Nauk. O. Zdrowiu 2012, 18, 47. [Google Scholar]
- Pąchalska, M. Rehabilitacja Neuropsychologiczna: Procesy Poznawcze i Emocjonalne; Wydawnictwo Uniwersytetu Marii Curie-Skłodowskiej: Lublin, Poland, 2009; ISBN 83-227-2741-0. [Google Scholar]
- Kot-Brycko, K.; Pietraszkiewicz, F. Psychologia w Medycynie. Część 1-Deficyty Poznawcze u Osób Po Udarze Mózgu. Med. Ogólna I Nauk. O. Zdrowiu 2012, 18, 54. [Google Scholar]
- Kwolek, A. Rehabilitacja w Udarze Mózgu; Wydawnictwo Uniwersytetu Rzeszowskiego: Rzeszów, Poland, 2011; ISBN 978-83-7338-690-7. [Google Scholar]
- Li, Y.; Tang, A.; Ge, L.; Zhang, L.; Chen, L.; Xu, Y.; Wang, L.; Zhu, X.; Wu, Q. The Relationship between Social and Psychological Factors with Cognitive Impairment after Stroke: A Prospective Study. Front. Psychiatry 2024, 15, 1403027. [Google Scholar] [CrossRef] [PubMed]
- Björck, A.; Matérne, M.; Arvidsson Lindvall, M.; Jarl, G. Investigating Cognitive Impairment, Biopsychosocial Barriers, and Predictors of Return to Daily Life among Older Stroke Survivors. Front. Neurol. 2024, 15, 1403567. [Google Scholar] [CrossRef]
- Carlsson, G.E.; Möller, A.; Blomstrand, C.; Ueda, T.; Mizushige, K.; Yukiiri, K.; Takahashi, T.; Kohno, M.; Kuo, T.B.; Chern, C.-M. European Stroke Initiative Recommendations for Stroke Management–Update 2003. Cerebrovasc. Dis. 2003, 16, 311–337. [Google Scholar]
- Oros, R.I.; Popescu, C.A.; Iova, C.A.; Mihancea, P.; Iova, S.O. The Impact of Cognitive Impairment after Stroke on Activities of Daily Living. Hum. Vet. Med. 2016, 8, 41–44. [Google Scholar]
- Yoon, J.A.; Kim, D.Y.; Sohn, M.K.; Lee, J.; Lee, S.-G.; Lee, Y.-S.; Han, E.Y.; Joo, M.C.; Oh, G.-J.; Han, J. Factors Associated with Improvement or Decline in Cognitive Function after an Ischemic Stroke in Korea: The Korean Stroke Cohort for Functioning and Rehabilitation (KOSCO) Study. BMC Neurol. 2017, 17, 9. [Google Scholar]
- Suzuki, M.; Sugimura, Y.; Yamada, S.; Omori, Y.; Miyamoto, M.; Yamamoto, J. Predicting Recovery of Cognitive Function Soon after Stroke: Differential Modeling of Logarithmic and Linear Regression. PLoS ONE 2013, 8, e53488. [Google Scholar]
- Kasner, S.E. Clinical Interpretation and Use of Stroke Scales. Lancet Neurol. 2006, 5, 603–612. [Google Scholar] [CrossRef]
- Horoszkiewicz, K. Contemporary Approaches to Assessing Psychomotor Efficiency: A Study in Sports Psychology and Transportation. Adv. Cogn. Psychol. 2024, 20, 275–286. [Google Scholar] [CrossRef]
- Horoszkiewicz, K.; Horoszkiewicz, B.; Załęski, G. Psychomotor Performance in Video Games. J. Educ. Health Sport. 2022, 12, 667–682. [Google Scholar] [CrossRef]
- Horoszkiewicz, K.; Horoszkiewicz, B. Cognitive and Psychomotor Performance of Polish and Ukrainian Drivers. J. Educ. Health Sport. 2022, 12, 616–624. [Google Scholar] [CrossRef]
- Samėnienė, J.; Kriščiūnas, A.; Endzelytė, E. The Evaluation of the Rehabilitation Effects on Cognitive Dysfunction and Changes in Psychomotor Reactions in Stroke Patients. Medicina 2008, 44, 860. [Google Scholar] [CrossRef]
- Nowakowska, K.; Adamiak, G.; Jabłkowska, K.; Lewandowska, A.; Stetkiewicz, A.; Borkowska, A. Deficyty Poznawcze i Zaburzenia Depresyjne u Chorych Po Udarze Mózgu. Post. Psychiatr. Neurol. 2009, 18, 255–262. [Google Scholar]
- Borkowska, A.; Warwas, I.; Wiłkość, M.; Dróżdż, W. Neuropsychologiczna Ocena Dysfunkcji Poznawczych w Depresji Po Udarze Mózgu. Psychiatria 2007, 4, 39–44. [Google Scholar]
- Rajtar-Zembaty, A.; Starowicz-Filip, A.; Bober-Płonka, B.; Przewoźnik, D.; Nowak, R. Analiza Wykonania “Testu Łączenia Punktów” Przez Osoby Po Udarze Mózgu o Różnej Lokalizacji Ogniska Uszkodzenia. Neuropsychiatr. I Neuropsychol. 2015, 10, 5–10. [Google Scholar]
- Yoo, C.; Yong, M.; Chung, J.; Yang, Y. Effect of Computerized Cognitive Rehabilitation Program on Cognitive Function and Activities of Living in Stroke Patients. J. Phys. Ther. Sci. 2015, 27, 2487–2489. [Google Scholar] [CrossRef]
- Chen, S.; Thomas, J.; Glueckauf, R.; Bracy, O. The Effectiveness of Computer-Assisted Cognitive Rehabilitation for Persons with Traumatic Brain Injury. Brain Inj. 1997, 11, 197–210. [Google Scholar] [PubMed]
- Lee, I.-H.; Park, S.-Y.; Son, C.-S.; Kim, Y.-N. Clinical Trial Evaluating an Online Cognitive Dysfunction Evaluation System for Stroke Patients. J. Phys. Ther. Sci. 2012, 24, 503–507. [Google Scholar] [CrossRef]
- Antonioni, A.; Cellini, N.; Baroni, A.; Fregna, G.; Lamberti, N.; Koch, G.; Manfredini, F.; Straudi, S. Characterizing Practice-Dependent Motor Learning after a Stroke. Neurol. Sci. 2025, 46, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Total (n = 86) | Early Rehabilitation (n = 56) | Late Rehabilitation (n = 30) | p | |
|---|---|---|---|---|---|
| Age (years) [M (SD)] | 66 (10.6) | 65.7 (10.9) | 66.6 (9.9) | 0.964 | |
| NIHSS [M (SD)] | 6.72 (8.7) | 7.34 (9.10) | 6.10 (8.30) | 0.525 | |
| Barthel Index [M (SD)] | 54.9 (34.1) | 57.4 (37.3) | 52.5 (31.0) | 0.541 | |
| Rankin Scale [M (SD)] | 2.75 (1.35) | 2.84 (1.50) | 2.66 (1.20) | 0.572 | |
| Gender [n (%)] | Males | 55 (64) | 38 (68) | 17 (57) | 0.303 |
| Females | 31 (36) | 18 (32) | 13 (43) | ||
| Education [n (%)] | Primary | 14 (16.3) | 10 (17.9) | 4 (13.3) | 0.839 |
| Vocational | 32 (37.2) | 20 (35.7) | 12 (40) | ||
| Secondary | 31 (36) | 21 (37.5) | 10 (33.3) | ||
| Higher | 9 (10.5) | 5 (8.9) | 4 (13.3) | ||
| Marital status [n (%)] | Single | 9 (10.5) | 6 (10.7) | 3 (10) | 0.168 |
| Married | 54 (62.8) | 37 (66.1) | 17 (56.7) | ||
| Divorced | 6 (6.9) | 4 (7.1) | 2 (6.7) | ||
| Widowed | 17 (19.8) | 9 (16.1) | 8 (26.7) | ||
| Type of work [n (%)] | Mental | 29 (33.7) | 16 (28.6) | 13 (43.3) | 0.705 |
| Physical | 57 (66.3) | 40 (71.4) | 17 (56.7) | ||
| Comorbidities [n (%)] | Ischaemic heart disease | 13 (15.1) | 6 (10.7) | 7 (23.3) | 0.119 |
| Circulatory failure | 1 (1.2) | 1 (1.8) | 0 (0) | 0.462 | |
| Diabetes mellitus | 33 (38.4) | 21 (37.5) | 12 (40) | 0.820 | |
| Chronic kidney disease | 2 (2.3) | 2 (3.6) | 0 (0) | 0.295 | |
| Stroke site [n (%)] | Right brain hemisphere | 36 (42) | 24 (43) | 12 (40) | * 0.786 |
| Left brain hemisphere | 43 (50) | 27 (48) | 16 (53) | ||
| Brain stem | 7 (8) | 5 (9) | 2 (7) | ||
| Parameter | Early Rehabilitation (n = 56) | Late Rehabilitation (n = 30) | U | p | ||||
|---|---|---|---|---|---|---|---|---|
| M | Md | SD | M | Md | SD | |||
| Total Time of the Addition Test 1 (s) | 130.62 | 125.52 | 51.78 | 118.06 | 99.83 | 54.96 | 679 | 0.146 |
| Average Reaction Time of the Addition Test 1 (s) | 13.06 | 12.55 | 5.18 | 11.81 | 9.98 | 5.50 | 679 | 0.146 |
| Number of Errors of the Addition Test 1 | 0.55 | 0.00 | 1.09 | 0.67 | 0.00 | 1.15 | 787 | 0.562 |
| Total Time of the Addition Test 2 (s) | 89.37 | 86.37 | 41.77 | 76.87 | 70.84 | 39.64 | 656 | 0.096 |
| Average Reaction Time of the Addition Test 2 (s) | 8.94 | 8.64 | 4.18 | 8.47 | 7.61 | 4.69 | 737 | 0.353 |
| Number of Errors of the Addition Test 2 | 0.32 | 0.00 | 0.69 | 0.40 | 0.00 | 0.86 | 820 | 0.807 |
| Total Time of the Number Test (s) | 297.44 | 293.68 | 160.26 | 251.38 | 238.30 | 126.67 | 703 | 0.264 |
| Average Reaction Time of the Number Test (s) | 32.91 | 30.75 | 19.36 | 27.95 | 26.43 | 14.08 | 723 | 0.351 |
| Number of Number Test Returns | 1.38 | 1.00 | 1.78 | 0.87 | 0.50 | 1.17 | 726 | 0.335 |
| Total Time of the Line Test (s) | 136.15 | 114.56 | 66.75 | 130.75 | 118.67 | 61.99 | 784 | 0.710 |
| Average Reaction Time of the Line Test (s) | 14.24 | 11.83 | 7.46 | 14.20 | 12.49 | 7.12 | 809 | 0.887 |
| Number of Errors of the Line Test | 1.42 | 0.00 | 2.32 | 1.90 | 1.00 | 2.59 | 723 | 0.313 |
| Average Reaction Time of the Simple Coordination Test (s) | 2.23 | 2.07 | 0.88 | 2.19 | 1.90 | 0.95 | 762 | 0.565 |
| Number of Reaction of the Simple Coordination Test | 29.78 | 28.00 | 11.44 | 31.17 | 30.50 | 9.76 | 735 | 0.410 |
| Total Time of the Complex Coordination Test (s) | 25.08 | 22.77 | 13.54 | 22.91 | 20.86 | 13.20 | 729 | 0.380 |
| Average Reaction Time of the Complex Coordination Test (s) | 5.18 | 4.55 | 3.18 | 5.59 | 4.27 | 5.44 | 778 | 0.669 |
| Average Value of Average Reaction Times (s) | 12.60 | 12.27 | 5.30 | 11.70 | 11.45 | 4.97 | 734 | 0.339 |
| Parameter | Before Rehabilitation | After Rehabilitation | Δ | Z | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| M | Md | SD | M | Md | SD | ||||
| Total Time of the Addition Test 1 (s) | 130.62 | 125.52 | 51.78 | 110.31 | 95.74 | 84.09 | −20.30 | −4.747 | <0.001 |
| Average Reaction Time of the Addition Test 1 (s) | 13.06 | 12.55 | 5.18 | 12.34 | 9.57 | 15.55 | −0.72 | −4.356 | <0.001 |
| Number of Errors of the Addition Test 1 | 0.55 | 0.00 | 1.09 | 0.55 | 0.00 | 1.23 | 0.00 | −0.154 | 0.877 |
| Total Time of the Addition Test 2 (s) | 89.37 | 86.37 | 41.77 | 79.99 | 68.10 | 42.75 | −9.38 | −3.361 | 0.001 |
| Average Reaction Time of the Addition Test 2 (s) | 8.94 | 8.64 | 4.18 | 8.08 | 6.86 | 4.23 | −0.86 | −3.022 | 0.003 |
| Number of Errors of the Addition Test 2 | 0.32 | 0.00 | 0.69 | 0.77 | 0.00 | 1.21 | 0.45 | 2.481 | 0.013 |
| Total Time of the Number Test (s) | 297.44 | 293.68 | 160.26 | 240.07 | 211.04 | 142.84 | −57.37 | −3.494 | <0.001 |
| Average Reaction Time of the Number Test (s) | 32.91 | 30.75 | 19.36 | 26.82 | 21.84 | 16.87 | −6.09 | −3.268 | 0.001 |
| Number of Number Test Returns | 1.38 | 1.00 | 1.78 | 0.91 | 0.00 | 1.27 | −0.47 | −1.987 | 0.047 |
| Total Time of the Line Test (s) | 136.15 | 114.56 | 66.75 | 123.71 | 110.42 | 57.62 | −12.44 | −2.656 | 0.008 |
| Average Reaction Time of the Line Test (s) | 14.24 | 11.83 | 7.46 | 12.84 | 11.37 | 6.40 | −1.40 | −2.648 | 0.008 |
| Number of Errors of the Line Test | 1.42 | 0.00 | 2.32 | 1.76 | 1.00 | 2.52 | 0.35 | 0.656 | 0.512 |
| Average Reaction Time of the Simple Coordination Test (s) | 2.23 | 2.07 | 0.88 | 2.20 | 1.90 | 1.35 | −0.03 | −2.376 | 0.017 |
| Number of Reaction of the Simple Coordination Test | 29.78 | 28.00 | 11.44 | 32.95 | 31.50 | 12.95 | 3.16 | 3.444 | 0.001 |
| Total Time of the Complex Coordination Test (s) | 25.08 | 22.77 | 13.54 | 22.07 | 18.23 | 13.74 | −3.01 | −3.167 | 0.002 |
| Average Reaction Time of the Complex Coordination Test (s) | 5.18 | 4.55 | 3.18 | 4.55 | 3.59 | 3.55 | −0.62 | −3.276 | 0.001 |
| Average Value of Average Reaction Times (s) | 12.60 | 12.27 | 5.30 | 11.10 | 9.60 | 6.91 | −1.49 | −3.614 | <0.001 |
| Parameter | Before Rehabilitation | After Rehabilitation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M | Md | SD | M | Md | SD | Δ | Z | p | |
| Total Time of the Addition Test 1 (s) | 118.06 | 99.83 | 54.96 | 100.13 | 83.97 | 53.46 | −17.93 | −2.602 | 0.009 |
| Average Reaction Time of the Addition Test 1 (s) | 11.81 | 9.98 | 5.50 | 9.88 | 8.40 | 5.01 | −1.92 | −2.869 | 0.004 |
| Number of Errors of the Addition Test 1 | 0.67 | 0.00 | 1.15 | 0.83 | 0.00 | 1.46 | 0.17 | 0.637 | 0.524 |
| Total Time of the Addition Test 2 (s) | 76.87 | 70.84 | 39.64 | 72.64 | 61.25 | 50.51 | −4.23 | −2.195 | 0.028 |
| Average Reaction Time of the Addition Test 2 (s) | 8.47 | 7.61 | 4.69 | 7.43 | 6.05 | 5.23 | −1.05 | −2.324 | 0.020 |
| Number of Errors of the Addition Test 2 | 0.40 | 0.00 | 0.86 | 0.69 | 0.00 | 1.56 | 0.29 | 0.975 | 0.330 |
| Total Time of the Number Test (s) | 251.38 | 238.30 | 126.67 | 229.64 | 211.62 | 123.95 | −21.74 | −1.491 | 0.136 |
| Average Reaction Time of the Number Test (s) | 27.95 | 26.43 | 14.08 | 25.38 | 22.09 | 15.97 | −2.57 | −1.306 | 0.192 |
| Number of Number Test Returns | 0.87 | 0.50 | 1.17 | 0.97 | 1.00 | 1.52 | 0.10 | 0.381 | 0.703 |
| Total Time of the Line Test (s) | 130.75 | 118.67 | 61.99 | 108.55 | 95.28 | 44.74 | −22.20 | −2.355 | 0.019 |
| Average Reaction Time of the Line Test (s) | 14.20 | 12.49 | 7.12 | 11.22 | 9.77 | 4.94 | −2.98 | −2.417 | 0.016 |
| Number of Errors of the Line Test | 1.90 | 1.00 | 2.59 | 1.47 | 1.00 | 1.68 | −0.43 | −0.774 | 0.439 |
| Average Reaction Time of the Simple Coordination Test (s) | 2.19 | 1.90 | 0.95 | 1.91 | 1.60 | 0.87 | −0.28 | −2.627 | 0.009 |
| Number of Reaction of the Simple Coordination Test | 31.17 | 30.50 | 9.76 | 34.80 | 36.50 | 11.31 | 3.63 | 3.207 | 0.001 |
| Total Time of the Complex Coordination Test (s) | 22.91 | 20.86 | 13.20 | 20.92 | 16.26 | 12.27 | −1.98 | −0.162 | 0.871 |
| Average Reaction Time of the Complex Coordination Test (s) | 5.59 | 4.27 | 5.44 | 4.63 | 3.24 | 3.39 | −0.96 | −0.442 | 0.658 |
| Average Value of Average Reaction Times (s) | 11.70 | 11.45 | 4.97 | 10.03 | 8.61 | 5.05 | −1.67 | −2.931 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korchut, A.; Sternal, D.; Krzemińska, S.; Marcisz-Dyla, E.; Bąk, E. Cognitive and Psychomotor Performance of Patients After Ischemic Stroke Undergoing Early and Late Rehabilitation. J. Clin. Med. 2025, 14, 2122. https://doi.org/10.3390/jcm14062122
Korchut A, Sternal D, Krzemińska S, Marcisz-Dyla E, Bąk E. Cognitive and Psychomotor Performance of Patients After Ischemic Stroke Undergoing Early and Late Rehabilitation. Journal of Clinical Medicine. 2025; 14(6):2122. https://doi.org/10.3390/jcm14062122
Chicago/Turabian StyleKorchut, Aleksander, Danuta Sternal, Sylwia Krzemińska, Ewa Marcisz-Dyla, and Ewelina Bąk. 2025. "Cognitive and Psychomotor Performance of Patients After Ischemic Stroke Undergoing Early and Late Rehabilitation" Journal of Clinical Medicine 14, no. 6: 2122. https://doi.org/10.3390/jcm14062122
APA StyleKorchut, A., Sternal, D., Krzemińska, S., Marcisz-Dyla, E., & Bąk, E. (2025). Cognitive and Psychomotor Performance of Patients After Ischemic Stroke Undergoing Early and Late Rehabilitation. Journal of Clinical Medicine, 14(6), 2122. https://doi.org/10.3390/jcm14062122







