Rethinking Balanced Resuscitation in Trauma
Abstract
1. Introduction
2. Evolution of Resuscitation: A By-Product of War and Necessity
3. Damage Control and the Role of Balanced Fluid Resuscitation
4. Coagulopathy—A Game Changing Consideration
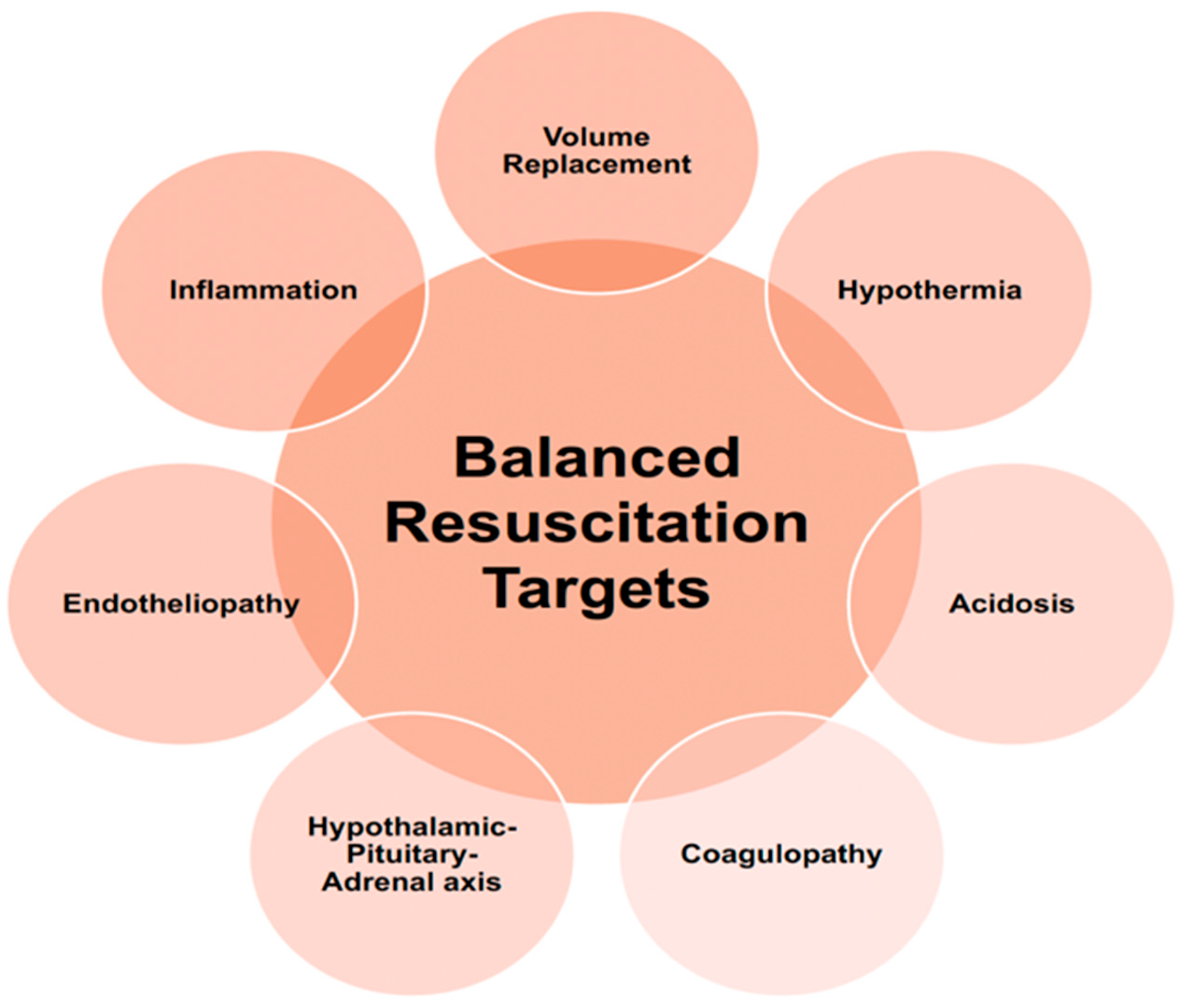
5. Additional Balanced Resuscitation Considerations
5.1. Endotheliopathy of Trauma
5.2. Inflammatory Response After Traumatic Injury
6. Hypothalamic–Pituitary–Adrenal (HPA) Axis in Traumatic Injury
7. Targeted and Balanced Resuscitation
8. Balanced Resuscitation Revamped—Resuscitation Adjuncts and Whole Blood
8.1. Tranexamic Acid
8.2. Calcium
8.3. Whole Blood, Components, and Concentrates
8.3.1. Factor Concentrates
8.3.2. Fresh Frozen Plasma
9. Whole Blood
10. Balanced Resuscitation Protocol
11. Special Populations
11.1. Pediatric Patients
11.2. Injured Older Adults
12. Conclusions
13. Future Perspectives
Funding
Conflicts of Interest
References
- DiMaggio, C.; Ayoung-Chee, P.; Shinseki, M.; Wilson, C.; Marshall, G.; Lee, D.C.; Wall, S.; Maulana, S.; Pachter, H.L.; Frangos, S. Traumatic injury in the United States: In-patient epidemiology 2000–2011. Injury 2016, 47, 1393–1403. [Google Scholar] [CrossRef]
- Jacob, M.; Kumar, P. The challenge in management of hemorrhagic shock in trauma. Med. J. Armed Forces India 2014, 70, 163–169. [Google Scholar] [CrossRef]
- Eastridge, B.J.; Holcomb, J.B.; Shackelford, S. Outcomes of traumatic hemorrhagic shock and the epidemiology of preventable death from injury. Transfusion 2019, 59 (Suppl. S2), 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, J.B.; Helling, T.S.; Hirshberg, A. Military, civilian, and rural application of the damage control philosophy. Mil. Med. 2001, 166, 490–493. [Google Scholar] [CrossRef]
- Pidcoke, H.F.; Aden, J.K.; Mora, A.G.; Borgman, M.A.; Spinella, P.C.; Dubick, M.A.; Blackbourne, L.H.; Cap, A.P. Ten-year analysis of transfusion in Operation Iraqi Freedom and Operation Enduring Freedom: Increased plasma and platelet use correlates with improved survival. J. Trauma Acute Care Surg. 2012, 73 (Suppl. 5), S445–S452. [Google Scholar] [CrossRef]
- Moore, E.E.; Moore, H.B. Historical Perspective of Trauma-Induced Coagulopathy. In Trauma Induced Coagulopathy; Moore, H.B., Neal, M.D., Moore, E.E., Eds.; Springer International Publishing: New York, NY, USA, 2021; pp. 3–11. [Google Scholar] [CrossRef]
- Boulton, F. Blood transfusion and the World Wars. Med. Confl. Surviv. 2015, 31, 57–68. [Google Scholar] [CrossRef]
- Holcomb, J.B.; Tilley, B.C.; Baraniuk, S.; Fox, E.E.; Wade, C.E.; Podbielski, J.M.; del Junco, D.J.; Brasel, K.J.; Bulger, E.M.; Callcut, R.A.; et al. Transfusion of Plasma, Platelets, and Red Blood Cells in a 1:1:1 vs a 1:1:2 Ratio and Mortality in Patients with Severe Trauma: The PROPPR Randomized Clinical Trial. JAMA 2015, 313, 471–482. [Google Scholar] [CrossRef]
- Holcomb, J.B.; Jenkins, D.; Rhee, P.; Johannigman, J.; Mahoney, P.; Mehta, S.; Cox, E.D.; Gehrke, M.J.; Beilman, G.J.; Schreiber, M.; et al. Damage Control Resuscitation: Directly Addressing the Early Coagulopathy of Trauma. J. Trauma Acute Care Surg. 2007, 62, 307. [Google Scholar] [CrossRef]
- Diebel, L.N.; Martin, J.V.; Liberati, D.M. Early tranexamic acid administration ameliorates the endotheliopathy of trauma and shock in an in vitro model. J. Trauma Acute Care Surg. 2017, 82, 1080–1086. [Google Scholar] [CrossRef]
- Joseph, B.; Haider, A.A.; Pandit, V.; Kulvatunyou, N.; Orouji, T.; Khreiss, M.; Tang, A.; O’Keeffe, T.; Friese, R.; Rhee, P. Impact of Hemorrhagic Shock on Pituitary Function. J. Am. Coll. Surg. 2015, 221, 502–508. [Google Scholar] [CrossRef]
- Cabrales, P.; Vázquez, B.Y.S.; Tsai, A.G.; Intaglietta, M. Microvascular and capillary perfusion following glycocalyx degradation. J. Appl. Physiol. 2007, 102, 2251–2259. [Google Scholar] [CrossRef]
- Torres, L.N.; Sondeen, J.L.; Dubick, M.A.; Torres Filho, I.P. Systemic and microvascular effects of resuscitation with blood products after severe hemorrhage in rats. J. Trauma Acute Care Surg. 2014, 77, 716–723. [Google Scholar] [CrossRef]
- Janatpour, K.; Holland, P. A Brief History of Blood Transfusion. In Blood Banking and Transfusion Medicine: Basic Principles and Practice; Elsevier Health Sciences: Amsterdam, The Netherlands, 2007; pp. 1–11. [Google Scholar]
- Robertson, O.H. Transfusion with preserved red blood cells. Br. Med. J. 1918, 1, 691–695. [Google Scholar] [CrossRef]
- Strumia, M.M.; Wagner, J.A.; Monaghan, J.F. The intravenous use of serum and plasma, fresh and preserved. Ann. Surg. 1940, 111, 623–629. [Google Scholar] [CrossRef]
- Cantle, P.M.; Cotton, B.A. Balanced Resuscitation in Trauma Management. Surg. Clin. N. Am. 2017, 97, 999–1014. [Google Scholar] [CrossRef]
- Pearce, F.J.; Lyons, W.S. Logistics of Parenteral Fluids in Battlefield Resuscitation. Mil. Med. 1999, 164, 653–655. [Google Scholar] [CrossRef]
- Proctor, H.J.; Ballantine, T.V.; Broussard, N.D. An analysis of pulmonary function following non-thoracic trauma, with recommendations for therapy. Ann. Surg. 1970, 172, 180–189. [Google Scholar] [CrossRef]
- Parekh, D.; Dancer, R.C.; Thickett, D.R. Acute lung injury. Clin. Med. 2011, 11, 615–618. [Google Scholar] [CrossRef]
- Rotondo, M.F.; Zonies, D.H. The damage control sequence and underlying logic. Surg. Clin. N. Am. 1997, 77, 761–777. [Google Scholar] [CrossRef]
- Holcomb, J.B.; del Junco, D.J.; Fox, E.E.; Wade, C.E.; Podbielski, J.M.; del Junco, D.J.; Brasel, K.J.; Bulger, E.M.; Callcut, R.A.; Cohen, M.J.; et al. The Prospective, Observational, Multicenter, Major Trauma Transfusion (PROMMTT) Study: Comparative Effectiveness of a Time-Varying Treatment with Competing Risks. JAMA Surg. 2013, 148, 127–136. [Google Scholar] [CrossRef]
- Brohi, K.; Singh, J.; Heron, M.; Coats, T. Acute traumatic coagulopathy. J. Trauma 2003, 54, 1127–1130. [Google Scholar] [CrossRef]
- Hess, J.R.; Brohi, K.; Dutton, R.P.; Hauser, C.J.; Holcomb, J.B.; Kluger, Y.; Mackway-Jones, K.; Parr, M.J.; Rizoli, S.B.; Yukioka, T.; et al. The coagulopathy of trauma: A review of mechanisms. J. Trauma 2008, 65, 748–754. [Google Scholar] [CrossRef]
- MacLeod, J.B.A.; Lynn, M.; McKenney, M.G.; Cohn, S.M.; Murtha, M. Early Coagulopathy Predicts Mortality in Trauma. J. Trauma Acute Care Surg. 2003, 55, 39. [Google Scholar] [CrossRef]
- Schreiber, M.A.; Tieu, B. Hemostasis in Operation Iraqi Freedom III. Surgery 2007, 142 (Suppl. 4), S61–S66. [Google Scholar] [CrossRef] [PubMed]
- Sixta, S.L.; Hatch, Q.M.; Matijevic, N.; Wade, C.E.; Holcomb, J.B.; Cotton, B.A. Mechanistic determinates of the acute coagulopathy of trauma (ACoT) in patients requiring emergency surgery. Int. J. Burns Trauma 2012, 2, 158–166. [Google Scholar]
- Moore, H.B.; Moore, E.E.; Gonzalez, E.; Chapman, M.P.; Chin, T.L.; Silliman, C.C.; Banerjee, A.; Sauaia, A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: The spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J. Trauma Acute Care Surg. 2014, 77, 811–817. [Google Scholar] [CrossRef]
- Moore, H.B.; Moore, E.E.; Liras, I.N.; Gonzalez, E.; Harvin, J.A.; Holcomb, J.B.; Sauaia, A.; Cotton, B.A. Acute Fibrinolysis Shutdown after Injury Occurs Frequently and Increases Mortality: A Multicenter Evaluation of 2,540 Severely Injured Patients. J. Am. Coll. Surg. 2016, 222, 347–355. [Google Scholar] [CrossRef]
- Jakubowski, H.V.; Owen, W.G. Macromolecular Specificity Determinants on Thrombin for Fibrinogen and Thrombomodulin. J. Biol. Chem. 1989, 264, 11117–11121. [Google Scholar] [CrossRef]
- Brohi, K.; Cohen, M.J.; Ganter, M.T.; Matthay, M.A.; Mackersie, R.C.; Pittet, J.F. Acute traumatic coagulopathy: Initiated by hypoperfusion: Modulated through the protein C pathway? Ann. Surg. 2007, 245, 812–818. [Google Scholar] [CrossRef]
- Hayakawa, M. Pathophysiology of trauma-induced coagulopathy: Disseminated intravascular coagulation with the fibrinolytic phenotype. J. Intensive Care 2017, 5, 14. [Google Scholar] [CrossRef]
- Meng, Z.H.; Wolberg, A.S.; Monroe, D.M.; Hoffman, M. The effect of temperature and pH on the activity of factor VIIa: Implications for the efficacy of high-dose factor VIIa in hypothermic and acidotic patients. J. Trauma 2003, 55, 886–891. [Google Scholar] [CrossRef]
- Wolberg, A.S.; Meng, Z.H.; Monroe, D.M.; Hoffman, M. A systematic evaluation of the effect of temperature on coagulation enzyme activity and platelet function. J. Trauma 2004, 56, 1221–1228. [Google Scholar] [CrossRef]
- Henriksen, H.H.; McGarrity, S.; SigurÐardóttir, R.S.; Nemkov, T.; D’Alessandro, A.; Palsson, B.O.; Stensballe, J.; Wade, C.E.; Rolfsson, Ó.; Johansson, P.I. Metabolic Systems Analysis of Shock-Induced Endotheliopathy (SHINE) in Trauma: A New Research Paradigm. Ann. Surg. 2020, 272, 1140–1148. [Google Scholar] [CrossRef]
- Barakat, A.I. Dragging along: The glycocalyx and vascular endothelial cell mechanotransduction. Circ. Res. 2008, 102, 747–748. [Google Scholar] [CrossRef]
- Butler, M.J.; Down, C.J.; Foster, R.R.; Satchell, S.C. The Pathological Relevance of Increased Endothelial Glycocalyx Permeability. Am. J. Pathol. 2020, 190, 742–751. [Google Scholar] [CrossRef]
- Hofmann, N.; Zipperle, J.; Jafarmadar, M.; Ashmwe, M.; Keibl, C.; Penzenstadler, C.; Ponschab, M.; Jafarmadar, B.; Redl, H.; Bahrami, S.; et al. Experimental Models of Endotheliopathy: Impact of Shock Severity. Shock. Inj. Inflamm. Sepsis Lab. Clin. Approaches 2018, 49, 564–571. [Google Scholar] [CrossRef]
- Gonzalez Rodriguez, E.; Ostrowski, S.R.; Cardenas, J.C.; Baer, L.A.; Tomasek, J.S.; Henriksen, H.H.; Stensballe, J.; Cotton, B.A.; Holcomb, J.B.; Johansson, P.I.; et al. Syndecan-1: A Quantitative Marker for the Endotheliopathy of Trauma. J. Am. Coll. Surg. 2017, 225, 419–427. [Google Scholar] [CrossRef]
- Johansson, P.I.; Henriksen, H.H.; Stensballe, J.; Gybel-Brask, M.; Cardenas, J.C.; Baer, L.A.; Cotton, B.A.; Holcomb, J.B.; Wade, C.E.; Ostrowski, S.R. Traumatic endotheliopathy: A prospective observational study of 424 severely injured patients. Ann. Surg. 2017, 265, 597–603. [Google Scholar] [CrossRef]
- Johansson, P.I.; Stensballe, J.; Rasmussen, L.S.; Ostrowski, S.R. A high admission syndecan-1 Level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann. Surg. 2011, 254, 194–200. [Google Scholar] [CrossRef]
- Barry, M.; Pati, S. Targeting repair of the vascular endothelium and glycocalyx after traumatic injury with plasma and platelet resuscitation. Matrix Biol. Plus 2022, 14, 100107. [Google Scholar] [CrossRef]
- Abdullah, S.; Karim, M.; Legendre, M.; Rodriguez, L.; Friedman, J.; Cotton-Betteridge, A.; Drury, R.; Packer, J.; Guidry, C.; Duchesne, J.; et al. Hemorrhagic Shock and Resuscitation Causes Glycocalyx Shedding and Endothelial Oxidative Stress Preferentially in the Lung and Intestinal Vasculature. Shock 2021, 56, 803–812. [Google Scholar] [CrossRef]
- Van Zyl, N.; Milford, E.M.; Diab, S.; Dunster, K.; McGiffin, P.; Rayner, S.G.; Staib, A.; Reade, M.C.; Fraser, J.F. Activation of the protein C pathway and endothelial glycocalyx shedding is associated with coagulopathy in an ovine model of trauma and hemorrhage. J. Trauma Acute Care Surg. 2016, 81, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, S.R.; Henriksen, H.H.; Stensballe, J.; Gybel-Brask, M.; Cardenas, J.C.; Baer, L.A.; Cotton, B.A.; Holcomb, J.B.; Wade, C.E.; Johansson, P.I. Sympathoadrenal activation and endotheliopathy are drivers of hypocoagulability and hyperfibrinolysis in trauma: A prospective observational study of 404 severely injured patients. J. Trauma Acute Care Surg. 2017, 82, 293–301. [Google Scholar] [CrossRef]
- Adamson, R.H.; Clough, G. Plasma proteins modify the endothelial cell glycocalyx of frog mesenteric microvessels. J. Physiol. 1992, 445, 473–486. [Google Scholar] [CrossRef]
- Diebel, L.N.; Martin, J.V.; Liberati, D.M. Microfluidics: A high-throughput system for the assessment of the endotheliopathy of trauma and the effect of timing of plasma administration on ameliorating shock-associated endothelial dysfunction. J. Trauma Acute Care Surg. 2018, 84, 575–581. [Google Scholar] [CrossRef]
- Gruen, D.S.; Brown, J.B.; Guyette, F.X.; Johansson, P.I.; Stensballe, J.; Li, S.R.; Leeper, C.M.; Eastridge, B.J.; Nirula, R.; Vercruysse, G.A.; et al. Prehospital tranexamic acid is associated with a dose-dependent decrease in syndecan-1 after trauma: A secondary analysis of a prospective randomized trial. J. Trauma Acute Care Surg. 2023, 95, 642–648. [Google Scholar] [CrossRef]
- Peng, Z.; Pati, S.; Potter, D.; Brown, R.; Holcomb, J.B.; Grill, R.; Wataha, K.; Park, P.W.; Xue, H.; Kozar, R.A. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan-1. Shock 2013, 40, 195–202. [Google Scholar] [CrossRef]
- Lenz, A.; Franklin, G.A.; Cheadle, W.G. Systemic inflammation after trauma. Injury 2007, 38, 1336–1345. [Google Scholar] [CrossRef]
- Palmer, J.; Pandit, V.; Zeeshan, M.; Kulvatunyou, N.; Hamidi, M.; Hanna, K.; Fain, M.; Nikolich-Zugich, J.; Zakaria, E.-R.; Joseph, B. The acute inflammatory response after trauma is heightened by frailty: A prospective evaluation of inflammatory and endocrine system alterations in frailty. J. Trauma Acute Care Surg. 2019, 87, 54–60. [Google Scholar] [CrossRef]
- Sauaia, A.; Moore, F.A.; Moore, E.E. Postinjury Inflammation and Organ Dysfunction. Crit. Care Clin. 2017, 33, 167–191. [Google Scholar] [CrossRef]
- Gopal, S. Syndecans in Inflammation at a Glance. Front. Immunol. 2020, 11, 227. [Google Scholar] [CrossRef]
- Ganter, M.T.; Brohi, K.; Cohen, M.J.; Shaffer, L.A.; Walsh, M.C.; Stahl, G.L.; Pittet, J.-F. Role of the alternative pathway in the early complement activation following major trauma. Shock 2007, 28, 29. [Google Scholar] [CrossRef]
- Rigby, A.C.; Grant, M.A. Protein S: A conduit between anticoagulation and inflammation. Crit. Care Med. 2004, 32 (Suppl. 5), S336–S341. [Google Scholar] [CrossRef]
- Bulger, E.M.; Cuschieri, J.; Warner, K.; Maier, R.V. Hypertonic Resuscitation Modulates the Inflammatory Response in Patients With Traumatic Hemorrhagic Shock. Ann. Surg. 2007, 245, 635. [Google Scholar] [CrossRef]
- Cai, B.; Deitch, E.A.; Grande, D.; Ulloa, L. Anti-Inflammatory Resuscitation Improves Survival in Hemorrhage with Trauma. J. Trauma Acute Care Surg. 2009, 66, 1632–1640. [Google Scholar] [CrossRef]
- Makley, A.T.; Goodman, M.D.; Belizaire, R.M.; Friend, L.A.; Johannigman, J.A.; Dorlac, W.C.; Lentsch, A.B.; Pritts, T.A. Damage Control Resuscitation Decreases Systemic Inflammation After Hemorrhage. J. Surg. Res. 2012, 175, e75–e82. [Google Scholar] [CrossRef]
- Johansson, P.I.; Stensballe, J.; Rasmussen, L.S.; Ostrowski, S.R. High circulating adrenaline levels at admission predict increased mortality after trauma. J. Trauma Acute Care Surg. 2012, 72, 428–436. [Google Scholar] [CrossRef]
- Hilton, J.G.; Marullo, D.S. Trauma induced increases in plasma vasopressin and angiotensin II. Life Sci. 1987, 41, 2195–2200. [Google Scholar] [CrossRef]
- Li, T.; Fang, Y.; Zhu, Y.; Fan, X.; Liao, Z.; Chen, F.; Liu, L. A small dose of arginine vasopressin in combination with norepinephrine is a good early treatment for uncontrolled hemorrhagic shock after hemostasis. J. Surg. Res. 2011, 169, 76–84. [Google Scholar] [CrossRef]
- Xu, L.; Yu, W.K.; Lin, Z.L.; Tan, S.J.; Bai, X.W.; Ding, K.; Li, N. Chemical sympathectomy attenuates inflammation, glycocalyx shedding and coagulation disorders in rats with acute traumatic coagulopathy. Blood Coagul. Fibrinolysis 2015, 26, 152–160. [Google Scholar] [CrossRef]
- Altura, B.M. Evidence that endogenous vasopressin plays a protective role in circulatory shock. Role for reticuloendothelial system using Brattleboro rats. Experientia 1980, 36, 1080–1082. [Google Scholar] [CrossRef]
- Oliver, J.A.; Landry, D.W. Endogenous and exogenous vasopressin in shock. Curr. Opin. Crit. Care 2007, 13, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Sims, C.A.; Guan, Y.; Bergey, M.; Jaffe, R.; Holmes-Maguire, L.; Martin, N.; Reilly, P. Arginine vasopressin, copeptin, and the development of relative AVP deficiency in hemorrhagic shock. Am. J. Surg. 2017, 214, 589–595. [Google Scholar] [CrossRef]
- Anand, T.; Skinner, R. Arginine vasopressin: The future of pressure-support resuscitation in hemorrhagic shock. J. Surg. Res. 2012, 178, 321–329. [Google Scholar] [CrossRef]
- Cohn, S.M.; DeRosa, M.; McCarthy, J.; Song, J.; White, C.; Louden, C.; Ehler, B.; Michalek, J.; Landry, D.W. Characterizing vasopressin and other vasoactive mediators released during resuscitation of trauma patients. J. Trauma Acute Care Surg. 2013, 75, 620–628. [Google Scholar] [CrossRef]
- Cohn, S.; McCarthy, J.; Stewart, R.; Jonas, R.B.; Dent, D.L.; Michalek, J.E. Impact of low-dose vasopressin on trauma outcome: Prospective randomized study. World J. Surg. 2011, 35, 430–439. [Google Scholar] [CrossRef]
- Sims, C.A.; Holena, D.; Kim, P.; Pascual, J.; Smith, B.; Martin, N.; Seamon, M.; Shiroff, A.; Raza, S.; Kaplan, L.; et al. Effect of Low-Dose Supplementation of Arginine Vasopressin on Need for Blood Product Transfusions in Patients with Trauma and Hemorrhagic Shock: A Randomized Clinical Trial. JAMA Surg. 2019, 154, 994–1003. [Google Scholar] [CrossRef]
- Morales, D.; Madigan, J.; Cullinane, S.; Chen, J.; Heath, M.; Oz, M.; Oliver, J.A.; Landry, D.W. Reversal by vasopressin of intractable hypotension in the late phase of hemorrhagic shock. Circulation 1999, 100, 226–229. [Google Scholar] [CrossRef]
- Tapia, N.M.; Chang, A.; Norman, M.; Welsh, F.; Scott, B.; Wall, M.J., Jr.; Mattox, K.L.; Suliburk, J. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J. Trauma Acute Care Surg. 2013, 74, 378–385; discussion 385–386. [Google Scholar] [CrossRef]
- Gazmuri, R.J.; Whitehouse, K.; Whittinghill, K.; Baetiong, A.; Shah, K.; Radhakrishnan, J. Early and sustained vasopressin infusion augments the hemodynamic efficacy of restrictive fluid resuscitation and improves survival in a liver laceration model of hemorrhagic shock. J. Trauma Acute Care Surg. 2017, 82, 317–327. [Google Scholar] [CrossRef]
- Sperry, J.L.; Minei, J.P.; Frankel, H.L.; West, M.A.; Harbrecht, B.G.; Moore, E.E.; Maier, R.V.; Nirula, R. Early use of vasopressors after injury: Caution before constriction. J. Trauma 2008, 64, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.R.; Ivatury, R.R.; Wayne Barbee, R. Endpoints of Resuscitation for the Victim of Trauma. J. Intensive Care Med. 2001, 16, 55–75. [Google Scholar] [CrossRef]
- Napolitano, L.M. Resuscitation Endpoints in Trauma. Transfus. Altern. Transfus. Med. 2005, 6, 6–14. [Google Scholar] [CrossRef]
- Anand, T.; Reyes, A.A.; Sjoquist, M.C.; Magnotti, L.; Joseph, B. Resuscitating the Endothelial Glycocalyx in Trauma and Hemorrhagic Shock. Ann. Surg. Open 2023, 4, e298. [Google Scholar] [CrossRef]
- Carge, M.; Diebel, L.N.; Liberati, D.M. The effect of tranexamic acid dosing regimen on trauma/hemorrhagic shock-related glycocalyx degradation and endothelial barrier permeability: An in vitro model. J. Trauma Acute Care Surg. 2022, 92, 812–820. [Google Scholar] [CrossRef]
- Pati, S.; Potter, D.R.; Baimukanova, G.; Farrel, D.H.; Holcomb, J.B.; Schreiber, M.A. Modulating the endotheliopathy of trauma: Factor concentrate versus fresh frozen plasma. J. Trauma Acute Care Surg. 2016, 80, 576. [Google Scholar] [CrossRef]
- Dunn, C.J.; Goa, K.L. Tranexamic Acid. Drugs 1999, 57, 1005–1032. [Google Scholar] [CrossRef]
- Wu, F.; Chipman, A.; Pati, S.; Miyasawa, B.; Corash, L.; Kozar, R.A. Resuscitative Strategies to Modulate the Endotheliopathy of Trauma: From Cell to Patient. Shock 2020, 53, 575–584. [Google Scholar] [CrossRef]
- Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. Lancet 2010, 376, 23–32. [CrossRef]
- Neeki, M.M.; Dong, F.; Toy, J.; West, M.A.; Harbrecht, B.G.; Moore, E.E.; Maier, R.V.; Nirula, R. Efficacy and Safety of Tranexamic Acid in Prehospital Traumatic Hemorrhagic Shock: Outcomes of the Cal-PAT Study. West. J. Emerg. Med. 2017, 18, 673–683. [Google Scholar] [CrossRef]
- Stansfield, R.; Morris, D.; Jesulola, E. The Use of Tranexamic Acid (TXA) for the Management of Hemorrhage in Trauma Patients in the Prehospital Environment: Literature Review and Descriptive Analysis of Principal Themes. Shock 2020, 53, 277. [Google Scholar] [CrossRef] [PubMed]
- Huebner, B.R.; Dorlac, W.C.; Cribari, C. Tranexamic Acid Use in Prehospital Uncontrolled Hemorrhage. Wilderness Environ. Med. 2017, 28, S50–S60. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, A.M.; Abramovich, A.; Nadler, R.; Feinstein, U.; Shaked, G.; Kreiss, Y.; Glassberg, E. Tranexamic acid in the prehospital setting: Israel Defense Forces’ initial experience. Injury 2014, 45, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Vasudeva, M.; Mathew, J.K.; Groombridge, C.; Tee, J.W.; Johnny, C.S.; Maini, A.; Fitzgerald, M.C. Hypocalcemia in trauma patients: A systematic review. J. Trauma Acute Care Surg. 2021, 90, 396. [Google Scholar] [CrossRef]
- Kronstedt, S.; Roberts, N.; Ditzel, R.; Elder, J.; Steen, A.; Thompson, K.; Anderson, J.; Siegler, J. Hypocalcemia as a predictor of mortality and transfusion. A scoping review of hypocalcemia in trauma and hemostatic resuscitation. Transfusion 2022, 62 (Suppl. 1), S158–S166. [Google Scholar] [CrossRef]
- Jackson-Weaver, O.; Friedman, J.K.; Rodriguez, L.A.; Hoof, M.A.; Drury, R.H.; Packer, J.T.; Smith, A.; Guidry, C.; Duchesne, J.C. Hypoxia/reoxygenation decreases endothelial glycocalyx via reactive oxygen species and calcium signaling in a cellular model for shock. J. Trauma Acute Care Surg. 2019, 87, 1070–1076. [Google Scholar] [CrossRef]
- Mortazavi, C.M.; Hoyt, J.M.; Patel, A.; Chignalia, A.Z. Chapter Two—The glycocalyx and calcium dynamics in endothelial cells. In Current Topics in Membranes; Fancher, I.S., Chignalia, A.Z., Eds.; The Cardiovascular Glycocalyx in Health and Disease; Academic Press: Cambridge, MA, USA, 2023; Volume 91, pp. 21–41. [Google Scholar] [CrossRef]
- Helsloot, D.; Fitzgerald, M.; Lefering, R.; Groombridge, C.; Becaus, N.; Verelst, S.; Missant, C. Calcium supplementation during trauma resuscitation: A propensity score-matched analysis from the TraumaRegister DGU®. Crit. Care 2024, 28, 222. [Google Scholar] [CrossRef]
- Giancarelli, A.; Birrer, K.L.; Alban, R.F.; Hobbs, B.P.; Liu-DeRyke, X. Hypocalcemia in trauma patients receiving massive transfusion. J. Surg. Res. 2016, 202, 182–187. [Google Scholar] [CrossRef]
- Wray, J.P.; Bridwell, R.E.; Schauer, S.G.; Shackelford, S.A.; Bebarta, V.S.; Wright, F.L.; Bynum, J.; Long, B. The diamond of death: Hypocalcemia in trauma and resuscitation. Am. J. Emerg. Med. 2021, 41, 104–109. [Google Scholar] [CrossRef]
- Chanthima, P.; Yuwapattanawong, K.; Thamjamrassri, T.; Nathwani, R.; Stansbury, L.G.; Vavilala, M.S.; Arbabi, S.; Hess, J.R. Association Between Ionized Calcium Concentrations During Hemostatic Transfusion and Calcium Treatment with Mortality in Major Trauma. Anesth. Analg. 2021, 132, 1684–1691. [Google Scholar] [CrossRef]
- Genét, G.F.; Johansson, P.I.; Meyer, M.A.S.; Sølbeck, S.; Sørensen, A.M.; Larsen, C.F.; Welling, K.L.; Windeløv, N.A.; Rasmussen, L.S.; Ostrowski, S.R. Trauma-induced coagulopathy: Standard coagulation tests, biomarkers of coagulopathy, and endothelial damage in patients with traumatic brain injury. J. Neurotrauma 2013, 30, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Shandaliy, Y.; Busey, K.; Scaturo, N. Impact of a calcium replacement protocol during massive transfusion in trauma patients at a level 2 trauma center. Am. J. Health-Syst. Pharm. AJHP Off. J. Am. Soc. Health-Syst. Pharm 2024, 81 (Suppl. 4), S160–S165. [Google Scholar] [CrossRef]
- Wade, D.J.; Pilkington, C.; Henson, J.C.; Jensen, H.K.; Kalkwarf, K.; Bhavaraju, A.; Bruce, N.; Bowman, S.; Margolick, J. Higher Doses of Calcium Associated with Survival in Trauma Patients. J. Surg. Res. 2024, 303, 788–794. [Google Scholar] [CrossRef]
- Gruen, D.S.; Guyette, F.X.; Brown, J.B.; Okonkwo, D.O.; Puccio, A.M.; Campwala, I.K.; Tessmer, M.T.; Daley, B.J.; Miller, R.S.; Harbrecht, B.G.; et al. Association of Prehospital Plasma with Survival in Patients with Traumatic Brain Injury: A Secondary Analysis of the PAMPer Cluster Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2016869. [Google Scholar] [CrossRef]
- Douin, D.J.; Fernandez-Bustamante, A. Early Fibrinogen Replacement to Treat the Endotheliopathy of Trauma: Novel Resuscitation Strategies in Severe Trauma. Anesthesiology 2023, 139, 675. [Google Scholar] [CrossRef]
- Sunde, G.A.; Vikenes, B.; Strandenes, G.; Flo, K.C.; Hervig, T.A.; Kristoffersen, E.K.; Heltne, J.K. Freeze dried plasma and fresh red blood cells for civilian prehospital hemorrhagic shock resuscitation. J. Trauma Acute Care Surg. 2015, 78, S26–S30. [Google Scholar] [CrossRef]
- Beattie, G.; Cohan, C.M.; Ng, V.L.; Victorino, G.P. Liquid plasma: A solution to optimizing early and balanced plasma resuscitation in massive transfusion. J. Trauma Acute Care Surg. 2020, 89, 488. [Google Scholar] [CrossRef]
- Marsden, M.; Benger, J.; Brohi, K.; Curry, N.; Foley, C.; Green, L.; Lucas, J.; Rossetto, A.; Stanworth, S.; Thomas, H.; et al. Coagulopathy, cryoprecipitate and CRYOSTAT-2: Realising the potential of a nationwide trauma system for a national clinical trial. Br. J. Anaesth. 2019, 122, 164–169. [Google Scholar] [CrossRef]
- Tama, M.A.; Stone, M.E., Jr.; Blumberg, S.M.; Reddy, S.H.; Conway, E.E., Jr.; Meltzer, J.A. Association of Cryoprecipitate Use with Survival After Major Trauma in Children Receiving Massive Transfusion. JAMA Surg. 2021, 156, 453–460. [Google Scholar] [CrossRef]
- Sugiyama, K.; Fujita, H.; Nishimura, S. Effects of in-house cryoprecipitate on transfusion usage and mortality in patients with multiple trauma with severe traumatic brain injury: A retrospective cohort study. Blood Transfus. 2020, 18, 6–12. [Google Scholar] [CrossRef]
- Davenport, R.; Curry, N.; Fox, E.E.; Thomas, H.; Lucas, J.; Evans, A.; Shanmugaranjan, S.; Sharma, R.; Deary, A.; Edwards, A.; et al. Early and Empirical High-Dose Cryoprecipitate for Hemorrhage After Traumatic Injury: The CRYOSTAT-2 Randomized Clinical Trial. JAMA 2023, 330, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, J.B.; Pati, S. Optimal trauma resuscitation with plasma as the primary resuscitative fluid: The surgeon’s perspective. Hematology 2013, 2013, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Aubron, C.; Reade, M.C.; Fraser, J.F.; Cooper, D.J. Efficacy and safety of fibrinogen concentrate in trauma patients—A systematic review. J. Crit. Care 2014, 29, e11–e471. [Google Scholar] [CrossRef] [PubMed]
- Curry, N.; Foley, C.; Wong, H.; Mora, A.; Curnow, E.; Zarankaite, A.; Hodge, R.; Hopkins, V.; Deary, A.; Ray, J.; et al. Early fibrinogen concentrate therapy for major haemorrhage in trauma (E-FIT 1): Results from a UK multi-centre, randomised, double blind, placebo-controlled pilot trial. Crit. Care 2018, 22, 164. [Google Scholar] [CrossRef]
- Nascimento, B.; Callum, J.; Tien, H.; Peng, H.; Rizoli, S.; Karanicolas, P.; Alam, A.; Xiong, W.; Selby, R.; Garzon, A.-M.; et al. Fibrinogen in the initial resuscitation of severe trauma (FiiRST): A randomized feasibility trial. BJA Br. J. Anaesth. 2016, 117, 775–782. [Google Scholar] [CrossRef]
- Winearls, J.; Wullschleger, M.; Wake, E.; McQuilten, Z.; Reade, M.; Hurn, C.; Ryan, G.; Trout, M.; Walsham, J.; Holley, A.; et al. Fibrinogen Early in Severe Trauma studY (FEISTY): Results from an Australian multicentre randomised controlled pilot trial. Crit. Care Resusc. J. Australas. Acad. Crit. Care Med. 2021, 23, 32–46. [Google Scholar] [CrossRef]
- Ziegler, B.; Bachler, M.; Haberfellner, H.; Niederwanger, C.; Innerhofer, P.; Hell, T.; Kaufmann, M.; Maegele, M.; Martinowitz, U.; Nebl, C.; et al. Efficacy of prehospital administration of fibrinogen concentrate in trauma patients bleeding or presumed to bleed (FIinTIC): A multicentre, double-blind, placebo-controlled, randomised pilot study. Eur. J. Anaesthesiol. EJA 2021, 38, 348–357. [Google Scholar] [CrossRef]
- Gruen, D.S.; Guyette, F.X.; Brown, J.B.M.; Daley, B.J.; Miller, R.S.; Harbrecht, B.G.; Claridge, J.A.; Phelan, H.A.; Yazer, M.H.; Neal, M.D.; et al. Characterization of unexpected survivors following a prehospital plasma randomized trial. J. Trauma Acute Care Surg. 2020, 89, 908–914. [Google Scholar] [CrossRef]
- Garrigue, D.; Godier, A.; Glacet, A.; Labreuche, J.; Kipnis, E.; Paris, C.; Duhamel, A.; Resch, E.; Bauters, A.; Machuron, F.; et al. French lyophilized plasma versus fresh frozen plasma for the initial management of trauma-induced coagulopathy: A randomized open-label trial. J. Thromb. Haemost. 2018, 16, 481–489. [Google Scholar] [CrossRef]
- Nederpelt, C.J. Fresh Frozen Plasma-to-Packed Red Blood Cell Ratio and Mortality in Traumatic Hemorrhage: Nationwide Analysis of 4,427 Patients. J. Am. Coll. Surg. 2020, 230, 893–901. [Google Scholar] [CrossRef]
- Sperry, J.L.; Guyette, F.X.; Brown, J.B.; Yazer, M.H.; Triulzi, D.J.; Early-Young, B.J.; Adams, P.W.; Daley, B.J.; Miller, R.S.; Harbrecht, B.G.; et al. Prehospital Plasma during Air Medical Transport in Trauma Patients at Risk for Hemorrhagic Shock. N. Engl. J. Med. 2018, 379, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Baskett, T.F. James Blundell: The first transfusion of human blood. Resuscitation 2002, 52, 229–233. [Google Scholar] [CrossRef]
- Murdock, A.D.; Berséus, O.; Hervig, T.; Strandenes, G.; Lunde, T.H. Whole Blood: The Future of Traumatic Hemorrhagic Shock Resuscitation. Shock 2014, 41, 62. [Google Scholar] [CrossRef] [PubMed]
- Brill, J.B.; Tang, B.; Hatton, G.; Mueck, K.M.; McCoy, C.C.; Kao, L.S.; Cotton, B.A. Impact of Incorporating Whole Blood into Hemorrhagic Shock Resuscitation: Analysis of 1,377 Consecutive Trauma Patients Receiving Emergency-Release Uncrossmatched Blood Products. J. Am. Coll. Surg. 2022, 234, 408. [Google Scholar] [CrossRef] [PubMed]
- Hazelton, J.P.D.; Cannon, J.W.; Zatorski, C.; Roman, J.S.; Moore, S.A.; Young, A.J.; Subramanian, M.; Guzman, J.F.B.; Fogt, F.; Moran, A.; et al. Cold-stored whole blood: A better method of trauma resuscitation? J. Trauma Acute Care Surg. 2019, 87, 1035–1041. [Google Scholar] [CrossRef]
- Lee, J.S.; Khan, A.D.; Wright, F.L.; McIntyre, R.C.; Dorlac, W.C.; Cribari, C.; Brockman, V.; Vega, S.A.; Cofran, J.M.; Schroeppel, T.J. Whole Blood Versus Conventional Blood Component Massive Transfusion Protocol Therapy in Civilian Trauma Patients. Am. Surg. 2022, 88, 880–886. [Google Scholar] [CrossRef]
- Ngatuvai, M.; Zagales, I.; Sauder, M.; Andrade, R.; Santos, R.G.; Bilski, T.; Kornblith, L.; Elkbuli, A. Outcomes of Transfusion with Whole Blood, Component Therapy, or Both in Adult Civilian Trauma Patients: A Systematic Review and Meta-Analysis. J. Surg. Res. 2023, 287, 193–201. [Google Scholar] [CrossRef]
- Malkin, M.; Nevo, A.; Brundage, S.I.; Schreiber, M. Effectiveness and safety of whole blood compared to balanced blood components in resuscitation of hemorrhaging trauma patients—A systematic review. Injury 2021, 52, 182–188. [Google Scholar] [CrossRef]
- Mohammed, A.D.; Ntambwe, P.; Crawford, A.M. Barriers to Effective Transfusion Practices in Limited-Resource Settings: From Infrastructure to Cultural Beliefs. World J. Surg. 2020, 44, 2094–2099. [Google Scholar] [CrossRef]
- Geneen, L.J.; Brunskill, S.J.; Doree, C.; Estcourt, L.J.; Green, L. The Difference in Potential Harms between Whole Blood and Component Blood Transfusion in major Bleeding: A Rapid Systematic Review and Meta-Analysis of RCTs. Transfus. Med. Rev. 2021, 36, 7–15. [Google Scholar] [CrossRef]
- Ciaraglia, A.; Myers, J.C.; Braverman, M.D.; Barry, J.B.; Eastridge, B.; Stewart, R.; Nicholson, S.; Jenkins, D. Transfusion-related cost comparison of trauma patients receiving whole blood versus component therapy. J. Trauma Acute Care Surg. 2023, 95, 62–68. [Google Scholar] [CrossRef]
- Murphy, R.C.; Johnson, T.W.; Mack, T.J.; Burke, R.E.; Damiano, N.P.; Heger, L.; Minner, N.; German, E.; Wilson, A.; Mount, M.G.; et al. Cost Savings of Whole Blood Versus Component Therapy at a Community Level 1 Trauma Center. Am. Surg. 2024, 90, 2156–2159. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, R.M.D.; Bank, E.A.A.; Krohmer, J.R.; Haskell, A.; Taylor, A.L.; Jenkins, D.H.; Holcomb, J.B. Removing the barriers to prehospital blood: A roadmap to success. J. Trauma Acute Care Surg. 2024, 97, S138–S144. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.T.; Leeper, C.M.; Spinella, P.C. Damage-control resuscitation in pediatric trauma: What you need to know. J. Trauma Acute Care Surg. 2023, 95, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.A.; Yang, J.C.; Winchell, R.J.; Simons, R.K.; Fortlage, D.A.; Hollingsworth-Fridlund, P.; Hoyt, D.B. Lethal injuries and time to death in a level I trauma center. J. Am. Coll. Surg. 1998, 186, 528–533. [Google Scholar] [CrossRef]
- Anand, T.; Obaid, O.; Nelson, A.; Chehab, M.; Ditillo, M.D.; Hammad, A.; Douglas, M.; Bible, L.; Joseph, B. Whole blood hemostatic resuscitation in pediatric trauma: A nationwide propensity-matched analysis. J. Trauma Acute Care Surg. 2021, 91, 573–578. [Google Scholar] [CrossRef]
- Cannon, J.W.; Johnson, M.A.; Caskey, R.C.; Borgman, M.A.; Neff, L.P. High ratio plasma resuscitation does not improve survival in pediatric trauma patients. J. Trauma Acute Care Surg. 2017, 3, 211. [Google Scholar] [CrossRef]
- Akl, M.; Anand, T.; Reina, R.; El-Qawaqzeh, K.; Ditillo, M.; Hosseinpour, H.; Nelson, A.; Obaid, O.; Friese, R.; Joseph, B. Balanced hemostatic resuscitation for bleeding pediatric trauma patients: A nationwide quantitative analysis of outcomes. J. Pediatr. Surg. 2022, 57, 986–993. [Google Scholar] [CrossRef]
- Kornelsen, E.; Kuppermann, N.; Nishijima, D.K.; Ren, L.Y.; Rumantir, M.; Gill, P.J.; Finkelstein, Y. Effectiveness and safety of tranexamic acid in pediatric trauma: A systematic review and meta-analysis. Am. J. Emerg. Med. 2022, 55, 103–110. [Google Scholar] [CrossRef]
- Maeda, T.; Michihata, N.; Sasabuchi, Y.; Matsui, H.; Ohnishi, Y.; Miyata, S.; Yasunaga, H. Safety of Tranexamic Acid During Pediatric Trauma: A Nationwide Database Study. Pediatr. Crit. Care Med. 2018, 19, e637–e642. [Google Scholar] [CrossRef]
- Thomson, J.M.; Huynh, H.H.; Drone, H.M.; Jantzer, J.L.; Tsai, A.K.; Jancik, J.T. Experience in an Urban Level 1 Trauma Center with Tranexamic Acid in Pediatric Trauma: A Retrospective Chart Review. J. Intensiv. Care Med. 2020, 36, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Eckert, M.J.; Wertin, T.M.; Tyner, S.D.; Nelson, D.W.; Izenberg, S.; Martin, M.J. Tranexamic acid administration to pediatric trauma patients in a combat setting: The pediatric trauma and tranexamic acid study (PED-TRAX). J Trauma Acute Care Surg. 2014, 77, 852. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.B.; Gestring, M.L.; Forsythe, R.M.; Stassen, N.A.; Billiar, T.R.; Peitzman, A.B.; Sperry, J.L. Systolic blood pressure criteria in the National Trauma Triage Protocol for geriatric trauma: 110 is the new 90. J. Trauma Acute Care Surg. 2015, 78, 352. [Google Scholar] [CrossRef]
- Neideen, T.; Lam, M.; Brasel, K.J. Preinjury Beta Blockers are Associated with Increased Mortality in Geriatric Trauma Patients. J. Trauma Acute Care Surg. 2008, 65, 1016. [Google Scholar] [CrossRef]
- Joseph, B.; Hassan, A. Geriatric Trauma Patients: What is the Difference? Curr. Surg. Rep. 2015, 4, 1. [Google Scholar] [CrossRef]
- Quick, J.A.; Bartels, A.N.; Coughenour, J.P.; Barnes, S.L. Experience with prothrombin complex for the emergent reversal of anticoagulation in rural geriatric trauma patients. Surgery 2012, 152, 722–728. [Google Scholar] [CrossRef]
- Al Ma’ani, M.; Nelson, A.; Castillo Diaz, F.; Specner, A.L.; Khurshid, M.H.; Anand, T.; Hejazi, O.; Ditillo, M.; Magnotti, L.J.; Joseph, B. A narrative review: Resuscitation of older adults with hemorrhagic shock. Transfusion 2025. [Google Scholar] [CrossRef]
- Hosseinpour, H.; Anand, T.; Hejazi, O.; Colosimo, C.; Bhogadi, S.K.; Spencer, A.; Nelson, A.; Ditillo, M.; Magnotti, L.J.; Joseph, B. The Role of Whole Blood Hemostatic Resuscitation in Bleeding Geriatric Trauma Patients. J Surg Res. 2024, 299, 26–33. [Google Scholar] [CrossRef]
- Warner, R.; Mc Cullough, M.A.; Painter, M.D.; Hoth, J.J.; Meredith, W.J.; Miller, P.R.; Nunn, A.M. Whole Blood in Those with Old Blood: The Use of Whole Blood in the Geriatric Trauma Population. J. Am. Coll. Surg. 2021, 233, S307. [Google Scholar] [CrossRef]
- Moran, J.; Kahan, J.B.; Morris, J.; Joo, P.Y.; O’Connor, M.I. Tranexamic Acid Administration at Hospital Admission Decreases Transfusion Rates in Geriatric Hip Fracture Patients Undergoing Surgery. Geriatr. Orthop. Surg. Rehabil. 2022, 13, 21514593221124414. [Google Scholar] [CrossRef]
- Xie, J.; Hu, Q.; Huang, Q.; Chen, G.; Zhou, Z.; Pei, F. Efficacy and safety of tranexamic acid in geriatric hip fracture with hemiarthroplasty: A retrospective cohort study. BMC Musculoskelet. Disord. 2019, 20, 304. [Google Scholar] [CrossRef] [PubMed]
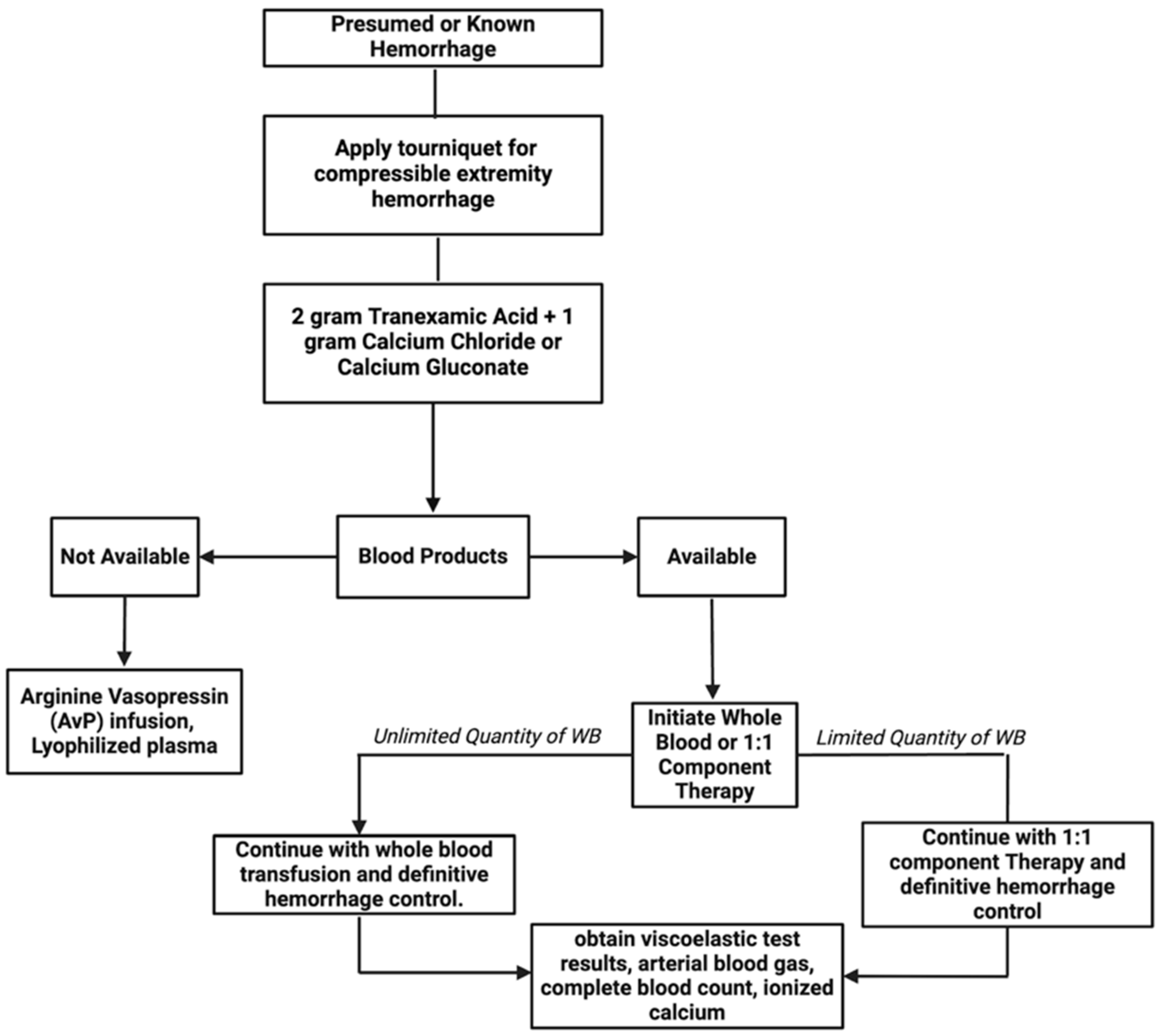
| Key Recommendations |
|---|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anand, T.; Shin, H.; Ratnasekera, A.; Tran, M.L.; Huckeby, R.; Butts, L.; Stejskal, I.; Magnotti, L.J.; Joseph, B. Rethinking Balanced Resuscitation in Trauma. J. Clin. Med. 2025, 14, 2111. https://doi.org/10.3390/jcm14062111
Anand T, Shin H, Ratnasekera A, Tran ML, Huckeby R, Butts L, Stejskal I, Magnotti LJ, Joseph B. Rethinking Balanced Resuscitation in Trauma. Journal of Clinical Medicine. 2025; 14(6):2111. https://doi.org/10.3390/jcm14062111
Chicago/Turabian StyleAnand, Tanya, Hannah Shin, Asanthi Ratnasekera, MyDuyen Luong Tran, Rebekah Huckeby, Lindsey Butts, Ivy Stejskal, Louis J. Magnotti, and Bellal Joseph. 2025. "Rethinking Balanced Resuscitation in Trauma" Journal of Clinical Medicine 14, no. 6: 2111. https://doi.org/10.3390/jcm14062111
APA StyleAnand, T., Shin, H., Ratnasekera, A., Tran, M. L., Huckeby, R., Butts, L., Stejskal, I., Magnotti, L. J., & Joseph, B. (2025). Rethinking Balanced Resuscitation in Trauma. Journal of Clinical Medicine, 14(6), 2111. https://doi.org/10.3390/jcm14062111






