Improving Perioperative Care in Gastric Surgery: Insights from the EUropean PErioperative MEdical Networking (EUPEMEN) Project
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Development
2.2. Consensus Formation
2.3. Technical Activities
- Development of multimodal rehabilitation manuals: Detailed manuals were created to provide comprehensive guidance on perioperative care for gastric cancer surgery and six other surgical disciplines, including esophageal, colorectal, liver, and bariatric surgery, as well as two commonly encountered conditions, including acute appendicitis and bowel obstruction. These manuals were translated into five languages (English, Spanish, Italian, Greek, and Czech) to ensure accessibility across Europe and made available through the EUPEMEN project website (https://eupemen.eu/eupemen-manuals/, accessed on 1 January 2025).
- Creation of an online learning platform: A user-friendly online platform, accessible free of charge through the EUPEMEN Learning Website (https://eupemen.eu/, accessed on 1 January 2025), was established to host evidence-based, standardized perioperative protocols. This platform offers healthcare professionals free access to learning resources, interactive modules, and practical tools to enhance recovery outcomes in surgical patients.
- Training future educators: A “train the trainer” approach was employed to ensure the sustainability of the project. Selected professionals were trained in the principles of enhanced recovery and equipped with the knowledge and skills to educate and mentor colleagues in their institutions.
- Project promotion through multiplier events: Five large-scale promotional events were organized across participating countries to disseminate the EUPEMEN protocol, raise awareness of its benefits, and encourage widespread adoption in clinical practice.
- International collaboration meetings: Four transnational meetings were held, bringing together experts from the participating institutions to refine the protocol, share experiences, and address challenges in implementation. These meetings facilitated consensus-building and strengthened the collaborative framework of the project.
- Revision and standardization of protocols: The English version of the Recovery Intensification for Optimal Care in Adult Surgery (RICA) protocol was revised and adapted to create a unified set of perioperative care guidelines [12]. This process ensured that the EUPEMEN protocols aligned with the latest evidence and were tailored for effective application across diverse healthcare settings.
2.4. Protocol Structure
- Preoperative phase: This phase emphasized comprehensive patient assessment, including nutritional evaluation, risk stratification, and psychological support. Multidisciplinary teams comprising surgeons, anesthesiologists, nurses, and dietitians worked collaboratively to optimize patient readiness through preoperative education, carbohydrate loading, and prophylactic measures to reduce surgical site infections and thromboembolic risks.
- Intraoperative phase: The intraoperative recommendations focused on minimizing surgical stress through techniques such as goal-directed fluid therapy, hemodynamic monitoring, and temperature control. Multimodal analgesia strategies were employed to reduce opioid use, and minimally invasive surgical approaches were prioritized to accelerate recovery.
- Postoperative phase: The postoperative phase prioritized early mobilization, oral intake initiation, and thromboembolism prevention through a combination of low-molecular-weight heparin and mechanical prophylaxis. Pain management relied on opioid-sparing multimodal analgesia, and patients were actively engaged in recovery through structured respiratory and functional physiotherapy sessions.
2.5. Objectives
- Enhancing recovery by minimizing surgical stress and promoting early mobilization and oral intake.
- Reducing postoperative complications, such as infections, thromboembolic events, and delayed gastric emptying.
- Shortening hospital stays without compromising patient safety or outcomes.
- Providing a comprehensive framework that supports multidisciplinary collaboration, ensuring that all aspects of perioperative care are addressed effectively.
- Facilitating the education and training of healthcare professionals to implement and disseminate enhanced recovery protocols consistently.
- Establishing a unified standard of care that can be adapted to diverse healthcare settings, ultimately improving outcomes for gastric surgery patients.
2.6. Clinical Impact
3. Results
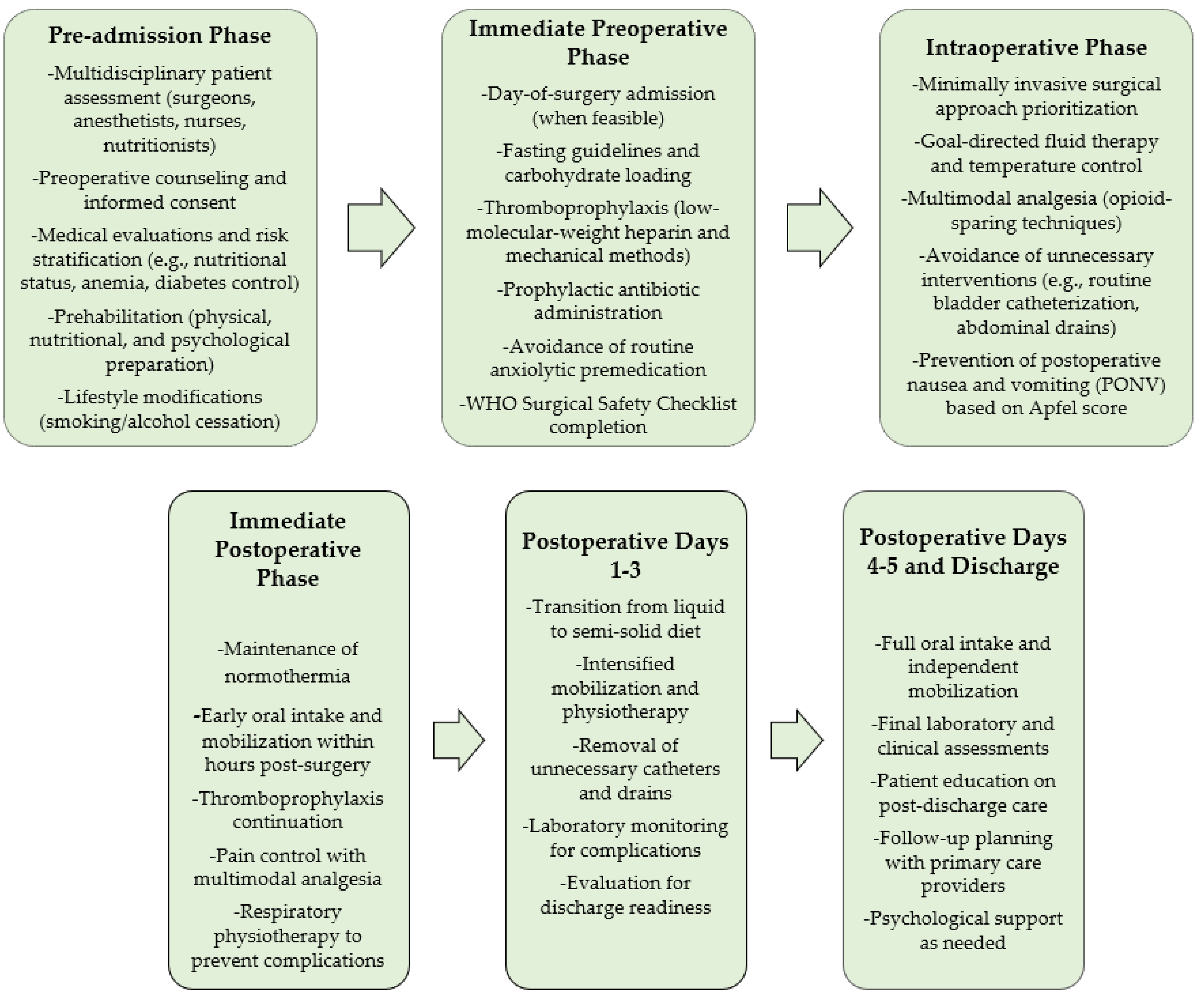
3.1. Pre-Admission Phase
3.2. Perioperative Phase
3.2.1. Immediate Preoperative Phase
3.2.2. Intraoperative Phase
3.2.3. Immediate Postoperative Phase
3.3. Postoperative Day 1
3.4. Postoperative Day 2
3.5. Postoperative Day 3
3.6. Postoperative Day 4
3.7. Discharge
4. Discussion
5. Future Research Directions
6. Challenges and Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiologists |
| ERAS | Enhanced Recovery After Surgery |
| EUPEMEN | European Perioperative Medical Networking |
| FiO2 | Fraction of Inspired Oxygen |
| HbA1c | Hemoglobin A1c |
| LMWH | Low-Molecular-Weight Heparin |
| MUST | Malnutrition Universal Screening Tool |
| PONV | Postoperative Nausea and Vomiting |
| PPC | Postoperative Pulmonary Complications |
| RICA | Recovery Intensification for Optimal Care in Adult Surgery |
| VAS | Visual Analog Scale |
| WHO | World Health Organization |
References
- Yang, W.; Zhao, H.; Yu, Y.; Wang, J.; Guo, L.; Liu, J.; Pu, J.; Lv, J. Updates on Global Epidemiology, Risk and Prognostic Factors of Gastric Cancer. World J. Gastroenterol. 2023, 29, 2452–2468. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.S. Treatment of Localized Gastric Cancer. Semin. Oncol. 2004, 31, 566–573. [Google Scholar] [CrossRef]
- Shannon, A.B.; Straker, R.J.; Fraker, D.L.; Roses, R.E.; Miura, J.T.; Karakousis, G.C. Ninety-Day Mortality after Total Gastrectomy for Gastric Cancer. Surgery 2021, 170, 603–609. [Google Scholar] [CrossRef]
- Desiderio, J.; Trastulli, S.; D’Andrea, V.; Parisi, A. Enhanced Recovery after Surgery for Gastric Cancer (ERAS-GC): Optimizing Patient Outcome. Transl. Gastroenterol. Hepatol. 2020, 5, 11. [Google Scholar] [CrossRef]
- Smith, T.W.J.; Wang, X.; Singer, M.A.; Godellas, C.V.; Vaince, F.T. Enhanced Recovery after Surgery: A Clinical Review of Implementation across Multiple Surgical Subspecialties. Am. J. Surg. 2020, 219, 530–534. [Google Scholar] [CrossRef]
- Rosa, F.; Longo, F.; Pozzo, C.; Strippoli, A.; Quero, G.; Fiorillo, C.; Mele, M.C.; Alfieri, S. Enhanced Recovery after Surgery (ERAS) versus Standard Recovery for Gastric Cancer Patients: The Evidences and the Issues. Surg. Oncol. 2022, 41, 101727. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, O.; Anestiadou, E.; Ramirez, J.M.; Fabbri, N.; Mart, J.; Feo, C.V.; Pesce, A.; Rosetzka, K.; Arroyo, A.; Koci, P.; et al. The EUPEMEN (EUropean PErioperative MEdical Networking) Protocol for Acute Appendicitis: Recommendations for Perioperative Care. J. Clin. Med. 2024, 13, 6943. [Google Scholar] [CrossRef] [PubMed]
- Pesce, A.; Ramírez, J.M.; Fabbri, N.; Ubieto, J.M.; Vittorio, F.C. The EUropean PErioperative MEdical Networking (EUPEMEN) Project and Recommendations for Perioperative Care in Colorectal Surgery: A Quality Improvement Study. Int. J. Surg. 2024, 110, 4796–4803. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Fish, L.S.; Busby, D.M. The Delphi Method; Guilford: New York, NY, USA, 2005. [Google Scholar]
- Ioannidis, O.; Ramirez, J.M.; Ubieto, J.M.; Feo, C.V.; Arroyo, A.; Kocián, P.; Sánchez-Guillén, L.; Bellosta, A.P.; Whitley, A.; Enguita, A.B.; et al. The EUPEMEN (EUropean PErioperative MEdical Networking) Protocol for Bowel Obstruction: Recommendations for Perioperative Care. J. Clin. Med. 2023, 12, 4185. [Google Scholar] [CrossRef]
- Calvo-Vecino, J.; Hernández, E.; Ramirez, J.; Loinaz, C.; Trapero, C.; Quintas, C.; Antolín, A.; Rodriguez-Cuellar, E.; Aguado, J.; Ruiz-López, P.; et al. Vía Clínica de Recuperación Intensificada En Cirugía Abdominal (RICA); Instituto Aragonés de Ciencias de la Salud: Zaragoza, Spain, 2014. [Google Scholar]
- Stenberg, E.; Dos Reis Falcão, L.F.; O’Kane, M.; Liem, R.; Pournaras, D.J.; Salminen, P.; Urman, R.D.; Wadhwa, A.; Gustafsson, U.O.; Thorell, A. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations: A 2021 Update. World J. Surg. 2022, 46, 729–751. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, M.; Noor, M.S.; Abdelfatah, E. The Multidisciplinary Approach and Surgical Management of GE Junction Adenocarcinoma. Cancers 2024, 16, 288. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Costantino, C.L.; Mullen, J.T. Morbidity and Mortality of Total Gastrectomy: A Comprehensive Analysis of 90-Day Outcomes. J. Gastrointest. Surg. 2019, 23, 1340–1348. [Google Scholar] [CrossRef]
- de Cássio Zequi, S.; Franca Silva, I.L.A.; Duprat, J.P.; Coimbra, F.J.F.; Gross, J.L.; Vartanian, J.G.; Makdissi, F.B.A.; Leite, F.P.M.; Costa, W.H.d.; Yazbek, G.; et al. Informed Consent and a Risk-Based Approach to Oncologic Surgery in a Cancer Center during the COVID-19 Pandemic. J. Surg. Oncol. 2021, 123, 1659–1668. [Google Scholar] [CrossRef]
- McNally, S.A.; El-Boghdadly, K.; Kua, J.; Moonesinghe, S.R. Preoperative Assessment and Optimisation: The Key to Good Outcomes after the Pandemic. Br. J. Hosp. Med. 2021, 82, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, S.; Thiagalingam, A.; Hillis, G.; Halliwell, R.; Pleass, H.C.C.; Chow, C.K. Cardiovascular Risk Management in the Peri-Operative Setting. Med. J. Aust. 2023, 219, 30–39. [Google Scholar] [CrossRef]
- Diaz-Fuentes, G.; Hashmi, H.R.T.; Venkatram, S. Perioperative Evaluation of Patients with Pulmonary Conditions Undergoing Non-Cardiothoracic Surgery. Health Serv. Insights 2016, 9, 9–23. [Google Scholar] [CrossRef]
- Kouyoumdjian, A.; Trepanier, M.; Al Shehhi, R.; Cools-Lartigue, J.; Ferri, L.E.; Lee, L.; Mueller, C.L. The Effect of Preoperative Anemia and Perioperative Transfusion on Surgical Outcomes After Gastrectomy for Gastric Cancer. J. Surg. Res. 2021, 259, 523–531. [Google Scholar] [CrossRef]
- Peng, D.; Xiang, Y.C.; Tang, K.L.; Qiu, Y.Y. Impact of Preoperative Type 2 Diabetes Mellitus on the Outcomes of Patients with Gastric Cancer Following Gastrectomy: Analysis of 834 Patients Using Propensity Score Matching. Biomed. Rep. 2023, 19, 1–6. [Google Scholar] [CrossRef]
- Hezkial, M.; Al-bazzaz, O.; Farag, M. Pre-Operative Haemoglobin A1c (HbA1c) in Diabetic Patients Undergoing Major Surgery: An Investigation into Current Practice. Perioper. Care Oper. Room Manag. 2018, 13, 6–11. [Google Scholar] [CrossRef]
- Cheisson, G.; Jacqueminet, S.; Cosson, E.; Ichai, C.; Leguerrier, A.-M.; Nicolescu-Catargi, B.; Ouattara, A.; Tauveron, I.; Valensi, P.; Benhamou, D. Perioperative Management of Adult Diabetic Patients. Preoperative Period. Anaesthesia, Crit. Care Pain Med. 2018, 37 (Suppl. S1), S9–S19. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Kwon, I.G.; Ryu, S.W. Assessment of Nutritional Status in Laparoscopic Gastrectomy for Gastric Cancer. Transl. Gastroenterol. Hepatol. 2017, 2, 85. [Google Scholar] [CrossRef]
- Matsui, R.; Inaki, N.; Tsuji, T. Impact of Preoperative Nutritional Assessment on Other-Cause Survival after Gastrectomy in Patients with Gastric Cancer. Nutrients 2023, 15, 3182. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-grillo, I.; Camilo, M.; Ravasco, P.; Bole, C. Validation of the Malnutrition Universal Screening Tool (MUST) in Cancer. Br. J. Nutr. 2021, 108, 343–348. [Google Scholar] [CrossRef]
- Oh, S.E.; Youn, H.G.; Oh, S.J.; Choi, M.-G.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; An, J.Y. Trends in Vitamin D Level and Risk of Vitamin D Deficiency after Gastrectomy for Gastric Cancer: A Retrospective Study of a Single High-Volume Center Experience. Clin. Nutr. ESPEN 2023, 53, 74–79. [Google Scholar] [CrossRef]
- Ao, M.; Awane, M.; Asao, Y.; Kita, S.; Miyawaki, T.; Tanaka, K. High Prevalence of Vitamin B-12 Deficiency before and Early after Gastrectomy in Patients with Gastric Cancer. Asia Pac. J. Clin. Nutr. 2023, 32, 275–281. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X.; Wang, X.; Zhu, C.; Hu, Y.; Yang, X.; Xu, C.; Shen, X. Preoperative Hyponatremia And Hypocalcemia Predict Poor Prognosis In Elderly Gastric Cancer Patients. Cancer Manag. Res. 2019, 11, 8765–8780. [Google Scholar] [CrossRef]
- Serra, F.; Pedrazzoli, P.; Brugnatelli, S.; Pagani, A.; Corallo, S.; Rosti, G.; Caccialanza, R.; Viganò, J.; Carminati, O. Nutritional Support Management in Resectable Gastric Cancer. Drugs Context 2022, 11, 1–11. [Google Scholar] [CrossRef]
- Ma, K.; Baloch, Z.; He, T.-T.; Xia, X. Alcohol Consumption and Gastric Cancer Risk: A Meta-Analysis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 238–246. [Google Scholar] [CrossRef]
- Smyth, E.C.; Capanu, M.; Janjigian, Y.Y.; Kelsen, D.K.; Coit, D.; Strong, V.E.; Shah, M.A. Tobacco Use Is Associated with Increased Recurrence and Death from Gastric Cancer. Ann. Surg. Oncol. 2012, 19, 2088–2094. [Google Scholar] [CrossRef]
- Bausys, A.; Luksta, M.; Kuliavas, J.; Anglickiene, G.; Maneikiene, V.; Gedvilaite, L.; Celutkiene, J.; Jamontaite, I.; Cirtautas, A.; Lenickiene, S.; et al. Personalized Trimodal Prehabilitation for Gastrectomy. Medicine 2020, 99, e20687. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tian, Y.; Li, Z.; Cao, S.; Liu, X.; Han, H.; Han, L.; Kong, L.; Zhang, X.; Liu, F.; et al. Multimodal Prehabilitation to Improve Functional Abilities and Reduce the Chronic Inflammatory Response of Frail Elderly Patients with Gastric Cancer: A Prospective Cohort Study. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2024, 51, 109563. [Google Scholar] [CrossRef]
- Carrillo, G.M.; Santamaría, N.P. Life after a Gastrectomy: Experience of Patients with Gastric Cancer. Enfermería Clínica Engl. Ed. 2019, 29, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Hallowell, N.; Lawton, J.; Badger, S.; Richardson, S.; Hardwick, R.H.; Caldas, C.; Fitzgerald, R.C. The Psychosocial Impact of Undergoing Prophylactic Total Gastrectomy (PTG) to Manage the Risk of Hereditary Diffuse Gastric Cancer (HDGC). J. Genet. Couns. 2017, 26, 752–762. [Google Scholar] [CrossRef]
- Tew, G.A.; Ayyash, R.; Durrand, J.; Danjoux, G.R. Clinical Guideline and Recommendations on Pre-Operative Exercise Training in Patients Awaiting Major Non-Cardiac Surgery. Anaesthesia 2018, 73, 750–768. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.; Stokmans, R.A.; Willigendael, E.M.; Nienhuijs, S.W.; Rosman, C.; van Ramshorst, B.; Teijink, J.A.W. Preoperative Exercise Therapy for Elective Major Abdominal Surgery: A Systematic Review. Int. J. Surg. 2014, 12, 134–140. [Google Scholar] [CrossRef]
- Avinash, S.H.; Krishna, H.M. The Impact of the Apfel Scoring System for Prophylaxis of Post-Operative Nausea and Vomiting: A Randomized Controlled Trial. J. Anaesthesiol. Clin. Pharmacol. 2023, 39, 463–467. [Google Scholar] [CrossRef]
- Rosa, F.; Tortorelli, A.P.; Quero, G.; Galiandro, F.; Fiorillo, C.; Sollazzi, L.; Alfieri, S. The Impact of Preoperative ASA-Physical Status on Postoperative Complications and Long-Term Survival Outcomes in Gastric Cancer Patients. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7383–7390. [Google Scholar] [CrossRef]
- Liu, L.-B.; Li, J.; Lai, J.-X.; Shi, S. Harnessing Interventions during the Immediate Perioperative Period to Improve the Long-Term Survival of Patients Following Radical Gastrectomy. World J. Gastrointest. Surg. 2023, 15, 520–533. [Google Scholar] [CrossRef]
- Irino, T.; Matsuda, S.; Wada, N.; Kawakubo, H.; Kitagawa, Y. Essential Updates 2019/2020: Perioperative and Surgical Management of Gastric Cancer. Ann. Gastroenterol. Surg. 2021, 5, 162–172. [Google Scholar] [CrossRef]
- Kulasegarah, J.; Lang, E.E.; Carolan, E.; Viani, L.; Gaffney, R.; Walsh, R.M. Day of Surgery Admission—Is This Safe Practise? Ir. Med. J. 2008, 101, 218–219. [Google Scholar] [PubMed]
- Dongare, P.A.; Bhaskar, S.B.; Harsoor, S.S.; Garg, R.; Kannan, S.; Goneppanavar, U.; Ali, Z.; Gopinath, R.; Sood, J.; Mani, K.; et al. Perioperative Fasting and Feeding in Adults, Obstetric, Paediatric and Bariatric Population: Practice Guidelines from the Indian Society of Anaesthesiologists. Indian J. Anaesth. 2020, 64, 556–584. [Google Scholar] [CrossRef]
- Faria, M.S.M.; de Aguilar-Nascimento, J.E.; Pimenta, O.S.; Alvarenga Jr, L.C.; Dock-Nascimento, D.B.; Slhessarenko, N. Preoperative Fasting of 2 Hours Minimizes Insulin Resistance and Organic Response to Trauma After Video-Cholecystectomy: A Randomized, Controlled, Clinical Trial. World J. Surg. 2009, 33, 1158. [Google Scholar] [CrossRef] [PubMed]
- Sukmono, B.; Hidayat, J.; Sugiarto, A.; Anggreni, M. Preoperative Fasting of Eight Hours Provide Better Gastric Emptying: Ultrasound Assessment of Gastric Volume. Asian J. Anesthesiol. 2023, 61. [Google Scholar] [CrossRef]
- Bartlett, M.A.; Mauck, K.F.; Stephenson, C.R.; Ganesh, R.; Daniels, P.R. Perioperative Venous Thromboembolism Prophylaxis. Mayo Clin. Proc. 2020, 95, 2775–2798. [Google Scholar] [CrossRef]
- Kakkos, S.K. Mechanical Thromboprophylaxis Is Effective in Reducing Postoperative Venous Thromboembolism. Br. J. Surg. 2022, 109, 667–668. [Google Scholar] [CrossRef]
- Yang, D.; Hou, X.; Fu, H.; Song, W.; Dong, W.; Wang, H.; Mao, Y.; Li, M.; Chen, J.; He, Y. Gastric Residual Volume, Safety, and Effectiveness of Drinking 250 ML of Glucose Solution 2–3 Hours before Surgery in Gastric Cancer Patients: A Multicenter, Single-Blind, Randomized–Controlled Trial. Gastroenterol. Rep. 2024, 12, goae077. [Google Scholar] [CrossRef]
- Yagmurdur, H.; Gunal, S.; Yildiz, H.; Gulec, H.; Topkaya, C. The Effects of Carbohydrate-Rich Drink on Perioperative Discomfort, Insulin Response and Arterial Pressure in Spinal Aesthesia. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2011, 16, 1483–1489. [Google Scholar]
- Benington, S. Preventing Aspiration and Regurgitation. Anaesth. Intensive Care Med. 2007, 8, 368–372. [Google Scholar] [CrossRef]
- Liu, D.S.; Stevens, S.G.; Watson, D.I.; Goh, S.K.; Muralidharan, V.; Wong, E.; Fong, J.; Wong, D.J. Optimal Timing of Perioperative Chemoprophylaxis in Patients With High Thromboembolic Risk Undergoing Major Abdominal Surgery: A Multicenter Cohort Study. Ann. Surg. 2023, 277, 79–86. [Google Scholar] [CrossRef]
- Cabral, F.; Ramos, P.; Monteiro, C.; Casaca, R. Impact of Perioperative Chemotherapy on Postoperative Morbidity after Gastrectomy for Gastric Cancer. Cirugía Española 2020, 9, 5–10. [Google Scholar]
- Bucx, M.J.L.; Krijtenburg, P.; Kox, M. Preoperative Use of Anxiolytic-Sedative Agents; Are We on the Right Track? J. Clin. Anesth. 2016, 33, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, A.; Saliou, P.; Lucet, J.C.; Mimoz, O.; Keita-perse, O.; Boisrenoult, P.; Lepelletier, D. Preoperative Hair Removal and Surgical Site Infections: Network Meta-Analysis of Randomized Controlled Trials. J. Hosp. Infect. 2015, 91, 100–108. [Google Scholar] [CrossRef]
- Mahajan, R.P. The WHO Surgical Checklist. Best Pract. Res. Clin. Anaesthesiol. 2011, 25, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.; Lumb, A.B.; Pelosi, P. Intraoperative Fraction of Inspired Oxygen: Bringing Back the Focus on Patient Outcome. Br. J. Anaesth. 2017, 119, 16–18. [Google Scholar] [CrossRef]
- Maitra, S.; Kirtania, J.; Pal, S.; Bhattacharjee, S.; Layek, A.; Ray, S. Intraoperative Blood Glucose Levels in Nondiabetic Patients Undergoing Elective Major Surgery under General Anaesthesia Receiving Different Crystalloid Solutions for Maintenance Fluid. Anesth. Essays Res. 2013, 7, 183–188. [Google Scholar] [CrossRef]
- Bruhn, J.; Myles, P.S.; Sneyd, R.; Struys, M.M.R.F. Depth of Anaesthesia Monitoring: What’s Available, What’s Validated and What’s Next? Br. J. Anaesth. 2006, 97, 85–94. [Google Scholar] [CrossRef]
- Anaesth, A. Intraoperative Management and Hemodynamic Monitoring for Ma—Jor Abdominal Surgery: A Narrative Review. Acta Anæsthesiologica Belg. 2021, 72, 63–71. [Google Scholar]
- Nicklas, J.Y.; Saugel, B. Non-Invasive Hemodynamic Monitoring for Hemodynamic Management in Perioperative Medicine. Front. Med. 2017, 4, 209. [Google Scholar] [CrossRef]
- Suh, J.; Lee, S.-W. Preoperative Prediction of the Need for Arterial and Central Venous Catheterization Using Machine Learning Techniques. Sci. Rep. 2022, 12, 11948. [Google Scholar] [CrossRef]
- Werneburg, G.T. Catheter-Associated Urinary Tract Infections: Current Challenges and Future Prospects. Res. Rep. Urol. 2022, 14, 109–133. [Google Scholar] [CrossRef]
- Lu, X.; Yu, Y.; Wang, Y.; Lyu, Y. Effect of Propofol or Etomidate as General Anaesthesia Induction on Gastric Cancer: A Retrospective Cohort Study with 10 Years’ Follow-Up. Cancer Manag. Res. 2022, 14, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Wetterslev, J.; Meyhoff, C.S.; Jørgensen, L.N.; Gluud, C.; Lindschou, J.; Rasmussen, L.S. The Effects of High Perioperative Inspiratory Oxygen Fraction for Adult Surgical Patients. Cochrane Database Syst. Rev. 2015, 2015, CD008884. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.C.-C.; Agarwala, A.; Bao, X. Perioperative Fluid Management in the Enhanced Recovery after Surgery (ERAS) Pathway. Clin. Colon Rectal Surg. 2019, 32, 114–120. [Google Scholar] [CrossRef]
- Gao, M.; Chen, M.; Dai, G.; Zhu, D.; Cai, Y. Clinical study: The impact of goal-directed fluid therapy on volume management during enhanced recovery after surgery in gastrointestinal procedures. Acta Biochim. Pol. 2024, 71, 12377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akyol, D.; Cukurova, Z.; Tulubas, E.K.; Yıldız, G.O.; Sabaz, M.S. Goal-Directed Fluid Therapy in Gastrointestinal Cancer Surgery: A Prospective Randomized Study. J. Acute Dis. 2022, 11, 52–58. [Google Scholar] [CrossRef]
- Harper, C.M.; McNicholas, T.; Gowrie-Mohan, S. Maintaining Perioperative Normothermia. BMJ 2003, 326, 721–722. [Google Scholar] [CrossRef]
- Liedl, H.J.C.; Lazenby, K.A.; Arimoto, R.S.; Singh, A.; Strelzow, J.A. Normothermia to Decrease Surgical Site Infection Risk: Silver Bullet or Fool’s Gold? A Retrospective Cohort Study. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2024, 8, e23. [Google Scholar] [CrossRef]
- Shao, L.; Zheng, H.; Jia, F.; Wang, H.; Liu, L.; Sun, Q.; An, M.; Zhang, X. Methods of Patient Warming during Abdominal Surgery. PLoS ONE 2012, 7, e39622. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, F.; Liang, C.; Huang, Y.; Zhao, Y.; Liu, C.; Lin, C.; Zhang, L.; Zhou, S.; Wang, Q.; et al. Fosaprepitant for Postoperative Nausea and Vomiting in Patients Undergoing Laparoscopic Gastrointestinal Surgery: A Randomised Trial. Br. J. Anaesth. 2023, 131, 673–681. [Google Scholar] [CrossRef]
- Choy, R.; Pereira, K.; Silva, S.G.; Thomas, N.; Tola, D.H. Use of Apfel Simplified Risk Score to Guide Postoperative Nausea and Vomiting Prophylaxis in Adult Patients Undergoing Same-Day Surgery. J. Perianesthesia Nurs. Off. J. Am. Soc. PeriAnesthesia Nurses 2022, 37, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Article, O.; Xu, L.; Yao, L.; Qin, J.; Xu, H. Efficacy of Multimodal Analgesia Based on the Concept of Enhanced Recovery after Surgery in Laparoscopic Radical Gastrectomy for Gastric Cancer. Pak. J. Med. Sci. 2024, 40, 2190–2195. [Google Scholar]
- Li, K.; Li, L.; Gao, M.; Zhu, Z.; Chen, P.; Yang, L.; Zhao, G. Application of Ultrasound-Guided Subcostal Transversus Abdominis Plane Block in Gastric Cancer Patients Undergoing Open Gastrectomy. Int. J. Clin. Exp. Med. 2015, 8, 13976–13982. [Google Scholar]
- Yoon, S.; Young, G.; Jihye, S.; Ho, L.; Lee, J.; Kong, S.H.; Kim, W.H.; Park, D.J.; Lee, H.J.; Yang, H.K. Ultrasound—Guided Bilateral Subcostal Transversus Abdominis Plane Block in Gastric Cancer Patients Undergoing Laparoscopic Gastrectomy: A Randomised—Controlled Double—Blinded Study. Surg. Endosc. 2022, 36, 1044–1052. [Google Scholar] [CrossRef]
- Rosen, D.R.; Wolfe, R.C.; Damle, A.; Atallah, C.; Chapman, W.C., Jr.; Vetter, J.M.; Mutch, M.G.; Hunt, S.R.; Glasgow, S.C.; Wise, P.E.; et al. Thoracic Epidural Analgesia: Does It Enhance Recovery? Dis. Colon Rectum 2019, 61, 1403–1409. [Google Scholar] [CrossRef]
- Hakkenbrak, N.A.G.; Jansma, E.P.; van der Wielen, N.; van der Peet, D.L.; Straatman, J. Laparoscopic versus Open Distal Gastrectomy for Gastric Cancer: A Systematic Review and Meta-Analysis. Surgery 2022, 171, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, J.; Su, K.; Dong, Z.; Wang, Z.; Chen, J.; Su, K.; Dong, Z. Abdominal Drainage versus No Drainage Post-Gastrectomy for Gastric Cancer. Cochrane Database Syst. Rev. 2015, 2015, CD008788. [Google Scholar] [CrossRef]
- Reasbeck, P. Routine Use of Nasogastric Drainage Tubes. Ann. R. Coll. Surg. Engl. 2019, 101, 537. [Google Scholar] [CrossRef] [PubMed]
- Kurz, A.; Sessler, D.I.; Lenhardt, R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N. Engl. J. Med. 1996, 334, 1209–1216. [Google Scholar] [CrossRef]
- Purwadadi, I.K.; Luh, N.; Inca, P.; Agustini, B.; Nuryanto, K. The Effectiveness of Blanket Warmer, Warm Intravenous Fluids, and Operating Room Temperature Modification in Preventing Perioperative Hypothermia. Jurnal Kesehat. 2024, 15, 134–143. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, N.K.; Baik, S.H.; Min, B.S.; Hur, H.; Lee, J.; Noh, H.-Y.; Lee, J.H.; Koo, B.-N. Effects of Postoperative Pain Management on Immune Function After Laparoscopic Resection of Colorectal Cancer: A Randomized Study. Medicine 2016, 95, e3602. [Google Scholar] [CrossRef]
- van der Veen, A.; Ramaekers, M.; Marsman, M.; Brenkman, H.J.F.; Seesing, M.F.J.; Luyer, M.D.P.; Nieuwenhuijzen, G.A.P.; Stoot, J.H.M.B.; Tegels, J.J.W.; Wijnhoven, B.P.L.; et al. Pain and Opioid Consumption After Laparoscopic Versus Open Gastrectomy for Gastric Cancer: A Secondary Analysis of a Multicenter Randomized Clinical Trial (LOGICA-Trial). J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2023, 27, 2057–2067. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Guo, K. Evaluation of the Effect of New Multimodal Analgesia Regimen for Cardiac Surgery: A Prospective, Randomized Controlled, Single-Center Clinical Study [Response to Letter]. Drug Des. Devel. Ther. 2023, 17, 2457–2460. [Google Scholar] [CrossRef]
- Jo, D.H.; Jeong, O.; Sun, J.W.; Jeong, M.R.; Ryu, S.Y.; Park, Y.K. Feasibility Study of Early Oral Intake after Gastrectomy for Gastric Carcinoma. J. Gastric Cancer 2011, 11, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Jønsson, L.R.; Foss, N.B.; Orbæk, J.; Lauritsen, M.L.; Sejrsen, H.N.; Kristensen, M.T. Early Intensive Mobilization after Acute High-Risk Abdominal Surgery: A Nonrandomized Prospective Feasibility Trial. Can. J. Surg. 2023, 66, E236–E245. [Google Scholar] [CrossRef]
- Porserud, A.; Aly, M.; Nygren-Bonnier, M.; Hagströmer, M. Association between Early Mobilisation after Abdominal Cancer Surgery and Postoperative Complications. Eur. J. Surg. Oncol. 2023, 49, 106943. [Google Scholar] [CrossRef] [PubMed]
- Tetzlaff, E.D.; Cheng, J.D.; Ajani, J.A. Thromboembolism in Gastrointestinal Cancers. Gastrointest. Cancer Res. 2008, 2, 267–272. [Google Scholar]
- Song, J.; Xuan, L.; Wu, W.; Huang, J.; Zhong, M. Low Molecular Weight Heparin Once versus Twice for Thromboprophylaxis Following Esophagectomy: A Randomised, Double-Blind and Placebo-Controlled Trial. J. Thorac. Dis. 2015, 7, 1158–1164. [Google Scholar] [CrossRef]
- Obrink, E.; Jildenstål, P.; Oddby, E.; Jakobsson, J.G. Post-Operative Nausea and Vomiting: Update on Predicting the Probability and Ways to Minimize Its Occurrence, with Focus on Ambulatory Surgery. Int. J. Surg. 2015, 15, 100–106. [Google Scholar] [CrossRef]
- Hur, H.; Kim, S.G.; Shim, J.H.; Song, K.Y.; Kim, W.; Park, C.H.; Jeon, H.M. Effect of Early Oral Feeding after Gastric Cancer Surgery: A Result of Randomized Clinical Trial. Surgery 2011, 149, 561–568. [Google Scholar] [CrossRef]
- Tegels, J.J.W.; De Maat, M.F.G.; Hulsewé, K.W.E.; Hoofwijk, A.G.M.; Stoot, J.H.M.B. Improving the Outcomes in Gastric Cancer Surgery. World J. Gastroenterol. 2014, 20, 13692–13704. [Google Scholar] [CrossRef] [PubMed]
- Willner, A.; Teske, C.; Hackert, T.; Welsch, T. Effects of Early Postoperative Mobilization Following Gastrointestinal Surgery: Systematic Review and Meta-Analysis. BJS Open 2023, 7, zrad102. [Google Scholar] [CrossRef] [PubMed]
- Gedda, C.; Nygren, J.; Garpenbeck, A.; Hoffström, L.; Thorell, A.; Soop, M. Multimodal Analgesia Bundle and Postoperative Opioid Use Among Patients Undergoing Colorectal Surgery. JAMA Netw. Open 2023, 6, e2332408. [Google Scholar] [CrossRef] [PubMed]
- Rourke, K.O.; Morrison, B.; Sen, S.; Jones, C. Fluid Management for Enhanced Recovery Surgery. Dig. Med. Res. 2019, 2, 37. [Google Scholar] [CrossRef]
- Meillat, H.; Magallon, C.; Brun, C.; Chaisemartin, C.D.; Moureau-zabotto, L. Coloproctology Systematic Early Urinary Catheter Removal Integrated in the Full Enhanced Recovery After Surgery (ERAS) Protocol After Laparoscopic Mid to Lower Rectal Cancer Excision: A Feasibility Study Coloproctology. Ann. Coloproctology 2021, 37, 204–211. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Ni, Y.; Liu, C. Analysis of postoperative pulmonary complications after gastrectomy for gastric cancer: Development and validation of a nomogram. Front. Surg. 2023, 10, 1308591. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Ravishankar, M.; Aravindan, U. Total Radical Gastrectomy under Continuous Thoracic Epidural Anaesthesia. Indian J. Anaesth. 2010, 54, 358–359. [Google Scholar] [CrossRef]
- Nakagawa, M.; Tokunaga, M.; Aburatani, T.; Sato, Y.; Matsuyama, T.; Nakajima, Y.; Kinugasa, Y. Feasibility and Safety of Early Oral Intake and Discharge After Total or Proximal Gastrectomy: An Analysis of Consecutive Cases Without Exclusion Criteria. Ann. Surg. Oncol. 2020, 27, 812–821. [Google Scholar] [CrossRef]
- Cananzi, F.C.M.; Biondi, A.; Agnes, A.; Ruspi, L.; Sicoli, F.; De Pascale, S.; Fumagalli, U.R.; D’Ugo, D.; Quagliuolo, V.; Persiani, R. Optimal Predictors of Postoperative Complications After Gastrectomy: Results from the Procalcitonin and C-Reactive Protein for the Early Diagnosis of Anastomotic Leakage in Esophagogastric Surgery (PEDALES) Study. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2023, 27, 478–488. [Google Scholar] [CrossRef]
- Guner, A.; Kim, K.Y.; Park, S.H.; Cho, M.; Kim, Y.M.; Hyung, W.J.; Kim, H.-I. Safe Discharge Criteria After Curative Gastrectomy for Gastric Cancer. J. Gastric Cancer 2022, 22, 395–407. [Google Scholar] [CrossRef]
- Choi, Y.Y. Decision for Safe Discharge After Gastric Cancer Surgery: The Finale of Enhanced Recovery After Surgery Program. J. Gastric Cancer 2022, 22, 261–263. [Google Scholar] [CrossRef]
- Kubota, T.; Shoda, K.; Konishi, H.; Okamoto, K.; Otsuji, E. Nutrition Update in Gastric Cancer Surgery. Ann. Gastroenterol. Surg. 2020, 4, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Karanicolas, P.J.; Graham, D.; Gönen, M.; Strong, V.E.; Brennan, M.F.; Coit, D.G. Quality of Life after Gastrectomy for Adenocarcinoma: A Prospective Cohort Study. Ann. Surg. 2013, 257, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Mithany, R.H.; Daniel, N.; Shahid, M.H.; Aslam, S.; Abdelmaseeh, M.; Gerges, F.; Gill, M.U.; Abdallah, S.B.; Hannan, A.; Saeed, M.T.; et al. Revolutionizing Surgical Care: The Power of Enhanced Recovery After Surgery (ERAS). Cureus 2023, 15, e48795. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, H. Enhanced Recovery After Surgery (ERAS): Good for Now, but What about the Future? Can. J. Anaesth. 2014, 62, 99–104. [Google Scholar] [CrossRef]
- Turaga, A.H. Enhanced Recovery After Surgery (ERAS) Protocols for Improving Outcomes for Patients Undergoing Major Colorectal Surgery. Cureus 2023, 15, e41755. [Google Scholar] [CrossRef]
- Hu, Y.; Hsu, A.W.; Strong, V.E. Enhanced Recovery After Major Gastrectomy for Cancer. Ann. Surg. Oncol. 2022, 28, 6947–6954. [Google Scholar] [CrossRef]
- Jeong, O.; Jang, A.; Jung, M.R.; Kang, J.H.; Ryu, S.Y. The Benefits of Enhanced Recovery after Surgery for Gastric Cancer: A Large before-and-after Propensity Score Matching Study. Clin. Nutr. 2021, 40, 2162–2168. [Google Scholar] [CrossRef]
- Sugisawa, N.; Tokunaga, M.; Makuuchi, R.; Miki, Y.; Tanizawa, Y.; Bando, E.; Kawamura, T.; Terashima, M. A Phase II Study of an Enhanced Recovery after Surgery Protocol in Gastric Cancer Surgery. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2016, 19, 961–967. [Google Scholar] [CrossRef]
- Liu, G.; Cao, S.; Liu, X.; Tian, Y.; Li, Z.; Sun, Y.; Zhong, H.; Wang, K.; Zhou, Y. Short- and Long-Term Outcomes Following Perioperative ERAS Management in Patients Undergoing Minimally Invasive Radical Gastrectomy after Neoadjuvant Chemotherapy: A Single-Center Retrospective Propensity Score Matching Study. Eur. J. Surg. Oncol. 2025, 51, 109459. [Google Scholar] [CrossRef]
- Jeong, O.; Jung, M.R. Enhanced Recovery After Surgery for Gastrectomy. Foregut Surg. 2023, 3, 65–74. [Google Scholar] [CrossRef]
- Davis, M.J.; Luu, B.C.; Raj, S.; Abu-Ghname, A.; Buchanan, E.P. Multidisciplinary Care in Surgery: Are Team-Based Interventions Cost-Effective? Surgeon 2021, 19, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Özçelik, M. Implementation of ERAS Protocols: In Theory and Practice. Turkish J. Anaesthesiol. Reanim. 2024, 52, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Wee, I.J.Y.; Syn, N.L.-X.; Shabbir, A.; Kim, G.; So, J.B.Y. Enhanced Recovery versus Conventional Care in Gastric Cancer Surgery: A Meta-Analysis of Randomized and Non-Randomized Controlled Trials. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2019, 22, 423–434. [Google Scholar] [CrossRef]
- Lombardi, P.M.; Mazzola, M.; Giani, A.; Baleri, S.; Maspero, M.; De Martini, P.; Gualtierotti, M.; Ferrari, G. ERAS Pathway for Gastric Cancer Surgery: Adherence, Outcomes and Prognostic Factors for Compliance in a Western Centre. Updates Surg. 2021, 73, 1857–1865. [Google Scholar] [CrossRef]
- Mariette, C. Role of the Nutritional Support in the ERAS Programme. J. Visc. Surg. 2015, 152, S18–S20. [Google Scholar] [CrossRef]
- Gillis, C.; Ljungqvist, O.; Carli, F. Prehabilitation, Enhanced Recovery after Surgery, or Both? A Narrative Review. Br. J. Anaesth. 2022, 128, 434–448. [Google Scholar] [CrossRef]
- Hart, N.H.; Crawford-Williams, F.; Crichton, M.; Yee, J.; Smith, T.J.; Koczwara, B.; Fitch, M.I.; Crawford, G.B.; Mukhopadhyay, S.; Mahony, J.; et al. Unmet Supportive Care Needs of People with Advanced Cancer and Their Caregivers: A Systematic Scoping Review. Crit. Rev. Oncol. Hematol. 2022, 176, 103728. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannidis, O.; Anestiadou, E.; Ramirez, J.M.; Fabbri, N.; Ubieto, J.M.; Feo, C.V.; Pesce, A.; Rosetzka, K.; Arroyo, A.; Kocián, P.; et al. Improving Perioperative Care in Gastric Surgery: Insights from the EUropean PErioperative MEdical Networking (EUPEMEN) Project. J. Clin. Med. 2025, 14, 2108. https://doi.org/10.3390/jcm14062108
Ioannidis O, Anestiadou E, Ramirez JM, Fabbri N, Ubieto JM, Feo CV, Pesce A, Rosetzka K, Arroyo A, Kocián P, et al. Improving Perioperative Care in Gastric Surgery: Insights from the EUropean PErioperative MEdical Networking (EUPEMEN) Project. Journal of Clinical Medicine. 2025; 14(6):2108. https://doi.org/10.3390/jcm14062108
Chicago/Turabian StyleIoannidis, Orestis, Elissavet Anestiadou, Jose M. Ramirez, Nicolò Fabbri, Javier Martínez Ubieto, Carlo Vittorio Feo, Antonio Pesce, Kristyna Rosetzka, Antonio Arroyo, Petr Kocián, and et al. 2025. "Improving Perioperative Care in Gastric Surgery: Insights from the EUropean PErioperative MEdical Networking (EUPEMEN) Project" Journal of Clinical Medicine 14, no. 6: 2108. https://doi.org/10.3390/jcm14062108
APA StyleIoannidis, O., Anestiadou, E., Ramirez, J. M., Fabbri, N., Ubieto, J. M., Feo, C. V., Pesce, A., Rosetzka, K., Arroyo, A., Kocián, P., Sánchez-Guillén, L., Bellosta, A. P., Whitley, A., Enguita, A. B., Teresa-Fernandéz, M., Bitsianis, S., & Symeonidis, S. (2025). Improving Perioperative Care in Gastric Surgery: Insights from the EUropean PErioperative MEdical Networking (EUPEMEN) Project. Journal of Clinical Medicine, 14(6), 2108. https://doi.org/10.3390/jcm14062108





