The Role of Neutrophil-to-Lymphocyte Ratio and Right Ventricular Dysfunction in Indonesian Patients with COVID-19: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Transthoracic Echocardiography
2.3. Data Collection
2.4. Clinical Outcomes and Statistical Analysis
3. Results
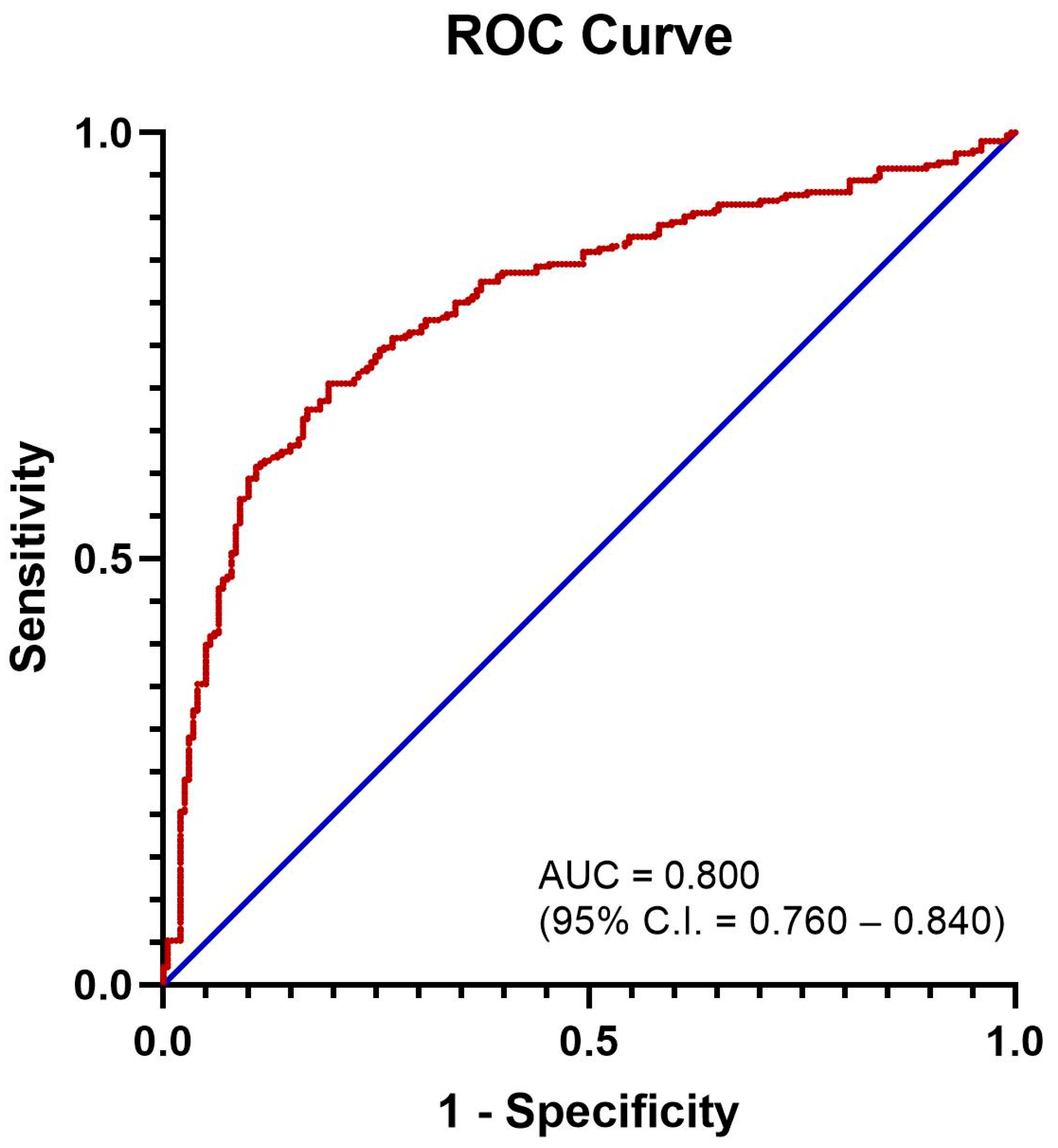
3.1. Defining an Elevated NLR
3.2. Factors Associated with RV Dysfunction
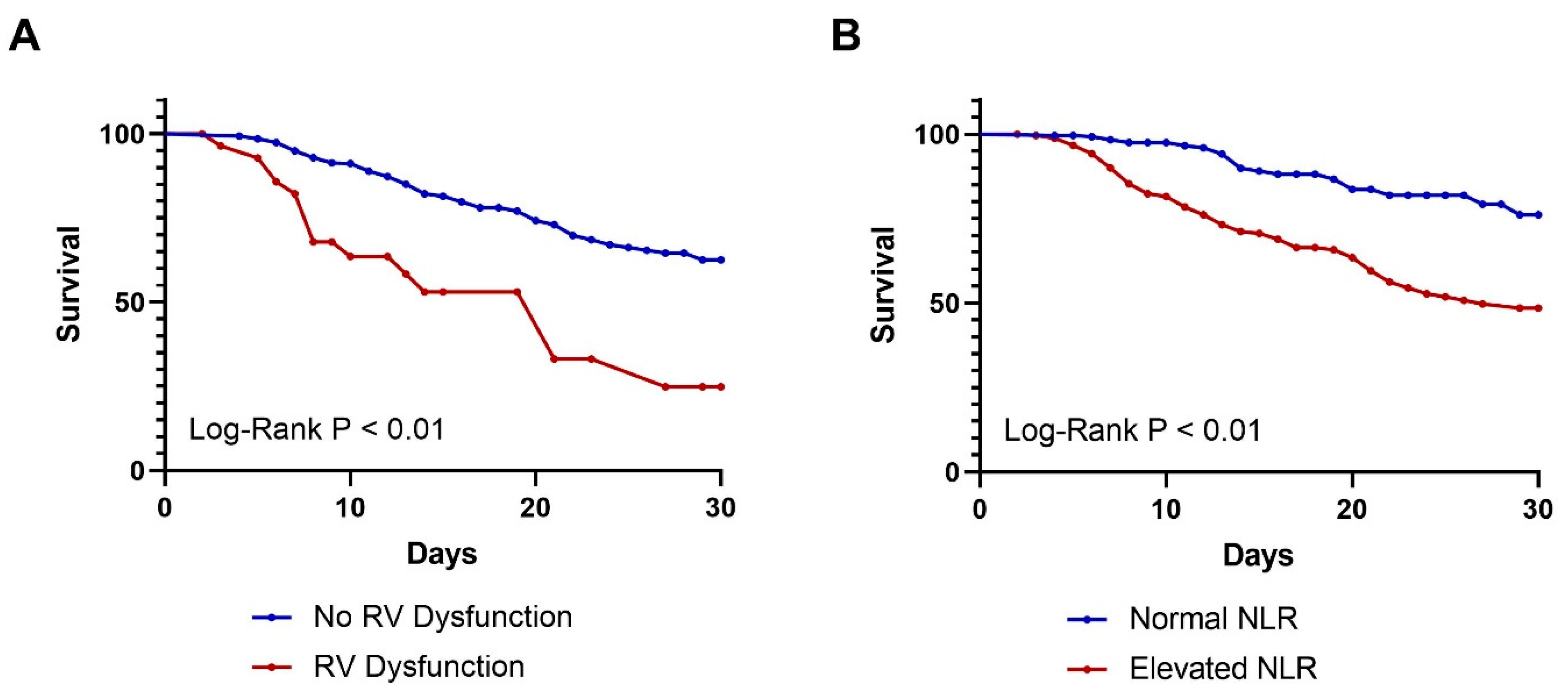
3.3. Analysis of 30-Day Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simadibrata, D.M.; Calvin, J.; Wijaya, A.D.; Ibrahim, N.A.A. Neutrophil-to-lymphocyte ratio on admission to predict the severity and mortality of COVID-19 patients: A meta-analysis. Am. J. Emerg. Med. 2021, 42, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Ssentongo, P.; Ssentongo, A.E.; Heilbrunn, E.S.; Ba, D.M.; Chinchilli, V.M. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0238215. [Google Scholar] [CrossRef]
- Kirkpatrick, J.N.; Swaminathan, M.; Adedipe, A.; Garcia-Sayan, E.; Hung, J.; Kelly, N.; Kort, S.; Nagueh, S.; Poh, K.K.; Sarwal, A.; et al. American Society of Echocardiography COVID-19 Statement Update: Lessons Learned and Preparation for Future Pandemics. J. Am. Soc. Echocardiogr. 2023, 36, 1127–1139. [Google Scholar] [CrossRef]
- Kim, J.; Volodarskiy, A.; Sultana, R.; Pollie, M.P.; Yum, B.; Nambiar, L.; Tafreshi, R.; Mitlak, H.W.; RoyChoudhury, A.; Horn, E.M.; et al. Prognostic Utility of Right Ventricular Remodeling Over Conventional Risk Stratification in Patients With COVID-19. J. Am. Coll. Cardiol. 2020, 76, 1965–1977. [Google Scholar] [CrossRef]
- Oweis, J.; Leamon, A.; Al-Tarbsheh, A.H.; Goodspeed, K.; Khorolsky, C.; Feustel, P.; Naseer, U.; Albaba, I.; Parimi, S.A.; Shkolnik, B.; et al. Influence of right ventricular structure and function on hospital outcomes in COVID-19 patients. Heart Lung 2023, 57, 19–24. [Google Scholar] [CrossRef]
- Corica, B.; Marra, A.M.; Basili, S.; Cangemi, R.; Cittadini, A.; Proietti, M.; Romiti, G.F. Prevalence of right ventricular dysfunction and impact on all-cause death in hospitalized patients with COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 17774. [Google Scholar] [CrossRef]
- Dweck, M.R.; Bularga, A.; Hahn, R.T.; Bing, R.; Lee, K.K.; Chapman, A.R.; White, A.; Salvo, G.D.; Sade, L.E.; Pearce, K.; et al. Global evaluation of echocardiography in patients with COVID-19. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Mboi, N.; Syailendrawati, R.; Ostroff, S.M.; Elyazar, I.R.; Glenn, S.D.; Rachmawati, T.; Nugraheni, W.P.; Ali, P.B.; Trisnantoro, L.; Adnani, Q.E.S.; et al. The state of health in Indonesia’s provinces, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Glob. Health 2022, 10, e1632–e1645. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, J.N.; Mitchell, C.; Taub, C.; Kort, S.; Hung, J.; Swaminathan, M. ASE Statement on Protection of Patients and Echocardiography Service Providers During the 2019 Novel Coronavirus Outbreak: Endorsed by the American College of Cardiology. J. Am. Soc. Echocardiogr. 2020, 33, 648–653. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Citu, C.; Gorun, F.; Motoc, A.; Sas, I.; Gorun, O.M.; Burlea, B.; Tuta-Sas, I.; Tomescu, L.; Neamtu, R.; Malita, D.; et al. The Predictive Role of NLR, d-NLR, MLR, and SIRI in COVID-19 Mortality. Diagnostics 2022, 12, 122. [Google Scholar] [CrossRef] [PubMed]
- Cakirca, G.; Cakirca, T.D.; Bindal, A.; Olcen, M. Inflammation-based Indices Predicting Mortality in COVID-19. J. Coll. Physicians Surg. Pak. 2023, 33, 112–114. [Google Scholar] [PubMed]
- Seyfi, S.; Azadmehr, A.; Ezoji, K.; Nabipour, M.; Babazadeh, A.; Saleki, K.; Mahmoodi, M.; Pouladi, A.H. Mortality in ICU COVID-19 Patients Is Associated with Neutrophil-to-Lymphocyte Ratio (NLR): Utility of NLR as a Promising Immunohematological Marker. Interdiscip. Perspect. Infect. Dis. 2023, 2023, 9048749. [Google Scholar] [CrossRef] [PubMed]
- Regolo, M.; Vaccaro, M.; Sorce, A.; Stancanelli, B.; Colaci, M.; Natoli, G.; Russo, M.; Alessandria, I.; Motta, M.; Santangelo, N.; et al. Neutrophil-to-Lymphocyte Ratio (NLR) Is a Promising Predictor of Mortality and Admission to Intensive Care Unit of COVID-19 Patients. J. Clin. Med. 2022, 11, 2235. [Google Scholar] [CrossRef]
- Andiani, F.; Herawati, R.; Triyani, Y. Correlation between NLR and PLR with the Severity of COVID-19 Inpatients. Indones. J. Clin. Pathol. Med. Lab. 2023, 29, 47–53. [Google Scholar] [CrossRef]
- Arini, I.A.; Masyeni, S.; Widhidewi, N.W. Relationship between neutrophil-lymphocyte ratio and platelet-lymphocyte ratio with the severity of COVID-19. Narra J. 2024, 4, e262. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Cavalcante-Silva, L.H.A.; Carvalho, D.C.M.; Lima, É.d.A.; Galvão, J.G.F.M.; da Silva, J.S.d.F.; Sales-Neto, J.M.d.; Rodrigues-Mascarenhas, S. Neutrophils and COVID-19: The road so far. Int. Immunopharmacol. 2021, 90, 107233. [Google Scholar] [CrossRef]
- Soulat-Dufour, L.; Fauvel, C.; Weizman, O.; Barbe, T.; Pezel, T.; Mika, D.; Cellier, J.; Geneste, L.; Panagides, V.; Marsou, W.; et al. Prognostic value of right ventricular dilatation in patients with COVID-19: A multicentre study. Eur. Heart J. Cardiovasc. Imaging 2022, 13, 569–577. [Google Scholar] [CrossRef]
- Zochios, V.; Parhar, K.; Tunnicliffe, W.; Roscoe, A.; Gao, F. The Right Ventricle in ARDS. Chest 2017, 152, 181–193. [Google Scholar] [CrossRef]
- Adeghate, E.A.; Eid, N.; Singh, J. Mechanisms of COVID-19-induced heart failure: A short review. Heart Fail Rev. 2021, 26, 363–369. [Google Scholar] [CrossRef] [PubMed]
| Entire Cohort (N = 488) | No RV Dysfunction (N = 459) | RV Dysfunction (N = 29) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Demographics and Past Medical History | |||||||
| Age, mean (SD) | 54.82 | 13.50 | 54.34 | 13.50 | 62.32 | 11.19 | 0.005 † |
| Males, N (%) | 283 | 58.0% | 261 | 56.9% | 22 | 75.9% | 0.044 |
| Obesity, N (%) | 225 | 46.1% | 214 | 46.6% | 11 | 37.9% | 0.362 |
| Hypertension, N (%) | 326 | 66.8% | 308 | 67.1% | 18 | 62.1% | 0.577 |
| Diabetes, N (%) | 188 | 38.5% | 172 | 37.5% | 16 | 55.2% | 0.057 |
| Dyslipidemia, N (%) | 29 | 5.9% | 28 | 6.1% | 1 | 3.4% | 1.000 ‡ |
| COPD, N (%) | 8 | 1.6% | 6 | 1.3% | 2 | 6.9% | 0.076 ‡ |
| CKD, N (%) | 99 | 20.3% | 90 | 19.6% | 9 | 31.0% | 0.138 |
| CHF, N (%) | 158 | 32.4% | 140 | 30.5% | 18 | 62.1% | <0.001 |
| CAD, N (%) | 149 | 30.5% | 131 | 28.5% | 18 | 62.1% | <0.000 |
| Malignancy, N (%) | 5 | 1.0% | 4 | 0.9% | 1 | 3.4% | 0.265 ‡ |
| Hematological Indices and Other Echocardiographic Findings | |||||||
| NLR at admission, mean (SD) | 6.85 | 6.33 | 6.52 | 5.91 | 11.93 | 9.82 | <0.001 † |
| Elevated NLR, N (%) | 241 | 49.5% | 218 | 47.6% | 23 | 79.3% | 0.001 |
| LA dilation, N (%) | 137 | 28.1% | 124 | 27.0% | 13 | 44.8% | 0.038 |
| LV systolic dysfunction, N (%) | 34 | 7.0% | 20 | 4.4% | 14 | 48.3% | <0.001 ‡ |
| LV diastolic dysfunction, N (%) | 220 | 45.2% | 210 | 45.9% | 10 | 34.5% | 0.233 |
| Admission outcomes | |||||||
| Severe COVID-19 disease, N (%) | 286 | 58.6% | 261 | 56.9% | 25 | 86.2% | 0.002 |
| 30-day mortality, N (%) | 121 | 24.8% | 105 | 22.9% | 16 | 55.2% | <0.001 |
| In-hospital mortality, N (%) | 134 | 27.5% | 118 | 25.7% | 16 | 55.2% | 0.001 |
| Length of stay, mean (SD) | 17.48 | 10.23 | 17.71 | 10.28 | 13.72 | 8.76 | 0.008 † |
| Covariates | Odds Ratio | p-Value |
|---|---|---|
| Male sex | 1.83 (0.65–5.14) | 0.249 |
| Age | 1.04 (0.99–1.08) | 0.094 |
| Obesity | 0.61 (0.24–1.55) | 0.302 |
| Hypertension | 0.99 (0.36–2.72) | 0.991 |
| Diabetes | 1.54 (0.62–3.81) | 0.354 |
| COPD | 2.63 (0.27–25.20) | 0.402 |
| CKD | 0.80 (0.29–2.23) | 0.669 |
| CHF | 1.60 (0.61–4.24) | 0.341 |
| CAD | 1.76 (0.69–4.51) | 0.237 |
| Dyslipidemia | 0.66 (0.06–7.47) | 0.736 |
| High NLR | 3.38 (1.19–9.59) | 0.022 |
| LA dilation | 1.21 (0.44–3.32) | 0.714 |
| LV systolic dysfunction | 9.76 (3.27–29.09) | <0.001 |
| LV diastolic dysfunction | 0.47 (0.18–1.24) | 0.127 |
| Variables | Hazard Ratio | p-Value |
|---|---|---|
| Male sex | 1.15 (0.80–1.65) | 0.462 |
| Age | 1.03 (1.01–1.04) | <0.001 |
| Obesity | 1.71 (1.19–2.46) | 0.004 |
| Hypertension | 0.94 (0.64–1.37) | 0.742 |
| Diabetes | 1.85 (1.29–2.66) | 0.001 |
| COPD | 0.84 (0.21–3.42) | 0.812 |
| CKD | 2.39 (1.65–3.46) | <0.001 |
| CHF | 1.05 (0.72–1.53) | 0.802 |
| CAD | 1.57 (1.09–2.26) | 0.014 |
| Malignancy | 0.77 (0.11–5.48) | 0.789 |
| Dyslipidemia | 0.92 (0.43–1.97) | 0.821 |
| High NLR | 3.38 (2.18–5.22) | <0.001 |
| RV dysfunction | 3.13 (1.85–5.30) | <0.001 |
| LA dilation | 1.39 (0.96–2.03) | 0.085 |
| LV systolic dysfunction | 2.38 (1.39–4.10) | 0.002 |
| LV diastolic dysfunction | 1.08 (0.75–1.54) | 0.691 |
| Variables | Hazard Ratio | p-Value |
|---|---|---|
| Age | 1.02 (1.01–1.04) | 0.010 |
| Obesity | 1.85 (1.28–2.67) | 0.001 |
| Diabetes | 1.37 (0.95–1.98) | 0.095 |
| CKD | 1.69 (1.13–2.52) | 0.010 |
| CAD | 1.19 (0.81–1.75) | 0.375 |
| High NLR | 2.75 (1.76–4.30) | <0.001 |
| RV dysfunction | 2.07 (1.14–3.76) | 0.017 |
| LV systolic dysfunction | 1.07 (0.57–2.02) | 0.830 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agarwal, R.; Krishnanda, S.I.; Yausep, O.E.; Nugraha, R.A.; Priyonugroho, G.; Hertine, S.; Wicaksono, S.H.; Almazini, P.; Zamroni, D.; Muliawan, H.S. The Role of Neutrophil-to-Lymphocyte Ratio and Right Ventricular Dysfunction in Indonesian Patients with COVID-19: A Retrospective Cohort Study. J. Clin. Med. 2025, 14, 2051. https://doi.org/10.3390/jcm14062051
Agarwal R, Krishnanda SI, Yausep OE, Nugraha RA, Priyonugroho G, Hertine S, Wicaksono SH, Almazini P, Zamroni D, Muliawan HS. The Role of Neutrophil-to-Lymphocyte Ratio and Right Ventricular Dysfunction in Indonesian Patients with COVID-19: A Retrospective Cohort Study. Journal of Clinical Medicine. 2025; 14(6):2051. https://doi.org/10.3390/jcm14062051
Chicago/Turabian StyleAgarwal, Raksheeth, Stanislaus Ivanovich Krishnanda, Oliver Emmanuel Yausep, Raka Aldy Nugraha, Gatut Priyonugroho, Siti Hertine, Sony Hilal Wicaksono, Prima Almazini, Dian Zamroni, and Hary Sakti Muliawan. 2025. "The Role of Neutrophil-to-Lymphocyte Ratio and Right Ventricular Dysfunction in Indonesian Patients with COVID-19: A Retrospective Cohort Study" Journal of Clinical Medicine 14, no. 6: 2051. https://doi.org/10.3390/jcm14062051
APA StyleAgarwal, R., Krishnanda, S. I., Yausep, O. E., Nugraha, R. A., Priyonugroho, G., Hertine, S., Wicaksono, S. H., Almazini, P., Zamroni, D., & Muliawan, H. S. (2025). The Role of Neutrophil-to-Lymphocyte Ratio and Right Ventricular Dysfunction in Indonesian Patients with COVID-19: A Retrospective Cohort Study. Journal of Clinical Medicine, 14(6), 2051. https://doi.org/10.3390/jcm14062051







