Abstract
Background: Systemic sclerosis (SSc) is a multisystem autoimmune disease characterised by fibrosis, vasculopathy, and immune dysfunction. Silica exposure has been associated with a more aggressive phenotype of the disease, including diffuse cutaneous involvement and interstitial lung disease. This study aims to identify proteomic differences between SSc patients exposed to silica and those not exposed to silica. Methods: An observational study of 32 SSc patients (11 silica-exposed and 21 non-exposed) was performed, with occupational history and quantitative proteomic analysis using SWATH-MS mass spectrometry. Differentially expressed proteins were analysed, and functional pathway enrichment was performed. Results: Eight proteins showed significant differences between groups, all with reduced levels in silica-exposed patients: adiponectin, immunoglobulins (IGLV3-19, IGLV2-18), complement C2, alpha-2-macroglobulin, vitronectin, cytoplasmic actin 2, and pigment epithelium-derived factor. Alterations in pathways related to fibrinolysis, complement activation, and inflammation were highlighted, suggesting that silica exposure may influence the pathogenesis of SSc and worsen its clinical course. Conclusions: This study supports the hypothesis that silica exposure is not only a triggering factor for SSc, but is also modulating its progression through inflammatory, procoagulant, and fibrotic pathways. The identification of proteomic biomarkers could contribute to the phenotypic classification of patients and the development of personalised therapies. Future studies should expand the cohort and further investigate the functional mechanisms of these proteins in SSc.
1. Introduction
Systemic sclerosis (SSc) is a systemic autoimmune disease that is much more common in women (ratio 9:1), with an estimated prevalence in our region’s population of 0.82/100,000 [1]. Its prognosis can vary widely due to the high variability of its clinical expression, with an overall 10-year survival rate of 65–92% [2].
SSc is a complex disease in which immunological, vascular, and fibrotic factors interact. The pathogenesis of SSc is based on three fundamental pillars: autoantibody production, endothelial damage, and fibrosis due to excessive collagen deposition. As a result of these mechanisms, the function of virtually any organ can be altered, with the most commonly affected organs being the skin, gastrointestinal tract, lungs, heart, and kidneys [3].
Although the cause of SSc is unknown, several hormonal (17β-estradiol and prolactin), genetic (some genes associated with the X chromosome, HLA, and non-HLA genetic regions), and environmental (exposure to toxins such as silica dust, organic solvents, or vinyl chloride) factors have been described [4,5,6,7]. Of all the toxicants involved, silica is the most common in most series. Furthermore, a higher proportion of diffuse skin disease, interstitial lung disease, and mortality have been described in patients with SSc and a history of exposure to silica [7], suggesting that the toxin may not only act as a triggering mechanism for the disease but may also condition its phenotypic expression by inducing the activation of a specific pathogenic pathway.
There are several hypotheses about the pathogenic mechanisms underlying silica exposure and the onset of autoimmune phenomena (Figure 1). However, little is known about the molecular mechanisms that trigger SSc in genetically predisposed individuals following exposure to the toxin.
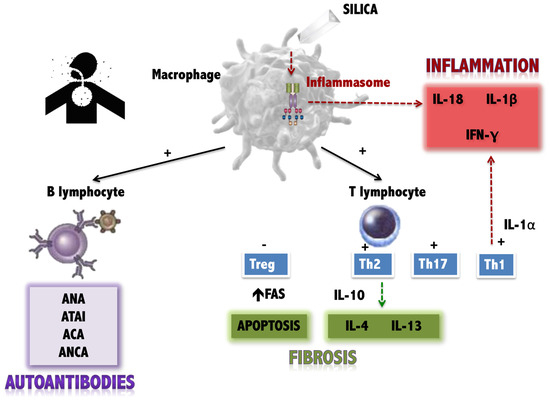
Figure 1.
Current hypotheses about the effects of silica on the immune system. Antigen-presenting cells (such as the macrophage) are stimulated by silica, producing three types of effects on the immune system: (1) Stimulation of the inflammasome, with the production of pro-inflammatory cytokines (IL-18, IL-1beta). (2) Stimulation of T lymphocytes (both the Th1 response, also pro-inflammatory, and the Th2 response and attenuation of regulatory T lymphocytes, which favour fibrosis. (3) Stimulation of the production of autoantibodies by B lymphocytes (own elaboration, based on data collected in [8,9,10].
Recently, Chairta et al. compiled and thoroughly analysed the candidate biomarkers in SSc discovered by mass spectrometry, collected from the 25 full-text studies published to date. This study showed that many proteins with different functions are involved in the pathogenesis of SSc, confirming the heterogeneity of the disease, as the reported deregulated proteins are involved in approximately 240 different pathogenic pathways. Therefore, we can say that different pathways and molecules are involved in different stages of SSc pathogenesis and different SSc subtypes [11]. Continued research into its molecular mechanisms is crucial to developing more effective therapies and improving the quality of life of patients.
The main objective of this work is to identify molecular patterns through proteomic analyses in a cohort of patients with SSc that will allow us to recognise a differentiating molecular signature between patients exposed and not exposed to silica dust. Identifying the predominant molecular pathways in the subset of exposed patients will allow hypotheses to be generated about the initial mechanisms of damage.
2. Material and Methods
2.1. Study Design
This observational study was conducted in patients with SSc who attended a routine follow-up consultation at the Systemic Autoimmune Diseases Unit in April 2018. All patients were diagnosed with SSc according to the ACR/EULAR 2013 criteria [12]. A work–life survey was performed, and exposure risk was assessed according to the specific recommendations for silicosis health surveillance of the Interterritorial Council of the National Health System of Spain [13]. Agriculture was also included among the occupations at risk of exposure to silica because digging in agriculture increases airborne silica exposure in farmworkers, as confirmed by lung autopsies showing higher mineral particle concentrations in agricultural workers [14,15]. furthermore, in our geographical environment, it is an activity carried out manually (digging with a hoe) on siliceous soil [16].
The inclusion and exclusion criteria were as follows:
- Inclusion criteria: patients exposed to silica dust were selected as cases (si-SSc) and patients not exposed as controls (nosi-SSc).
- The exclusion criteria were failure to provide written informed consent, comorbidity with another serious diagnosis or concomitant disease that could affect the results, pregnancy or lactation in women, or being a minor (<18 years).
2.2. Data Collection
- Working Life Epidemiological Survey.
Each patient was interviewed, and all the jobs they had in their life were recorded.
- Review of clinical and analytical data.
The computerised electronic history of each selected subject was accessed, and the most relevant clinical and analytical data were extracted.
2.3. Quantitative Proteomic Studies by Sequential Window Acquisition of All Theoretical Mass Spectrometry (SWATH MS) Method
- Protein Digestion.
The protein extract was loaded on a 10% SDS-PAGE (sodium dodecyl sulphate polyacrylamide gel electrophoresis) gel to the whole protein concentration. The gel was stained, and the band was exscinded and submitted to in-gel tryptic digestion, as described previously by our group [17,18], Peptides were extracted by carrying out three 20 min incubations in 40 μL of 60% ACN (acetonitrile) dissolved in 0.5% HCOOH (formic Acid), and then pooled, concentrated (SpeedVac, Thermo Fisher Scientific, Madrid, Spain), and stored at −2 °C.
- Mass Spectrometric Analysis by Sequential Window Acquisition of All Theoretical Mass Spectra (SWATH MS).
SWATH MS acquisition was performed on a TripleTOF® 6600 LC-MS/MS (liquid chromatography couplet to mas spectrometry) system (AB Sciex LLC, Framingham, MA, USA). To build the SWATH library, a pool of each condition was analysed by a shotgun data-dependent acquisition (DDA) approach by micro-LC-MS/MS, an analysis already described by our group [19,20,21,22,23,24,25]. After protein identification using ProteinPilot, the ion density found in the DDA runs was used to create the MSMS SWATH method, adjusting the 100 SWATH windows to this ion density. Therefore, all individual samples were analysed using a data-independent acquisition method. The targeted data extraction from the SWATH MS runs was performed by PeakView v.2.2 (AB Sciex LLC, Framingham, MA, USA) using the SWATH MS Acquisition MicroApp v.2.0 (AB Sciex LLC, Framingham, MA, USA), and the data were processed using the spectral library created from DDA. SWATH MS quantization was attempted for all proteins in the ion library, but only those that have 10 peptides and 7 transitions per peptide were used for protein quantization.
The integrated peak areas were processed by MarkerView software version v.1.3.1 (AB Sciex LLC, Framingham, MA, USA) for a data-independent method for relative quantitative analysis. To control for possible uneven sample loss across the different samples during the sample preparation process, we performed most likely ratio normalisation [26]. Unsupervised multivariate statistical analysis using PCA (principal component analysis) was performed to compare the data across the samples. A Student’s t-test analysis on the averaged area sums of all the transitions derived for each protein in every sample will indicate how well each variable distinguishes between the two groups, reported as a p-value. For each library, its set of differentially expressed proteins (p-value < 0.05) with a FCh > 2 or < 0.5 was selected.
- Functional Enrichment and Interaction Network Analysis:
We performed functional enrichment and interaction network proteomes using STRING: functional protein association networks, https://string-db.org/ (accessed on 8 January 2025) (free access at https://string-db.org). We conducted a pathway analysis using Reactome, https://reactome.org (accessed on 8 January 2025) (https://reactome.org). Volcano plots were performed using GraphPad Prism v.9.0.0 (GraphPad Software, San Diego, CA, USA).
2.4. Ethical Aspects
The study complies with the Declaration of Helsinki. The Ethical Committee from Pontevedra-Vigo-Ourense has approved (12 June 2017) the research protocol with the number 2017/303. Written informed consent has been obtained from the subjects.
3. Results
3.1. Selected Patients
Thirty-two patients with SSc were included in the study. Of these, 11 had a history of occupational silica exposure (si-SSc group). A further 21 patients had not been exposed (nosi-SSc group).
3.2. Occupational Exposure Study
Table 1 shows the details of the occupations of all patients. The most common occupation in the si-SSc group was agriculture (three patients, 27%), followed by marble working, stonemasonry, and foundry (three patients, 18% each); one patient worked in a paper mill and later in the manufacturing of dental prostheses, both occupations with a risk of exposure to silica; and one last patient worked in a stonemasonry workshop.

Table 1.
List of exposed and nonexposed patients with their professions.
3.3. Proteins with Statistically Significant Differences Between Exposed and Non-Exposed Groups
In the comparative study of the proteomic pattern between the si-SSc and nosi-SSc groups, statistically significant differences were found in eight proteins, all downregulated. (Table 2 and Figure 2): Adiponectin (log fold change −0.24), immunoglobulin lambda variable 3–19 (log fold change −0.21), immunoglobulin lambda variable 2–18 (log fold change −0.10), complement C2 (log fold change −0, 14), alpha-2-macroglobulin (log fold change −0.24), vitronectin (log fold change −0.12), actin, cytoplasmic 2 (log fold change −0.09), and pigment epithelium-derived factor (log fold change −0.37). A2M, PLG, and SERPINF1 stand out as hubs, indicating that they are essential in multiple processes related to the regulation of proteolysis, stress response, coagulation, and fibrinolysis (Figure 3). One part of the functional network is focused on coagulation and fibrinolysis (e.g., PLG, SERPINF1, CPB2), and another part is involved in the immune response (e.g., C4A, C9, CFH). The overlap between these domains suggests interrelated processes such as inflammation, tissue repair, and haemostatic regulation.

Table 2.
Differential proteins between si-SSc and nosi-SSc groups.
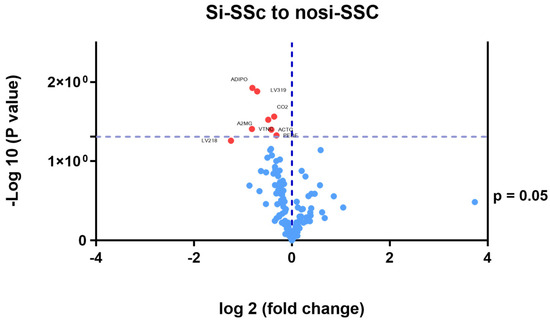
Figure 2.
Differential proteins between si-SSc and nosi-SSc groups. SSc; systemic sclerosis; Si-SSc: systemic sclerosis patients with silica exposure; nosi-SSc: systemic sclerosis patients without silica exposure; Adipo: adiponectin; LV319: immunoglobulin lambda variable 3–19; CO2: complement C2; A2MG: alfa 2-macroglobulin; VTNC: vitronectin; ACTG: cytoplasmic actin 2; PED: pigment epithelium-derived factor; LV218: immunoglobulin lambda variable 2–18.
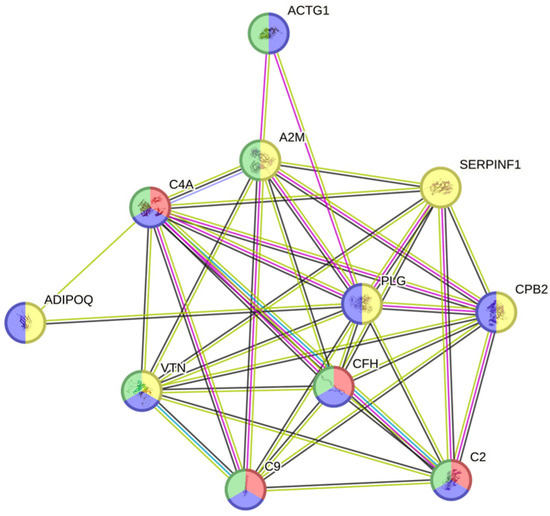
Figure 3.
Protein Interaction Network. ADIPOQ: adiponectin; ACTG1: actin, gamma 1; PLG: plasminogen; C9: complement component 9; C2: complement component 2; C4A: complement C4-A; SERPINF1: Serpin Family F Member 1; CPB2: Carboxypeptidase B2; VTN: vitronectin; A2M: alpha-2-macroglobulin; CFH: Complement Factor H.
- −
- Negative regulation of fibrinolysis (GO: 0051918)—Relevant proteins: A2M and PLG. Imbalances in this process may be associated with thrombosis or bleeding disorders. In autoimmune diseases, altered fibrinolysis may contribute to chronic inflammation.
- −
- Complement activation (GO: 0006956)—Relevant proteins: C4A, C2, and other components of the complement cascade. This pathway plays a key role in systemic autoimmune diseases. Its excessive activation can damage tissue and perpetuate inflammation.
- −
- Positive regulation of wound healing (GO: 0090303)—Relevant proteins: SERPINF1 and PLG. Critical for the resolution of inflammation and tissue remodelling. Deregulation could lead to fibrosis or repair defects.
- −
- Regulation of response to stimuli (GO: 0032102 and GO: 0048584)—Relevant proteins: A2M, SERPINF1, and C9. These proteins are involved in the modulation of inflammation. Changes in their function are associated with uncontrolled immune responses.
- −
- Coagulation and negative regulation of coagulation (GO: 0030195)—Relevant proteins: PLG and CPB2. Alterations in this balance contribute to thrombotic phenomena and cardiovascular disease.
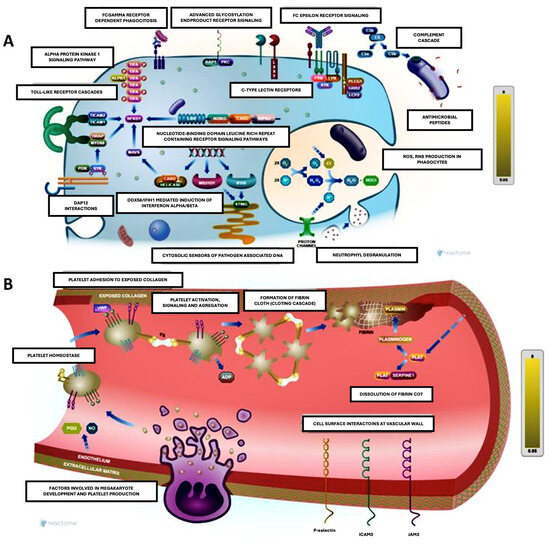
Figure 4.
Figure made with Reactome Pathway Browser. Both schemes integrate immune and inflammatory processes in defence and repair contexts, highlighting the interconnection between immunity and coagulation. (A): Immune activation and molecular recognition (left). This scheme shows immune activation pathways, including pattern recognition receptors (PRRs), which detect danger or damage signals (PAMPs and DAMPs). Some key points include the following: toll-like receptors (TLRs) to detect bacterial components such as lipopolysaccharide (LPS) and activate intracellular signalling, resulting in the production of inflammatory cytokines; Fc receptors to mediate the response to immune complexes (antibodies bound to antigens), activating phagocytes and other immune cells; complement cascade promotes the opsonization and destruction of pathogens, amplifying the inflammatory response; DAMP interactions involve mitochondrial or cytosolic DNA recognised by intracellular sensors, leading to inflammatory activation; and neutrophil degranulation releases antimicrobial factors and extracellular networks (NETs) to contain infection. (B): Hemostasis and platelet adhesion (right). This schematic illustrates platelet adhesion and clot formation in response to endothelial damage. Platelet adhesion: Platelets adhere to exposed collagen via receptors such as GPIb and GPIIb/IIIa, becoming activated. Platelet aggregation releases activating factors (e.g., ADP, thromboxane A2) that amplify platelet recruitment. Fibrin formation: thrombin converts fibrinogen to fibrin, stabilising the clot. Cell–vessel wall interactions: leukocytes adhere to endothelium via adhesion molecules such as selectins and integrins, contributing to inflammation.

Table 3.
Enrichment analysis.
Table 3.
Enrichment analysis.
| GO-Term | Description | Count in Network | Strength | Signal | False Discovery Rate |
|---|---|---|---|---|---|
| GO-0051918 | Negative regulation of fibrinolysis | 3 of 13 | 2.62 | 1.9 | 0.00032 |
| GO-0006956 | Complement activation | 4 of 60 | 2.08 | 1.78 | 0.00032 |
| GO-0090303 | Positive regulation of wound healing | 4 of 60 | 2.08 | 1.78 | 0.00032 |
| GO-0032102 | Negative regulation of response to external stimulus | 6 of 387 | 1.44 | 1.38 | 0.00032 |
| GO-0032101 | Regulation of response to external stimulus | 7 of 964 | 1.11 | 1.04 | 0.00032 |
| GO-0080134 | Regulation of response to stress | 8 of 1373 | 1.02 | 0.93 | 0.00032 |
| GO-0048584 | Positive regulation of response to stimulus | 9 of 2131 | 0.88 | 0.78 | 0.00032 |
| GO-0048583 | Regulation of response to stimulus | 11 of 3931 | 0.7 | 0.59 | 0.00032 |
| GO-0045861 | Negative regulation of proteolysis | 5 of 339 | 1.42 | 1.25 | 0.00082 |
| GO-0006958 | Complement activation, classical pathway | 3 of 40 | 2.13 | 1.47 | 0.0015 |
| GO-0006955 | Immune response | 7 of 1321 | 0.98 | 0.8 | 0.0015 |
| GO-0030195 | Negative regulation of blood coagulation | 3 of 46 | 2.07 | 1.42 | 0.0018 |
| GO-0002376 | Immune system process | 8 of 2121 | 0.83 | 0.66 | 0.0018 |
4. Discussion
SSc is a chronic, multisystem autoimmune disease characterised by cutaneous and visceral fibrosis, vasculopathy, and immune system changes. This results in a variety of changes in the molecular mechanisms underlying SSc, which are multifactorial and not yet fully understood in terms of how they interact to develop the different symptoms in these patients. The disease begins with microvascular injury, leading to endothelial cell dysfunction and profound molecular changes in the vascular system, such as endothelin-1 signalling [27].
SSc is also associated with a strong, dysregulated autoimmune response, including the production of specific autoantibodies (e.g., anti-topoisomerase I, anti-centromere, and anti-RNA polymerase III antibodies) or chronic inflammation with the high production of profibrotic cytokines (TGF-b, IL-3, IL-4, IL-6 and IL21) [28,29,30].
The present study focuses on analysing the proteomic differences between patients with systemic sclerosis (SSc), where we compared SSc patients exposed to silica dust with those not exposed. This research provides important information on the molecular mechanisms and pathogenic pathways involved, especially considering that silica exposure is associated with a higher prevalence of severe manifestations of the disease, such as diffuse cutaneous disease and interstitial lung disease. The findings add to the knowledge of the heterogeneity of SSc and how environmental factors influence its development and phenotypic expression.
4.1. Differential Proteins
This study identified eight proteins that were differentially regulated between the groups analysed. These proteins, all of which were reduced in the silica-exposed group, include adiponectin, immunoglobulins (IGLV3-19 and IGLV2-18), complement C2, alpha-2-macroglobulin (A2M), vitronectin (VTN), cytoplasmic actin 2, and pigment epithelium-derived factor (PEDF). Each of these molecules is involved in biological processes relevant to the pathogenesis of SSc, highlighting their potential contribution to the more severe phenotype observed in exposed patients.
- Adiponectin:
Its low expression in patients exposed to silica may contribute to endothelial dysfunction, chronic inflammation, and fibrosis, which are central processes in SSc. This protein has anti-inflammatory, anti-fibrotic, and vasculoprotective properties, which may be protective mechanisms lacking in the exposed group. Skin biopsies from patients with SSc have shown a reduction in the number and size of adipocytes (a major source of adiponectin) and their replacement by a fibrous matrix. In addition, lower levels of adiponectin have been reported in patients with SSc, both in the circulation and in skin, lung, and gastroscopic biopsies, compared to healthy controls, and significantly lower serum concentrations have been reported in patients with dcSSc compared to lcSSc [31,32,33]. Finally, an inverse correlation has been described between adiponectin levels in patients with SSc and the mRSS index, disease activity and progression, and a higher prevalence of scarring and pulmonary fibrosis [34,35].
- Immunoglobulin lambda variable 3–19 (IGLV3-19) and immunoglobulin lambda variable 2–18 (IGLV2-18):
This is a region of the variable domain of immunoglobulin light chains. Changwan Ryu et al. identified a proteomic profile in plasma samples from patients with SSc-ILD associated with platelet activation, cell adhesion, and immune responses, with IGLV3-19 being one of the thirty-eight proteins whose levels differed from healthy controls (upregulated, log 2-fold change 1.90) [36]. A positive correlation of IGLV3-19 with activated T cells and memory B cells has been reported in patients with SLE [37], which could imply its participation in immune hyperactivation and the production of autoantibodies, which could also be extended to other autoimmune diseases such as systemic sclerosis. Specifically, in this study, we describe IGLV-19 level differences between SSc patients exposed to silica and those not exposed. IGLV2-18, another variable domain region of immunoglobulin light chains, has never been reported as a differential marker in patients with SSc.
- Complement C2:
Reduced complement C2 may reflect the activation-associated consumption of the complement system, a common finding in autoimmune diseases. This activation perpetuates inflammation and tissue damage, which is likely to be exacerbated by exposure to toxicants. C2 deficiency has been linked to certain autoimmune diseases. In particular, complete genetic deficiency of complement component C2 is a strong risk factor for monogenic systemic lupus erythematosus (SLE), and partial deficiencies of C2 and C4A are important risk factors for SLE and primary Sjögren’s syndrome [27]. In a small study of 40 patients with scleroderma, Venneker et al. found that the incidence of partial C2 deficiency was higher than expected in patients with SSc [28]. On the other hand, complement activation is partly responsible for the tissue damage observed in autoimmune patients. Therefore, hypocomplementemia is a characteristic finding in patients with SLE, Sjögren’s syndrome, or antiphospholipid syndrome with active disease. However, in SSc, complement depletion is a less common finding, accounting for 15% of series, has been associated with the SSc activity index, functional disability, and the severity of general, cutaneous, vascular, cardiac, and pulmonary manifestations [29], and may be used to identify a specific subgroup of SSc patients with other overlapping connective tissue diseases [30].
- α2-macroglobulin (A2M) and vitronectin (VTN):
These proteins involved in coagulation and fibrinolysis are downregulated in exposed patients. Birkenmeier et al. [38] showed that patients with progressive systemic sclerosis have a state of hypercoagulation and hypofibrinolysis, suggesting that platelet and vascular dysfunction may play a key role in the pathogenesis of the disease, contributing to the characteristic microangiopathy. Our results are consistent with this hypothesis, since A2M could play a role in inhibiting plasmin (inhibiting fibrinolysis), thus contributing to the hypercoagulable state characteristic of the disease, and vitronectin could be related to the increase in fibrosis and endothelial dysfunction. On the other hand, in the study by Gundogdu et al. [39], serum levels of VTN were found to be lower in the SSc group compared to patients with SLE or healthy controls, suggesting the accumulation of VTN in fibrotic skin and subcutaneous tissues as a cause, which has already been demonstrated in experimental models of renal fibrosis [40].
- Actin, cytoplasmic 2:
Cytoplasmic actin 2, or gamma actin, is a protein that is widely expressed in the cellular cytoskeleton of muscle cells. Fibrosis is the hallmark of SSc and results from the excessive accumulation of the extracellular matrix in the skin and internal organs. This is associated with tissue damage, and one effect of this damage is the release of cytoskeletal proteins such as actin. Kimberly Showalter [41] mentions smooth muscle actin as a key marker in the cutaneous histology of SSc, as it is a protein expressed by myofibroblasts, cells involved in the cutaneous fibrosis characteristic of the disease, and it has been found that α-SMA levels in skin biopsies correlate with the severity of systemic sclerosis and with the response to treatment in clinical trials of antifibrotic drugs.
- Pigment epithelium-derived factor (PEDF):
This is a potent inhibitor of angiogenesis. Proteomic studies have identified PEDF as one of the most abundant proteins secreted by SSc skin fibroblasts compared to healthy controls [42]. A recent study showed that dermal fibroblasts in SSc play a direct role in the impairment of angiogenesis through the secretion of PEDF [43]. TGF-β signalling in fibroblasts was found to suppress angiogenesis via the secretion of PEDF, and this pathway remains active in explanted SSc skin fibroblasts. However, no increase in serum PEDF levels was found in dcSSc patients compared to healthy controls, consistent with the known paracrine mode of action of PEDF. Interestingly, PEDF has been shown to have an anti-fibrotic effect in a chemically induced liver fibrosis model, so it cannot be excluded that the TGF-β-induced PEDF expression observed in both SSc and idiopathic pulmonary fibrosis may represent an attempt to negatively feedback the fibrotic process [44].
4.2. Biological Pathway Analysis
Enrichment analysis highlights key processes such as the downregulation of fibrinolysis, complement activation, and immune response (Table 3 and Figure 4). These pathways reinforce the idea that silica exposure may not only act as a trigger for the disease but can also shape its clinical course by activating specific pathways that contribute to a more aggressive phenotype.
- Regulation of fibrinolysis and coagulation: Imbalances in these pathways may explain the thrombotic and vascular events observed in patients with SSc, particularly those with silica exposure.
- Complement activation: This key pathogenic mechanism in autoimmunity is relevant in the exposed subgroup, possibly associated with exacerbated inflammation and more pronounced tissue damage.
- Regulation of wound healing: Proteins related to this process, such as SERPINF1, suggest altered tissue remodelling favouring fibrosis, a characteristic finding in SSc.
4.3. Clinical Relevance
Treatment for SSc is multidisciplinary and focuses on controlling symptoms, preventing complications and delaying disease progression. As the disease develops for different reasons, treatment and even the response to drugs can vary. This work highlights the importance of identifying proteomic biomarkers that allow patients to be stratified into phenotypic subgroups, which could facilitate the development of targeted therapies. GLV3-19, associated with B-cell activation and autoantibody production, could be targeted through monoclonal antibodies (e.g., belimumab) to reduce immune hyperactivity. Complement C2 modulation via complement inhibitors (e.g., eculizumab) may help control inflammation and tissue damage. Adiponectin, with its anti-inflammatory and antifibrotic properties, could be therapeutically increased through receptor agonists or gene therapy. Alpha-2-macroglobulin (A2M) and vitronectin (VTN), both involved in coagulation and fibrosis, represent targets for thrombotic and vascular complications in autoimmune diseases. Finally, the pigment epithelium-derived factor (PEDF), implicated in angiogenesis regulation, could be modulated to improve vascular dysfunction in SSc.
Similarly, a blood-based biomarker panel measuring IGLV3-19, complement C2, adiponectin, A2M, VTN, and PEDF could aid in early disease detection and patient stratification. Prognostically, these markers could help predict disease progression and complications, such as fibrosis or vascular dysfunction. Additionally, they could be used to monitor treatment response, adjusting therapies in real-time based on biomarker levels, such as IGLV3-19 reduction after B-cell depletion therapy or adiponectin increases in antifibrotic treatment.
4.4. Limitations and Future Directions
Although this study provides a solid basis for investigating the effects of silica exposure on SSc, the relatively small sample size limits the generalisability of the results. Future research should include larger cohorts and assess the longitudinal effects of silica exposure on disease progression. In addition, functional studies investigating the specific roles of the identified proteins in SSc pathogenesis will be crucial to confirm their biological and clinical relevance.
To overcome the limitations of the current study, future research should focus on larger, multicentre studies to enhance the statistical power and generalizability of the findings. Expanding the sample size would allow for the better differentiation of disease subtypes and reduce biases introduced by small cohorts. Multicentric studies involving diverse populations would help validate the identified protein biomarkers across different genetic backgrounds and environmental exposures, ensuring their clinical relevance and reproducibility.
Additionally, a functional validation of the identified proteins is crucial to confirm their role in disease mechanisms and their potential as therapeutic targets. This could be achieved through in vitro and in vivo studies, including the following:
- −
- Cell culture experiments to analyse how these proteins influence immune cell activation, fibrosis, and vascular dysfunction.
- −
- Animal models of SSc to determine the impact of modulating these proteins on disease progression.
- −
- CRISPR-based gene editing or RNA interference (siRNA/shRNA) to assess the direct effects of silencing or overexpressing these biomarkers in relevant cell types.
- −
- Longitudinal patient studies to correlate protein expression levels with disease activity and treatment response over time.
5. Conclusions
This analysis reinforces the idea that silica exposure not only increases the risk of developing systemic sclerosis but also modulates its progression by altering pathways related to fibrinolysis, inflammation, coagulation, and fibrosis. In the words of Dr Steen, scleroderma is the disease of many faces [45]. Underlying this phenotypic variability may be different pathogenic pathways triggered by different external factors, many of which are toxic. Studying these interactions between environmental factors and molecular biology may open new doors to prognostic factors that will help in disease monitoring and more effective and personalised therapies.
Author Contributions
Conceptualization, A.L.; Methodology, B.S. and C.S.; Software, A.L.; Validation, A.L.; Formal analysis, S.B.; Investigation, M.F., S.B., C.S., A.A. and C.P.; Resources, A.A. and M.E.; Data curation, A.A., M.E. and C.P.; Writing—original draft, M.F. and M.N.; Writing—review & editing, A.G.-Q.; Visualization, M.N.; Supervision, B.S. and A.G.-Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study complies with the Declaration of Helsinki. The Ethical Committee from Pontevedra-Vigo-Ourense has approved the research protocol with the number 2017/303 (12 June 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available on request due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arias-Nuñez, M.C.; Llorca, J.; Vazquez-Rodriguez, T.R.; Gomez-Acebo, I.; Miranda-Filloy, J.A.; Martin, J.; Gonzalez-Juanatey, C.; Gonzalez-Gay, M.A. Systemic sclerosis in northwestern Spain: A 19-year epidemiologic study. Medicine 2008, 87, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Tyndall, A.J.; Bannert, B.; Vonk, M.; Airò, P.; Cozzi, F.; Carreira, P.E.; Bancel, D.F.; Allanore, Y.; Müller-Ladner, U.; Distler, O.; et al. Causes and risk factors for death in systemic sclerosis: A study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann. Rheum. Dis. 2010, 69, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Skarmoutsou, E.; Mazzarino, M.C. The sex bias in systemic sclerosis: On the possible mechanisms underlying the female disease preponderance. Clin. Rev. Allergy Immunol. 2014, 47, 334–343. [Google Scholar] [CrossRef]
- Chairta, P.; Nicolaou, P.; Christodoulou, K. Genomic and genetic studies of systemic sclerosis: A systematic review. Hum. Immunol. 2017, 78, 153–165. [Google Scholar] [CrossRef]
- Marie, I.; Menard, J.-F.; Duval-Modeste, A.-B.; Joly, P.; Dominique, S.; Bravard, P.; Noël, D.; Gehanno, J.-F.; Bubenheim, M.; Benichou, J.; et al. Association of occupational exposure with features of systemic sclerosis. J. Am. Acad. Dermatol. 2015, 72, 456–464. [Google Scholar] [CrossRef]
- Freire, M.; Alonso, M.; Rivera, A.; Sousa, A.; Soto, A.; Gómez-Sousa, J.M.; Baroja, A.; Vázquez-Triñanes, C.; Sopeña, B. Clinical peculiarities of patients with scleroderma exposed to silica: A systematic review of the literature. Semin. Arthritis Rheum. 2015, 45, 294–300. [Google Scholar] [CrossRef]
- Ferri, C.; Artoni, E.; Sighinolfi, G.L.; Luppi, F.; Zelent, G.; Colaci, M.; Giuggioli, D. High serum levels of silica nanoparticles in systemic sclerosis patients with occupational exposure: Possible pathogenetic role in disease phenotypes. Semin. Arthritis Rheum. 2018, 48, 475–481. [Google Scholar] [CrossRef]
- Lee, S.; Hayashi, H.; Mastuzaki, H.; Kumagai-Takei, N.; Otsuki, T. Silicosis and autoimmunity. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 78–84. [Google Scholar] [CrossRef]
- Pollard, K.M. Silica, Silicosis, and Autoimmunity. Front Immunol. 2016, 7, 97. [Google Scholar] [CrossRef]
- Chairta, P.P.; Nicolaou, P.; Christodoulou, K. Enrichr in silico analysis of MS-based extracted candidate proteomic biomarkers highlights pathogenic pathways in systemic sclerosis. Sci. Rep. 2023, 13, 1934. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Arana, V.; Arias, M.; Ariza, M.; Arzallus, M.; Cabrerizo, J.I.; Carballo, M.; Fernández, P.; Fernández, A.; Fernández, E.; Freijo, A.; et al. Protocolo de Vigilancia Sanitaria Específica. Silicosis Comisión de Salud Pública. Consejo Interterritorial del Sistema Nacional de Salud. MInisterio de Sanidad. Gobierno de España. Available online: https://www.sanidad.gob.es/ciudadanos/saludAmbLaboral/docs/silicosis.pdf (accessed on 13 March 2024).
- Leung, C.C.; Yu, I.T.S.; Chen, W. Silicosis. Lancet 2012, 379, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- Schenker, M.B.; Pinkerton, K.E.; Mitchell, D.; Vallyathan, V.; Elvine-Kreis, B.; Green, F.H. Pneumoconiosis from agricultural dust exposure among young California farmworkers. Environ. Health Perspect. 2009, 117, 988–994. [Google Scholar] [CrossRef]
- Ferrero Arias, Á.; Rubio Navas, J.; Pérez Cerdán, F.; Baltuille Martín, J.M.; Fernández Suárez, J. Mapa de Rocas y Minerales Industriales de Galicia; Instituto Geológico y Minero de España: Madrid, Spain, 2008. [Google Scholar]
- Alonso-Sampedro, M.; Feás, X.; Bravo, S.B.; Chantada-Vázquez, M.P.; Vidal, C. Proteomics of Vespa velutina nigrithorax Venom Sac Queens and Workers: A Quantitative SWATH-MS Analysis. Toxins 2023, 15, 266. [Google Scholar] [CrossRef]
- Feás, X.; Alonso-Sampedro, M.; Bravo, S.B.; Vidal, C. Peeking into the Stingers: A Comprehensive SWATH-MS Study of the European Hornet Vespa crabro (Linnaeus, 1758) (Hymenoptera: Vespidae) Venom Sac Extracts. Int. J. Mol. Sci. 2024, 25, 3798. [Google Scholar] [CrossRef]
- Álvarez, J.V.; Bravo, S.B.; Chantada-Vázquez, M.P.; Pena, C.; Colón, C.; Tomatsu, S.; Otero-Espinar, F.J.; Couce, M.L. Morquio A Syndrome: Identification of Differential Patterns of Molecular Pathway Interactions in Bone Lesions. Int. J. Mol. Sci. 2024, 25, 3232. [Google Scholar] [CrossRef]
- Casado-Fernández, L.; Laso-García, F.; Piniella, D.; Frutos, M.C.G.-D.; Otero-Ortega, L.; Bravo, S.-B.; Fuentes-Gimeno, B.; Docando, F.; Alonso-López, E.; Ruiz-Ares, G.; et al. The proteomic signature of circulating extracellular vesicles following intracerebral hemorrhage: Novel insights into mechanisms underlying recovery. Neurobiol. Dis. 2024, 201, 106665. [Google Scholar] [CrossRef]
- Fondevila, M.F.; Novoa, E.; Fernandez, U.; Dorta, V.; Parracho, T.; Kreimeyer, H.; Garcia-Vence, M.; Chantada-Vazquez, M.P.; Bravo, S.B.; Porteiro, B.; et al. Inhibition of hepatic p63 ameliorates steatohepatitis with fibrosis in mice. Mol. Metab. 2024, 85, 101962. [Google Scholar] [CrossRef]
- Pérez-Mato, M.; Dopico-López, A.; Akkoc, Y.; López-Amoedo, S.; Correa-Paz, C.; Candamo-Lourido, M.; Iglesias-Rey, R.; López-Arias, E.; Bugallo-Casal, A.; da Silva-Candal, A.; et al. Preclinical validation of human recombinant glutamate-oxaloacetate transaminase for the treatment of acute ischemic stroke. iScience 2024, 27, 111108. [Google Scholar] [CrossRef]
- Rodrigues, J.S.; Chenlo, M.; Bravo, S.B.; Perez-Romero, S.; Suarez-Fariña, M.; Sobrino, T.; Sanz-Pamplona, R.; González-Prieto, R.; Blanco Freire, M.N.; Nogueiras, R.; et al. dsRNAi-mediated silencing of PIAS2beta specifically kills anaplastic carcinomas by mitotic catastrophe. Nat. Commun. 2024, 15, 3736. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, G.T.; López-Molina, M.; Botella, L.; Laso-García, F.; Chamorro, B.; Fernández-Fournier, M.; Puertas, I.; Bravo, S.B.; Alonso-López, E.; Díez-Tejedor, E.; et al. Differential Protein Expression in Extracellular Vesicles Defines Treatment Responders and Non-Responders in Multiple Sclerosis. Int. J. Mol. Sci. 2024, 25, 10761. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Mera, S.; Martelo-Vidal, L.; Miguéns-Suárez, P.; Bravo, S.B.; Saavedra-Nieves, P.; Arias, P.; Ferreiro-Posse, A.; Vázquez-Lago, J.; Salgado, F.J.; González-Barcala, F.J.; et al. Exploring CD26−/lo subpopulations of lymphocytes in asthma phenotype and severity: A novel CD4+ T cell subset expressing archetypical granulocyte proteins. Allergy 2024, 79, 3005–3021. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.-P.; Ivosev, G.; Couzens, A.L.; Larsen, B.; Taipale, M.; Lin, Z.-Y.; Zhong, Q.; Lindquist, S.; Vidal, M.; Aebersold, R.; et al. Mapping differential interactomes by affinity purification coupled with data-independent mass spectrometry acquisition. Nat. Methods 2013, 10, 1239–1245. [Google Scholar] [CrossRef]
- Riccardi, M.T.; Chialà, A.; Lannone, F.; Grattagliano, V.; Covelli, M.; Lapadula, G. Treatment of digital ulcers in systemtic sclerosis with endothelin-1 receptor antagonist (bosentan). Reumatismo 2007, 59, 135–139. [Google Scholar] [CrossRef][Green Version]
- Alsulimani, A.; Das, S.; Akhter, N.; Ahmad, A.; Jawed, A.; Beigh, S.; Zamzami, M.A.; Al-Thawadi, S.; Alfoud, M.Y.; Banerjee, B.D.; et al. Pesticide exposure promotes disease activity by decreasing lymphoproliferative activity and increasing IL-4 production in systemic sclerosis patients. Immunopharmacol. Immunotoxicol. 2025, 47, 112–119. [Google Scholar] [CrossRef]
- Dlala, A.; Gabsi, A.; Salem, K.B.; Boutabba, A.; Nacer, I.; Missaoui, F.; Neili, B.; Saïd, F.; Smiti-Khanfir, M.; Triki-Marrakchi, R. Elevated Interleukin-21 expression is correlated with systemic sclerosis’ severity in Tunisian patients. Hum. Immunol. 2024, 85, 111154. [Google Scholar] [CrossRef]
- Kirichenko, T.V.; Bogatyreva, A.I.; Gerasimova, E.V.; Popkova, T.V.; Markina, Y.V.; Markin, A.M.; Gerasimova, D.A.; Orekhov, A.N. Inflammatory Response of Monocytes/Macrophages in Patients with Systemic Sclerosis. Front. Biosci.-Landmark 2024, 29, 259. [Google Scholar] [CrossRef]
- Brezovec, N.; Perdan-Pirkmajer, K.; Čučnik, S.; Sodin-šemrl, S.; Varga, J.; Lakota, K. Adiponectin deregulation in systemic autoimmune rheumatic diseases. Int. J. Mol. Sci. 2021, 22, 4095. [Google Scholar] [CrossRef]
- Arakawa, H.; Jinnin, M.; Muchemwa, F.C.; Makino, T.; Kajihara, I.; Makino, K.; Honda, N.; Sakai, K.; Fukushima, S.; Ihn, H. Adiponectin expression is decreased in the involved skin and sera of diffuse cutaneous scleroderma patients. Exp. Dermatol. 2011, 20, 764–766. [Google Scholar] [CrossRef]
- Neumann, E.; Lepper, N.; Vasile, M.; Riccieri, V.; Peters, M.; Meier, F.; Hülser, M.L.; Distler, O.; Gay, S.; Mahavadi, P.; et al. Adipokine expression in systemic sclerosis lung and gastrointestinal organ involvement. Cytokine 2019, 117, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Lakota, K.; Wei, J.; Carns, M.; Hinchcliff, M.; Lee, J.; Whitfield, M.L.; Sodin-Semrl, S.; Varga, J. Levels of adiponectin, a marker for PPAR-gamma activity, correlate with skin fibrosis in systemic sclerosis: Potential utility as biomarker? Arthritis Res. Ther. 2012, 14, R102. [Google Scholar] [CrossRef] [PubMed]
- Olewicz-Gawlik, A.; Danczak-Pazdrowska, A.; Kuznar-Kaminska, B.; Batura-Gabryel, H.; Katulska, K.; Wojciech, S.; Trzybulska, D.; Hrycaj, P. Circulating adipokines and organ involvement in patients with systemic sclerosis. Acta Reum. Port. 2015, 40, 156–162. [Google Scholar]
- Ryu, C.; Walia, A.; Ortiz, V.; Perry, C.; Woo, S.; Reeves, B.C.; Sun, H.; Winkler, J.; Kanyo, J.E.; Wang, W.; et al. Bioactive Plasma Mitochondrial DNA Is Associated With Disease Progression in Scleroderma-Associated Interstitial Lung Disease. Arthritis Rheumatol. 2020, 72, 1905–1915. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, E.; Lei, Y.; Luo, A.; Yan, Y.; Cai, M.; Liu, S.; Huang, Y.; Guan, H.; Zhong, M.; et al. Diagnostic Values of METTL1-Related Genes and Immune Characteristics in Systemic Lupus Erythematosus. J. Inflamm. Res. 2023, 16, 5367–5383. [Google Scholar] [CrossRef]
- Birkenmeier, G.; Frank, R. Plasma concentration of total and transformed alpha 2-macroglobulin in systemic sclerosis. Arch. Dermatol. Res. 1996, 288, 405–407. [Google Scholar] [CrossRef]
- Gundogdu, B.; Yolbas, S.; Yilmaz, M.; Aydin, S.; Koca, S.S. Serum osteopontin and vitronectin levels in systemic sclerosis. Adv. Clin. Exp. Med. 2017, 26, 1231–1236. [Google Scholar] [CrossRef]
- López-Guisa, J.M.; Rassa, A.C.; Cai, X.; Collins, S.J.; Eddy, A.A. Vitronectin accumulates in the interstitium but minimally impacts fibrogenesis in experimental chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2011, 300, F1244–F1254. [Google Scholar] [CrossRef]
- Showalter, K.; Gordon, J.K. Skin histology in systemic sclerosis: A relevant clinical biomarker. Curr. Rheumatol. Rep. 2020, 23, 3. [Google Scholar] [CrossRef]
- Del Galdo, F.; Shaw, M.A.; Jimenez, S.A. Proteomic analysis identification of a pattern of shared alterations in the secretome of dermal fibroblasts from systemic sclerosis and nephrogenic systemic fibrosis. Am. J. Pathol. 2010, 177, 1638–1646. [Google Scholar] [CrossRef]
- Liakouli, V.; Elies, J.; El-Sherbiny, Y.M.; Scarcia, M.; Grant, G.; Abignano, G.; Derrett-Smith, E.C.; Esteves, F.; Cipriani, P.; Emery, P.; et al. Scleroderma fibroblasts suppress angiogenesis via TGF-β/caveolin-1 dependent secretion of pigment epithelium-derived factor. Ann. Rheum. Dis. 2018, 77, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-H.; Shih, S.-C.; Ho, T.-C.; Ma, H.-I.; Liu, M.-Y.; Chen, S.-L.; Tsao, Y.-P. Pigment epithelium-derived factor 34-mer peptide prevents liver fibrosis and hepatic stellate cell activation through down-regulation of the PDGF receptor. PLoS ONE 2014, 9, e95443. [Google Scholar] [CrossRef] [PubMed]
- Steen, V.D. The many faces of scleroderma. Rheum. Dis. Clin. N. Am. 2008, 34, 1–15. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).