A Systematic Review and Meta-Analysis of Tourniquet Pressures in Upper Limb Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Inclusion/Exclusion Criteria
- Patient population undergoing upper limb surgery under regional or general anesthesia;
- Case series, cohort studies, or randomized controlled trials;
- Use of upper limb tourniquet during surgery;
- Any outcome measure reported.
- Upper limb surgeries carried out under local anesthesia;
- Articles not reporting original research, e.g., narrative review articles;
- Articles in which full text is not available in English;
- Conference abstracts that have not been published as full-text publications;
- Articles that are duplicate publications, i.e., have been previously published as full-text publications;
- Articles in which studies were conducted using data from non-human studies, or articles presenting cadaveric studies.
2.3. Study Selection and Data Extraction
2.4. Statistical Analysis
2.5. Quality Assessment
3. Results
3.1. Study Characteristics
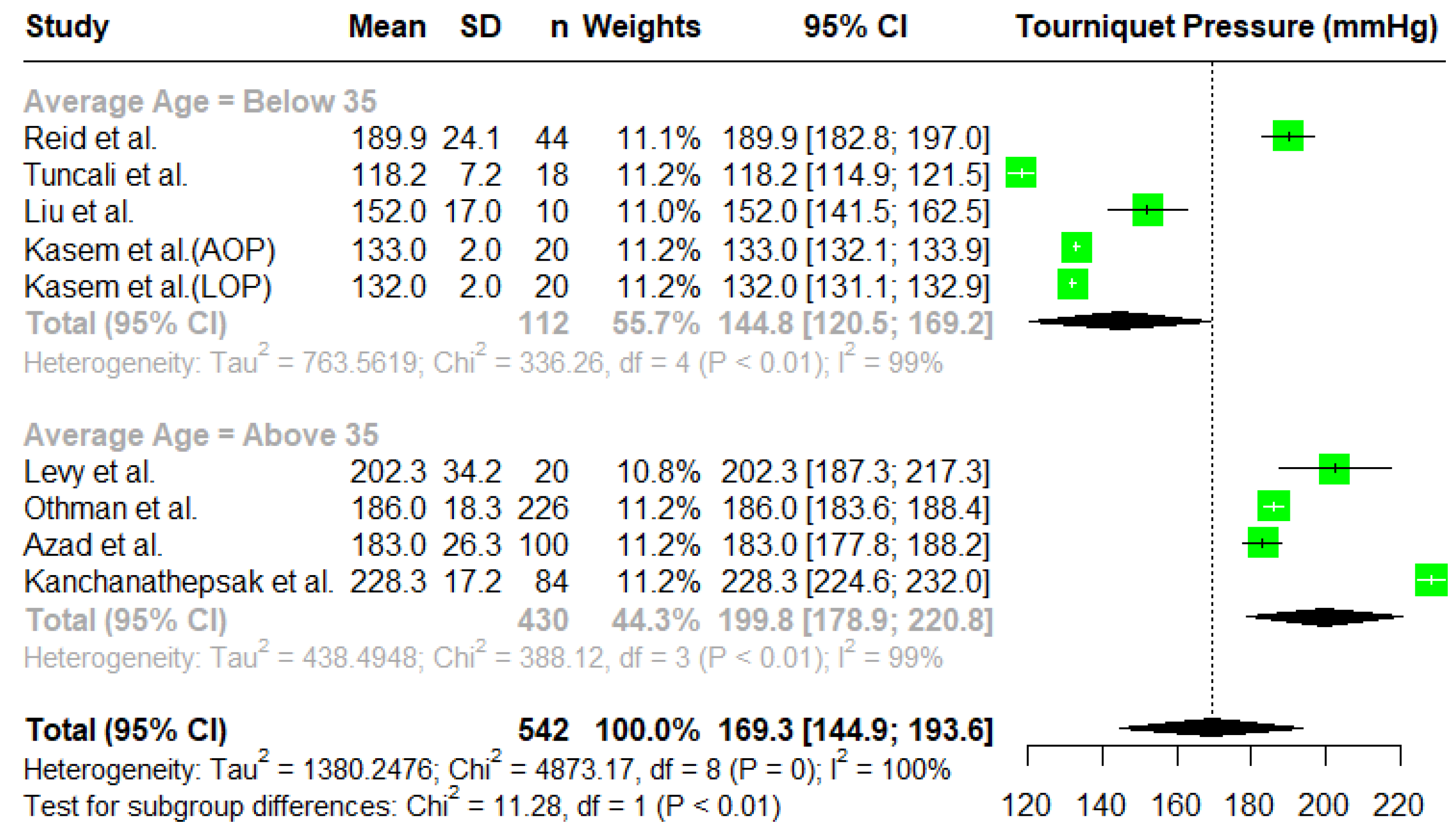
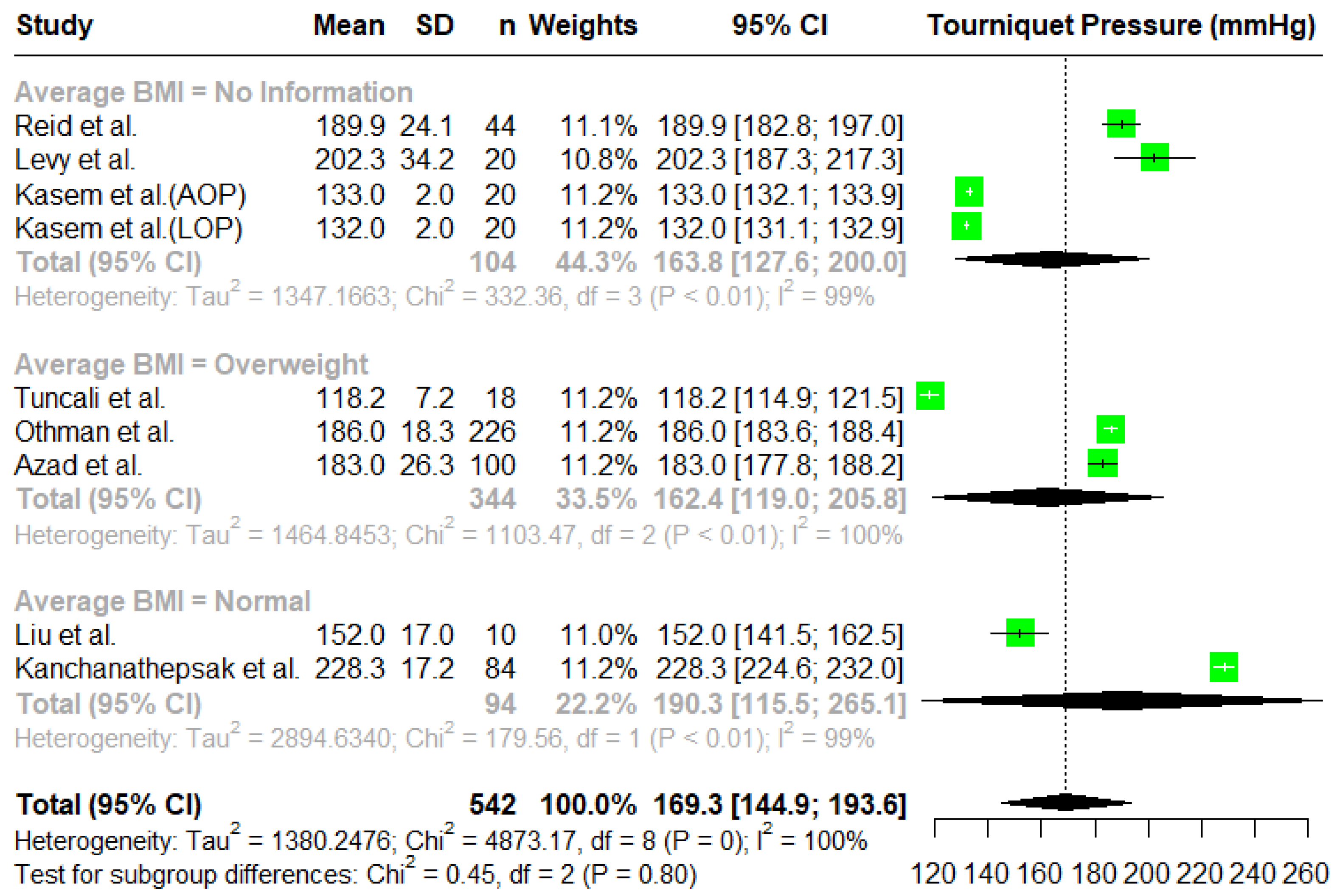
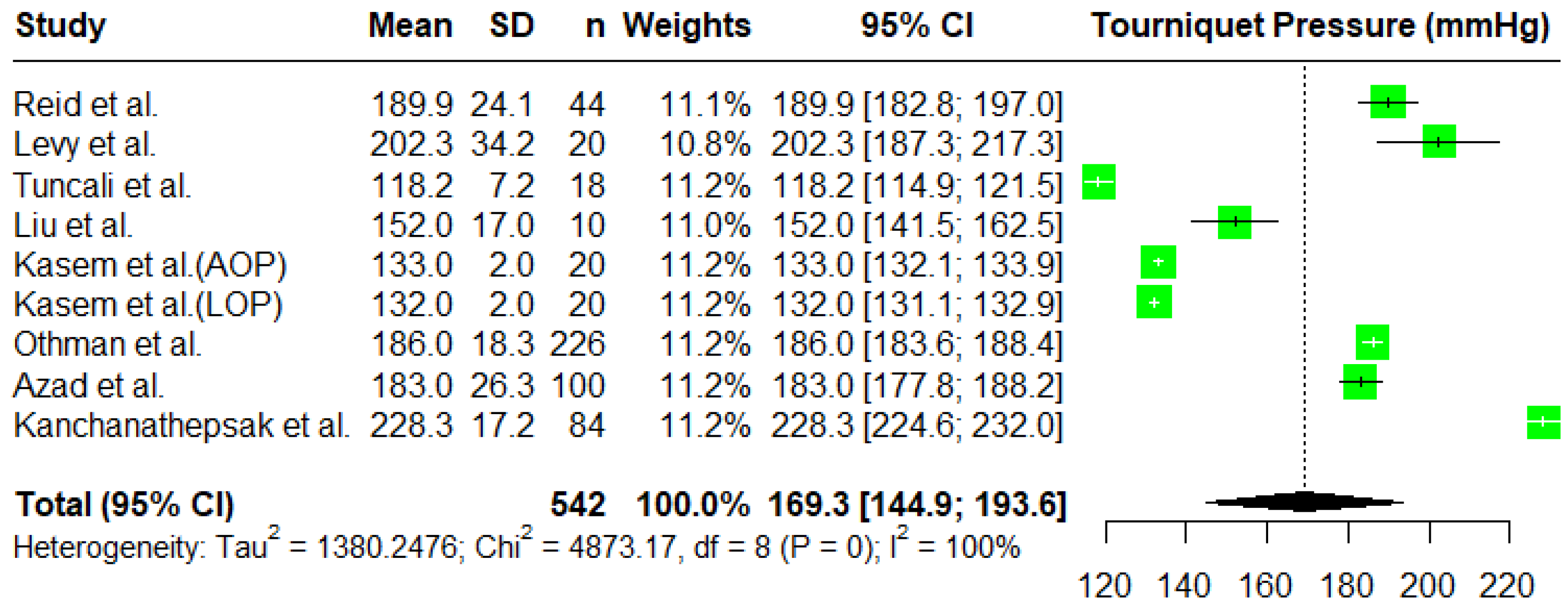
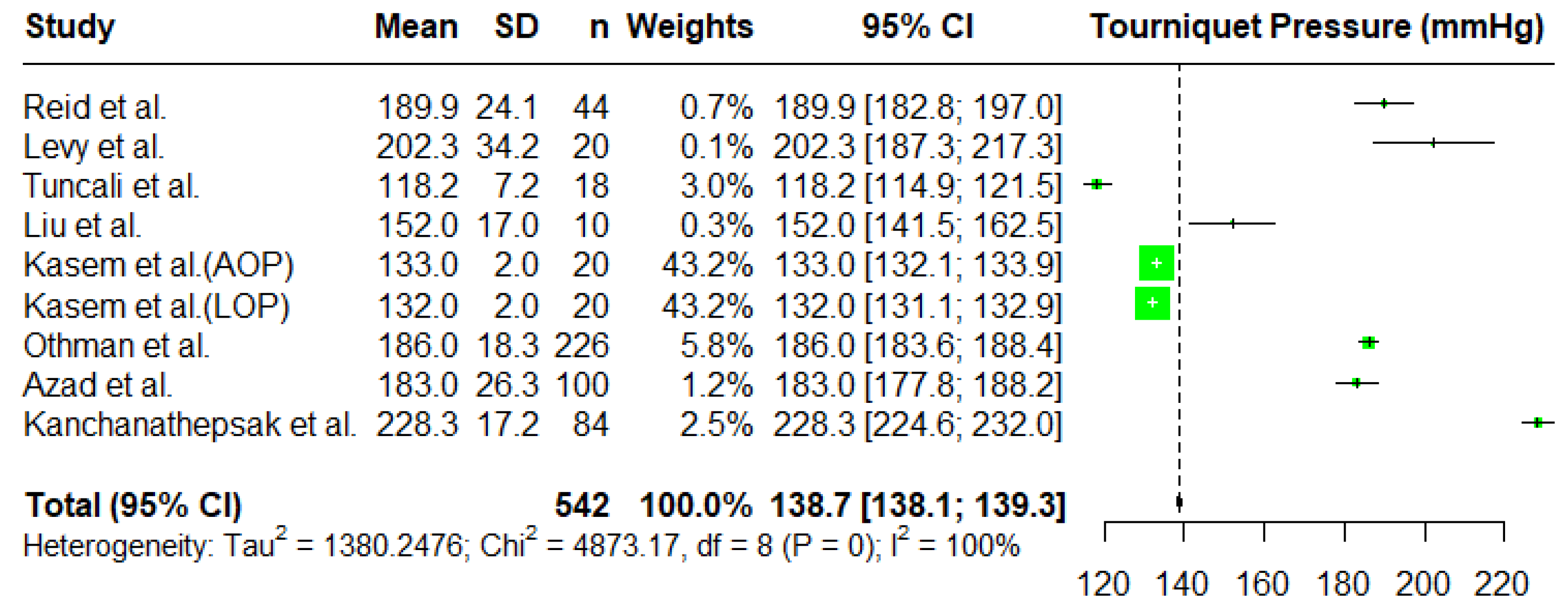
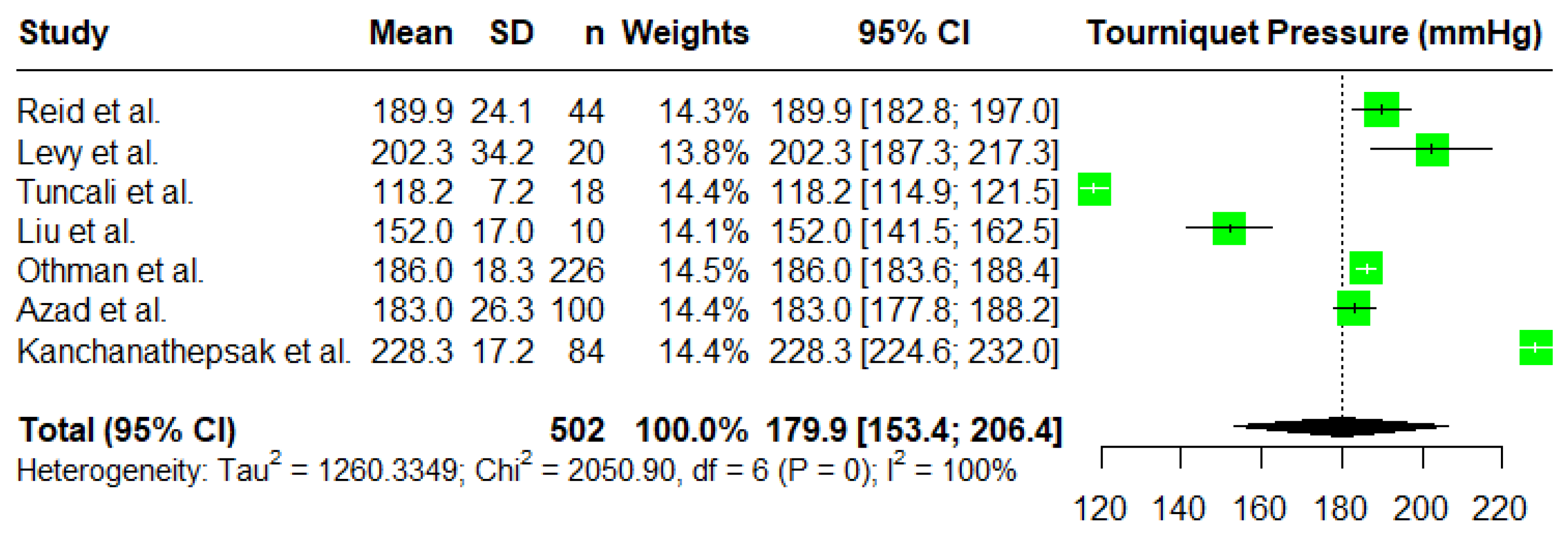
3.2. Tourniquet Pressures
3.3. Adequacy of Surgical Field
3.4. Secondary Outcomes
3.4.1. Complications
3.4.2. Operative Time and Tourniquet Time
3.4.3. Cuff Width
Meta-Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Kumar, K.; Railton, C.; Tawfic, Q. Tourniquet application during anesthesia: “What we need to know?”. J. Anaesthesiol. Clin. Pharmacol. 2016, 32, 424–430. [Google Scholar] [CrossRef]
- Azad, A.; Sager, B.; Gupta, S.; Ayalon, O.; Paksima, N. Reducing Tourniquet Pressures in Hand Surgery: Are Lower Pressures as Effective? J. Wrist Surg. 2022, 12, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Sarfani, S.; Cantwell, S.; Shin, A.Y.; Kakar, S. Challenging the Dogma of Tourniquet Pressure Requirements for Upper Extremity Surgery. J. Wrist Surg. 2016, 5, 120. [Google Scholar] [CrossRef] [PubMed]
- Tuncali, B.; Boya, H.; Kayhan, Z.; Arac, S. Tourniquet pressure settings based on limb occlusion pressure determination or arterial occlusion pressure estimation in total knee arthroplasty? A prospective, randomized, double blind trial. Acta Orthop. Traumatol. Turc. 2018, 52, 256. [Google Scholar] [CrossRef] [PubMed]
- Kasem, S.A.; Bassiouny, A.A.E.; Rashwan, D.A.E.; Bahr, M.H. Minimal Inflation Tourniquet Pressure Using Induced Hypotension with Limb Occlusion Pressure Determination or Arterial Occlusion Pressure Estimation in Upper Limb Surgery: A Randomized Double-Blinded Comparative Study. Anesthesiol. Pain Med. 2020, 10, e102124. [Google Scholar] [CrossRef]
- BOA; BSCOS; BSSH. BOAST—The Safe Use of Intraoperative Tourniquets. 2021. Available online: https://www.boa.ac.uk/resource/boast-the-safe-use-of-intraoperative-tourniquets.html (accessed on 5 November 2024).
- Reid, H.S.; Camp, R.A.; Jacob, W.H. Tourniquet hemostasis. A clinical study. Clin. Orthop. Relat. Res. 1983, 177, 222–234. [Google Scholar] [CrossRef]
- AORN Recommended Practices Committee. Recommended Practices for the Use of the Pneumatic Tourniquet in the Perioperative Practice Setting. AORN J. 2007, 86, 640–655. [Google Scholar] [CrossRef]
- Masri, B.A.; Day, B.; Younger, A.S.E.; Jeyasurya, J. Technique for Measuring Limb Occlusion Pressure that Facilitates Personalized Tourniquet Systems: A Randomized Trial. J. Med. Biol. Eng. 2016, 36, 644–650. [Google Scholar] [CrossRef]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; PRISMA-S Group. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Bramer, W.M.; Giustini, D.; De Jong, G.B.; Holland, L.; Bekhuis, T. De-duplication of database search results for systematic reviews in EndNote. J. Med. Libr. Assoc. 2016, 104, 240. [Google Scholar] [CrossRef] [PubMed]
- Cochran. RoB 2: A Revised Cochrane Risk-of-Bias Tool for Randomized Trials. 2019. Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 15 December 2024).
- Risk of Bias Tools—ROBINS-I V2 Tool. Available online: https://sites.google.com/site/riskofbiastool/welcome/robins-i-v2 (accessed on 15 December 2024).
- Van Roekel, H.E.; Thurston, A.J. Tourniquet Pressure: The Effect of Limb Circumference and Systolic Blood Pressure. J. Hand Surg. Br. Eur. Vol. 1984, 10, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.; Pistorio, A.L.; Lopez, S.; Orengia, A.; Born, M.W. Optimizing Tourniquet Pressure in Upper Extremity Surgery. J. Hand Surg. Asian Pac. Vol. 2021, 26, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.R.; Garfin, S.R.; Hargens, A.R. Wide tourniquets eliminate blood flow at low inflation pressures. J. Hand Surg. Am. 1987, 12, 1006–1011. [Google Scholar] [CrossRef]
- Drolet, B.C.; Okhah, Z.; Phillips, B.Z.; Christian, B.P.; Akelman, E.; Katarincic, J.; Schmidt, S.T. Evidence for safe tourniquet use in 500 consecutive upper extremity procedures. Hand 2014, 9, 494. [Google Scholar] [CrossRef]
- Levy, O.; David, Y.; Heim, M.; Eldar, I.; Chetrit, A.; Engel, J. Minimal tourniquet pressure to maintain arterial closure in upper limb surgery. J. Hand Surg. Br. 1993, 18, 204–206. [Google Scholar] [CrossRef]
- Kanchanathepsak, T.; Pukrittayakamee, N.C.; Woratanarat, P.; Tawonsawatruk, T.; Angsanuntsukh, C. Limb occlusion pressure versus standard tourniquet inflation pressure in minor hand surgery: A randomized controlled trial. J. Orthop. Surg. Res. 2023, 18, 539. [Google Scholar] [CrossRef]
- Liu, H.Y.; Guo, J.Y.; Zhang Zb Li, K.Y.; Wang, W.D. Development of adaptive pneumatic tourniquet systems based on minimal inflation pressure for upper limb surgeries. Biomed. Eng. Online 2013, 12, 92. [Google Scholar] [CrossRef]
- Salhotra, R.; Sharma, J. Tourniquets in orthopedic surgery. Indian J. Orthop. 2012, 46, 377. [Google Scholar]
- Estebe, J.P.; Le Naoures, A.; Chemaly, L.; Ecoffey, C. Tourniquet pain in a volunteer study: Effect of changes in cuff width and pressure. Anaesthesia 2000, 55, 21–26. [Google Scholar] [CrossRef]
- Crews, J.C.; Hilgenhurst, G.; Leavitt, B.; Denson, D.D.; Bridenbaugh, P.O.; Stuebing, R.C. Tourniquet pain: The response to the maintenance of tourniquet inflation on the upper extremity of volunteers. Reg. Anesth. 1991, 16, 314–371. [Google Scholar] [CrossRef] [PubMed]
- Siamdoust, S.A.S.; Barekati, M.; Zaman, B.; Noorizad, S.; Alimian, M. Comparison of the Effect of Intercostobrachial Nerve Block with and Without Ultrasound Guidance on Tourniquet Pain After Axillary Block of Brachial Plexus: A Randomized Clinical Trial. Anesth. Pain Med. 2023, 13, e134819. [Google Scholar] [CrossRef] [PubMed]
- Le-Wendling, L.; Ihnatsenka, B.; Jones, A.; Smith, C.R.; Helander, E.; Kedrowski, J.; Nin, O.C.; Gunnett, A.M.; Zasimovich, Y. Role of an Intercostobrachial Nerve Block in Alleviating Tourniquet Pain: A Randomized Clinical Trial. Cureus 2022, 14, e22196. [Google Scholar] [CrossRef]
- Albaker, A.B.; Almogbil, I.; Alkheraiji, A.F.; Alshahrani, A.H.; Alharbi, S.K.; AlSwaji, G.F.; Alotaibi, R.M.; Alrashidi, A. Tourniquet Practice Among Orthopaedic Surgeons in Saudi Arabia. Curēus 2023, 15, e45828. [Google Scholar] [CrossRef] [PubMed]
- Tuncali, B.; Karci, A.; Bacakoglu, A.K.; Tuncali, B.E.; Ekin, A. Controlled Hypotension and Minimal Inflation Pressure: A New Approach for Pneumatic Tourniquet Application in Upper Limb Surgery. Anesth. Analg. 2003, 97, 1529–1532. [Google Scholar] [CrossRef]
- Limb Occlusion Pressure Tourniquets to Decrease Pain After Surgery. 2025. Available online: https://ctv.veeva.com/study/limb-occlusion-pressure-tourniquets-to-decrease-pain-after-surgery (accessed on 14 January 2025).
- Seigler, S.W.; Quinn, K.M.; Holman, H.L.; Kim, J.Y.; Rajab, T.K. A single-center, nonblinded, clinical trial comparing blood pressures before and after tourniquet application in healthy humans: A study protocol. PLoS ONE 2023, 18, e0280139. [Google Scholar] [CrossRef]
- Pedowitz, R.A.; Gershuni, D.H.; Botte, M.J.; Kuiper, S.; Rydevik, B.L.; Hargens, A.R. The use of lower tourniquet inflation pressures in extremity surgery facilitated by curved and wide tourniquets and an integrated cuff inflation system. Clin. Orthop. Relat. Res. 1993, 287, 237–244. [Google Scholar] [CrossRef]
| Studies Assessed Using ROBINS-I Tool | Domain 1: Bias Due to Confounding | Domain 2: Bias Due to Selection of Participants | Domain 3: Bias in Classification of Interventions | Domain 4: Bias Due to Deviation from Intended Intervention | Domain 5: Bias Due to Missing Data | Domain 6: Bias in Outcome Measures | Domain 7: Bias in Selection of Reported Result | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Reid et al. [7] | no information | no information | moderate | low | low | serious | moderate | serious |
| Van Roekel and Thurston [15] | serious | no information | low | low | low | serious | moderate | serious |
| Othman et al. [16] | serious | low | moderate | low | low | serious | low | serious |
| Moore, Garfin, and Hargens [17] | serious | moderate | low | low | low | serious | moderate | serious |
| Drolet et al. [18] | serious | moderate | low | low | low | serious | low | serious |
| Levy et al. [19] | moderate | moderate | low | low | low | serious | low | serious |
| Sarfani et al. [3] | serious | low | low | low | low | serious | low | serious |
| Azad et al. [2] | serious | moderate | low | low | low | serious | low | serious |
| Studies Assessed Using ROB-2 Tool | Domain 1: Bias Arising from Randomization Process | Domain 2: Bias Due to Deviations from Intended Interventions | Domain 3: Bias Due to Missing Outcome Data | Domain 4: Risk of Bias in Measurement of Outcome | Domain 5: Risk of Bias in Selection of Reported Risk | Overall Risk of Bias |
|---|---|---|---|---|---|---|
| Kasem et al. [5] | low | low | low | high | low | high |
| Tuncali et al. [4] | low | low | low | high | low | high |
| Kanchanathepsak et al. [20] | low | low | low | high | low | high |
| Liu et al. [21] | no information | low | low | high | low | high |
| Author | Number of Participants Included in Review | Mean Age of Participants (Years) | Method for Determining Tourniquet Occlusion Pressure | Lowest Tourniquet Occlusion Pressure to Achieve Bloodless Field |
|---|---|---|---|---|
| Reid et al., 1983 [7] | 44 | Not stated | Doppler occlusion pressure + 50 mmHg and then adjusting accordingly | Mean: 189.9 mmHg Maximum: 225 mmHg |
| Van Roekel and Thurston, 1985 [15] | 15 | 36.9 | Commencing at 300 mmHg and then gradually decreasing pressure until capillary bleeding occurs | Maximum: 150 mmHg |
| Moore, Garfin, Hargens, 1987 [17] | 10 | Not stated | Elimination of Doppler flow | Range: 90 mmHg–160 mmHg |
| Levy et al., 1993 [19] | 50 | 39 | Based on mean BP: 1.68 × mean BP + 50 | Mean: 202.3 ± 34.2 mmHg |
| Liu et al., 2013 [21] | 10 | 31 | AOP = 17.9 + 3.158 (upper extremity circumference (cm)) + 0.408 (systolic BP (mmHg)) | Mean: 152.3 ± 16.7 mmHg |
| Drolet et al., 2014 [18] | 505 | 40.1 | Systolic BP of 250 mmHg, 225 mmHg and surgeon preference | Mean: 152.7 mmHg Maximum: 275 mmHg |
| Sarfani et al., 2016 [3] | 228 | 59 | Based on systolic BP—value of 125 mmHg, 150 mmHg, 175 mmHg, 200 mmHg, or 250 mmHg used | Range: 125 mmHg–250 mmHg |
| Kasem et al., 2020 [5] | 40 | 26.4 | AOP and LOP | Mean: 132 ± 2 mmHg |
| Othman et al., 2021 [16] | 226 | 57 | LOP | Mean: 187 mmHg |
| Tuncali et al., 2021 [4] | 115 | 56.9 | AOP + 20 mmHg and then elimination of Doppler flow | Mean: 175.5 ± 13.2 mmHg |
| Azad et al., 2022 [2] | 100 | 50 | Based on systolic BP—60 mmHg was added for BP < 130 mmHg, 80 mmHg for 131 and 190 mmHg, and 100 mmHg for >191 mmHg | Mean: 183 ± 26 mm Hg |
| Kanchanathepsak et al., 2023 [20] | 84 | 56 | LOP whilst monitoring distal arterial pulse | Mean: 228 ± 17.2 mmHg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, K.; Jaibaji, R.; Barker, E.; Talwar, C.; Pang, C. A Systematic Review and Meta-Analysis of Tourniquet Pressures in Upper Limb Surgery. J. Clin. Med. 2025, 14, 1938. https://doi.org/10.3390/jcm14061938
Chan K, Jaibaji R, Barker E, Talwar C, Pang C. A Systematic Review and Meta-Analysis of Tourniquet Pressures in Upper Limb Surgery. Journal of Clinical Medicine. 2025; 14(6):1938. https://doi.org/10.3390/jcm14061938
Chicago/Turabian StyleChan, Kayen, Rawan Jaibaji, Eleanor Barker, Cyrus Talwar, and Calver Pang. 2025. "A Systematic Review and Meta-Analysis of Tourniquet Pressures in Upper Limb Surgery" Journal of Clinical Medicine 14, no. 6: 1938. https://doi.org/10.3390/jcm14061938
APA StyleChan, K., Jaibaji, R., Barker, E., Talwar, C., & Pang, C. (2025). A Systematic Review and Meta-Analysis of Tourniquet Pressures in Upper Limb Surgery. Journal of Clinical Medicine, 14(6), 1938. https://doi.org/10.3390/jcm14061938