The Role of Immersive Virtual Reality in Upper Limb Rehabilitation for Subacute Stroke: A Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
- Studies with samples of subjects with subacute stroke sequelae (period from 7 days to 6 months following the occurrence of stroke) [19];
- Studies which applied immersive virtual reality as a rehabilitation strategy;
- Studies considering upper limb motor recovery as the outcome.
2.2. Search Strategy
2.3. Selection of Studies
2.4. Methodological Quality Assessment
2.5. Data Extraction and Analysis
3. Results
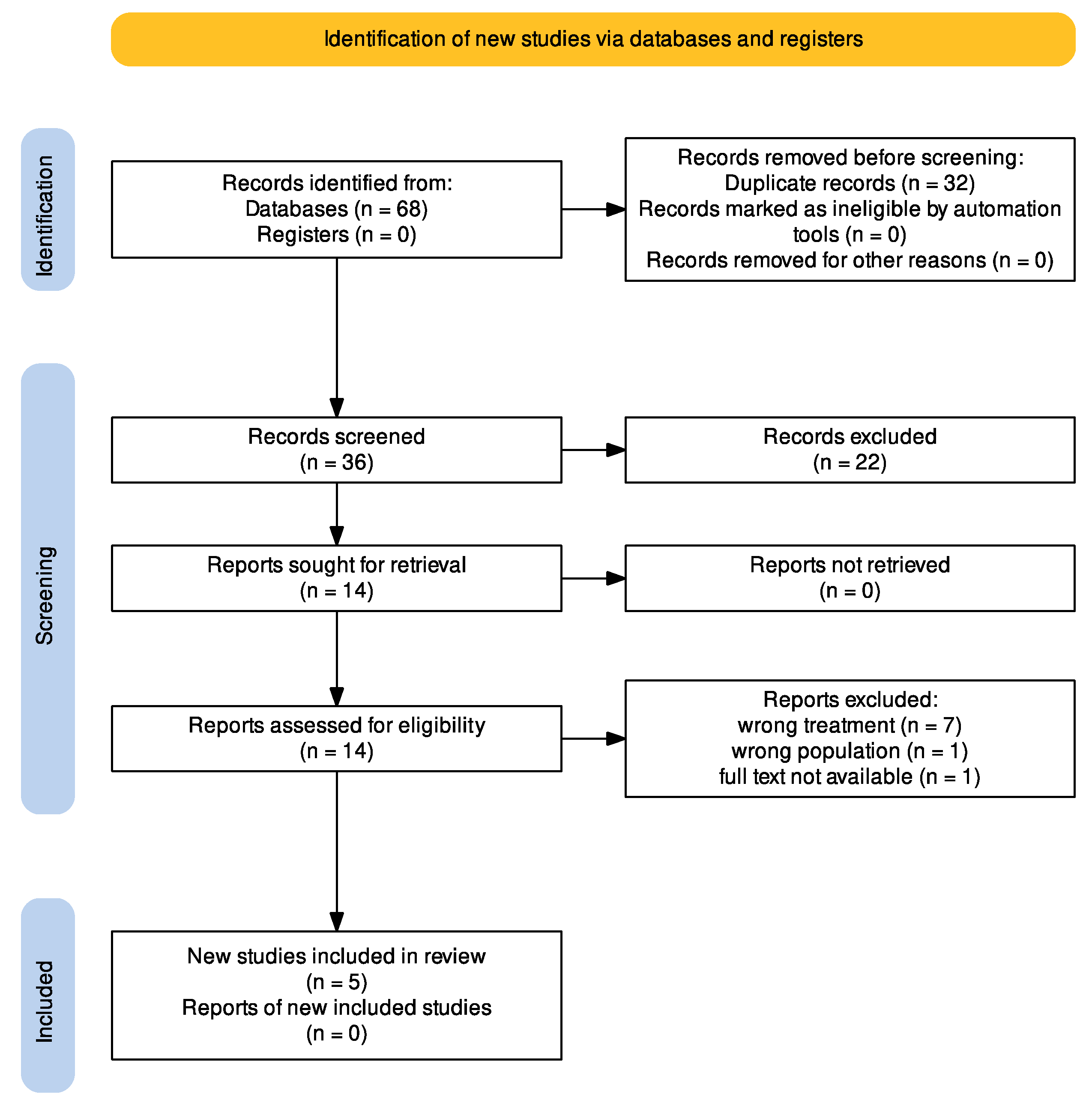
3.1. Search Results
3.2. Description of Studies
3.2.1. Synthesis of Results
3.2.2. The Aims of the Studies
3.2.3. The Intervention Protocols of the Experimental Group
3.2.4. Outcomes
3.2.5. Study Protocol
4. Discussion
4.1. The Interpretation of the Results
- -
- The immersive system used consist of an HMD device connected to HTC Vive motion tracking stations.
- -
- VR treatment assumes an intensive nature, with sessions ranging from 30 to 60 min of use, 3 to 5 times a week, for a period of 2, 3, or 4 weeks.
- -
- The use of VR is currently always integrated with conventional rehabilitation, so it must be considered as an additional therapy and not a substitute.
- -
- It is necessary that the patient does not present either cognitive disorders (e.g., neglect, aphasia, dementia) or sensory disorders of an auditory or visual nature.
- -
- The characteristics of patients eligible for VR treatment vary depending on the rationale underlying the use of VR. For example, if the rationale is related to motor imagery, the patient will simply have to imagine performing the task, standing still. Consequently, in this case, the necessary characteristics in terms of structural, functional, and postural aspects will differ from those expected for a patient undergoing VR therapy based on mirror therapy, who is required to move their limbs.
4.1.1. Comparison of VR Modalities in Stroke Rehabilitation
4.1.2. Effectiveness of VR Across Stroke Severity Levels
4.2. Limitations
5. Conclusions
Practical Guidelines for VR Integration
- Select the appropriate VR modality based on patient needs (e.g., immersive for intensive motor retraining, semi-immersive for moderate engagement, non-immersive for basic exercises).
- Ensure VR sessions align with conventional rehabilitation goals rather than replacing traditional therapy.
- Gradually introduce VR to prevent cybersickness and optimize patient adaptation.
- Use objective outcome measures (e.g., FM-UE scale, ARAT) to track progress and adjust therapy accordingly.
- Address financial and technical barriers by exploring cost-effective solutions, such as shared VR equipment and tele-rehabilitation options.
Author Contributions
Funding
Conflicts of Interest
References
- Teasell, R.; Hussein, N.; Longval, M. Stroke Rehabilitation Clinician Handbook 2020; Evidence-Based Review of Stroke Rehabilitation: London, ON, Canada, 2020. [Google Scholar]
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Pollock, A.; Farmer, S.E.; Brady, M.C.; Langhorne, P.; Mead, G.E.; Mehrholz, J.; van Wijck, F. Interventions for improving upper limb function after stroke. Cochrane Database Syst. Rev. 2014, 2014, CD010820. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, R.; Vita, F.; Platano, D.; Donati, D. Decision-Making in Adhesive Capsulitis: A Comprehensive Approach to the Diagnosis and Management of Frozen Shoulder. In Manuelle Medizin; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- Coleman, E.R.; Moudgal, R.; Lang, K.; Hyacinth, H.I.; Awosika, O.O.; Kissela, B.M.; Feng, W. Early Rehabilitation After Stroke: A Narrative Review. Curr. Atheroscler. Rep. 2017, 19, 59. [Google Scholar] [CrossRef]
- Patel, M.D.; Tilling, K.; Lawrence, E.; Rudd, A.G.; Wolfe, C.D.A.; McKevitt, C. Relationships between long-term stroke disability, handicap and health-related quality of life. Age Ageing 2006, 35, 273–279. [Google Scholar] [CrossRef]
- You, S.H.; Jang, S.H.; Kim, Y.-H.; Hallett, M.; Ahn, S.H.; Kwon, Y.-H.; Kim, J.H.; Lee, M.Y. Virtual reality-induced cortical reorganization and associated locomotor recovery in chronic stroke: An experimenter-blind randomized study. Stroke 2005, 36, 1166–1171. [Google Scholar] [CrossRef]
- León-Ruiz, M.; Pérez-Nieves, M.T.; Arce-Arce, S.; Benito-León, J.; Ezpeleta-Echávarri, D. Current evidence on virtual reality and its potential usefulness in post-stroke neurorehabilitation. Rev. Neurol. 2019, 69, 497–506. [Google Scholar] [CrossRef]
- Selzer, M.E.; Clarke, S.; Cohen, L.G.; Kwakkel, G.; Miller, R.H. (Eds.) Textbook of Neural Repair and Rehabilitation: Volume 2: Medical Neurorehabilitation, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; Volume 2, ISBN 978-1-107-01168-7. [Google Scholar]
- Slater, M. Place illusion and plausibility can lead to realistic behaviour in immersive virtual environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 3549–3557. [Google Scholar] [CrossRef]
- Tedeschi, R. Kinematic and plantar pressure analysis in Strumpell-Lorrain disease: A case report. Brain Disord. 2023, 11, 100097. [Google Scholar] [CrossRef]
- Riva, G.; Serino, S. Le Nuove Frontiere Della Riabilitazione: Perché la Realtà Virtuale Èsempre Più Realtà. Available online: https://www.researchgate.net/publication/273454626 (accessed on 26 October 2024).
- Levac, D.E.; Huber, M.E.; Sternad, D. Learning and transfer of complex motor skills in virtual reality: A perspective review. J. NeuroEngineering Rehabil. 2019, 16, 121. [Google Scholar] [CrossRef]
- Demeco, A.; Zola, L.; Frizziero, A.; Martini, C.; Palumbo, A.; Foresti, R.; Buccino, G.; Costantino, C. Immersive Virtual Reality in Post-Stroke Rehabilitation: A Systematic Review. Sensors 2023, 23, 1712. [Google Scholar] [CrossRef] [PubMed]
- Lange, B.; Koenig, S.; Chang, C.-Y.; McConnell, E.; Suma, E.; Bolas, M.; Rizzo, A. Designing informed game-based rehabilitation tasks leveraging advances in virtual reality. Disabil. Rehabil. 2012, 34, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W. Alan Craig Understanding Virtual Reality—2nd Edition. Available online: https://www.elsevier.com/books/understanding-virtual-reality/sherman/978-0-12-800965-9 (accessed on 26 February 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Bernhardt, J.; Hayward, K.S.; Kwakkel, G.; Ward, N.S.; Wolf, S.L.; Borschmann, K.; Krakauer, J.W.; Boyd, L.A.; Carmichael, S.T.; Corbett, D.; et al. Agreed Definitions and a Shared Vision for New Standards in Stroke Recovery Research: The Stroke Recovery and Rehabilitation Roundtable Taskforce. Int. J. Stroke 2017, 12, 444–450. [Google Scholar] [CrossRef]
- Rayyan—AI Powered Tool for Systematic Literature Reviews 2021. Available online: https://www.rayyan.ai/ (accessed on 26 October 2024).
- PEDro_scale_Italian.pdf. Available online: https://pedro.org.au/italian/resources/pedro-scale/ (accessed on 26 October 2024).
- Summary of Measurement Properties of the PEDro scale—PEDro 2019. Available online: https://pedro.org.au/english/summary-of-measurement-properties-of-the-pedro-scale/ (accessed on 26 October 2024).
- Mekbib, D.B.; Debeli, D.K.; Zhang, L.; Fang, S.; Shao, Y.; Yang, W.; Han, J.; Jiang, H.; Zhu, J.; Zhao, Z.; et al. A novel fully immersive virtual reality environment for upper extremity rehabilitation in patients with stroke. Ann. N. Y. Acad. Sci. 2021, 1493, 75–89. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, X.; Jin, Y.; Wu, B.; Vigotsky, A.D.; Fan, L.; Gu, P.; Tu, W.; Huang, L.; Jiang, S. Effectiveness of immersive VR-based rehabilitation on upper extremity recovery in subacute stroke: A randomized controlled trial. J. Neurol. 2024, 271, 1256–1266. [Google Scholar] [CrossRef]
- Mekbib, D.B.; Zhao, Z.; Wang, J.; Xu, B.; Zhang, L.; Cheng, R.; Fang, S.; Shao, Y.; Yang, W.; Han, J.; et al. Proactive Motor Functional Recovery Following Immersive Virtual Reality–Based Limb Mirroring Therapy in Patients with Subacute Stroke. Neurotherapeutics 2020, 17, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Shen, L.; Cai, Q.; Xiao, S.; Chen, Z. Novel Approach to Transfer of Coordination Skills to the Paretic Hand by Improving the Efficiency of Cortical Network. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 31, 326–334. [Google Scholar] [CrossRef]
- Sanchez-Cuesta, F.; Arroyo-Ferrer, A.; Gonzalez-Zamorano, Y.; Vourvopoulos, A.; Badia, S.; Figuereido, P.; Serrano, J.; Romero, J. Clinical Effects of Immersive Multimodal BCI-VR Training after Bilateral Neuromodulation with rTMS on Upper Limb Motor Recovery after Stroke. A Study Protocol for a Randomized Controlled Trial. Med. Kaunas Lith. 2021, 57, 736. [Google Scholar] [CrossRef]
- Hazelton, C.; McGill, K.; Campbell, P.; Todhunter-Brown, A.; Thomson, K.; Nicolson, D.J.; Cheyne, J.D.; Chung, C.; Dorris, L.; Gillespie, D.C.; et al. Perceptual Disorders After Stroke: A Scoping Review of Interventions. Stroke 2022, 53, 1772–1787. [Google Scholar] [CrossRef]
- Hazelton, C.; Thomson, K.; Todhunter-Brown, A.; Campbell, P.; Chung, C.S.; Dorris, L.; Gillespie, D.C.; Hunter, S.M.; McGill, K.; Nicolson, D.J.; et al. Interventions for perceptual disorders following stroke. Cochrane Database Syst. Rev. 2022, 2022, CD007039. [Google Scholar] [CrossRef]
- Tedeschi, R. Automated mechanical peripheral stimulation for gait rehabilitation in Parkinson’s disease: A comprehensive review. Clin. Park. Relat. Disord. 2023, 9, 100219. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, R.; Giorgi, F.; Donati, D. Harnessing Foot Mechanics: The Role of Lacing Techniques in Enhancing Comfort and Reducing Injury Risk. Appl. Sci. Switz. 2024, 14, 10190. [Google Scholar] [CrossRef]
- Tedeschi, R. Case study: Gait assessment of a patient with hallux rigidus before and after plantar modification. Int. J. Surg. Case Rep. 2024, 114, 109197. [Google Scholar] [CrossRef]
- Tedeschi, R.; Giorgi, F.; Donati, D. Footwear and Foot Health: Unveiling the Role of Proper Shoe Fit in Preventing Podiatric Issues and Enhancing Well-Being. Appl. Sci. Switz. 2024, 14, 9938. [Google Scholar] [CrossRef]
- Findlater, S.E.; Mazerolle, E.L.; Pike, G.B.; Dukelow, S.P. Proprioception and motor performance after stroke: An examination of diffusion properties in sensory and motor pathways. Hum. Brain Mapp. 2019, 40, 2995–3009. [Google Scholar] [CrossRef]
- Levin, M.F.; Weiss, P.L.; Keshner, E.A. Emergence of Virtual Reality as a Tool for Upper Limb Rehabilitation: Incorporation of Motor Control and Motor Learning Principles. Phys. Ther. 2015, 95, 415–425. [Google Scholar] [CrossRef]
- Nithianantharajah, J.; Hannan, A.J. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 2006, 7, 697–709. [Google Scholar] [CrossRef]
- Tedeschi, R. Mapping the Current Research on Mindfulness Interventions for Individuals with Cerebral Palsy: A Scoping Review. Neuropediatrics 2024, 55, 77–82. [Google Scholar] [CrossRef]
- Ricci, V.; Mezian, K.; Cocco, G.; Donati, D.; Naňka, O.; Farì, G.; Özçakar, L. Anatomy and ultrasound imaging of the tibial collateral ligament: A narrative review. Clin. Anat. 2022, 35, 571–579. [Google Scholar] [CrossRef]
- Farì, G.; Mancini, R.; Dell’Anna, L.; Ricci, V.; Della Tommasa, S.; Bianchi, F.P.; Ladisa, I.; De Serio, C.; Fiore, S.; Donati, D.; et al. Medial or Lateral, That Is the Question: A Retrospective Study to Compare Two Injection Techniques in the Treatment of Knee Osteoarthritis Pain with Hyaluronic Acid. J. Clin. Med. 2024, 13, 1141. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, V.; Sorriento, A.; Cafarelli, A.; Donati, D.; Papalexis, N.; Russo, A.; Lisignoli, G.; Ricotti, L.; Spinnato, P. Ultrasound Imaging in Knee Osteoarthritis: Current Role, Recent Advancements, and Future Perspectives. J. Clin. Med. 2024, 13, 4930. [Google Scholar] [CrossRef] [PubMed]
- Verheyden, G.; Nieuwboer, A.; Wit, L.D.; Feys, H.; Schuback, B.; Baert, I.; Jenni, W.; Schupp, W.; Thijs, V.; Weerdt, W.D. Trunk performance after stroke: An eye catching predictor of functional outcome. J. Neurol. Neurosurg. Psychiatry 2007, 78, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Santisteban, L.; Térémetz, M.; Bleton, J.-P.; Baron, J.-C.; Maier, M.A.; Lindberg, P.G. Upper Limb Outcome Measures Used in Stroke Rehabilitation Studies: A Systematic Literature Review. PLoS ONE 2016, 11, e0154792. [Google Scholar] [CrossRef]
- Passarelli, L.; Gamberini, M.; Fattori, P. The superior parietal lobule of primates: A sensory-motor hub for interaction with the environment. J. Integr. Neurosci. 2021, 20, 157–171. [Google Scholar] [CrossRef]
- Kalaska, J.F. Parietal cortex area 5 and visuomotor behavior. Can. J. Physiol. Pharmacol. 1996, 74, 483–498. [Google Scholar] [CrossRef]
- Andersen, R.A.; Snyder, L.H.; Bradley, D.C.; Xing, J. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu. Rev. Neurosci. 1997, 20, 303–330. [Google Scholar] [CrossRef]
- Filimon, F. Human cortical control of hand movements: Parietofrontal networks for reaching, grasping, and pointing. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2010, 16, 388–407. [Google Scholar] [CrossRef]
- Pisella, L. Visual perception is dependent on visuospatial working memory and thus on the posterior parietal cortex. Ann. Phys. Rehabil. Med. 2017, 60, 141–147. [Google Scholar] [CrossRef]
| CINAHL | (stroke subacute) AND (immersive virtual reality OR hmd) AND (upper extremity OR arm OR hand) |
| PubMed | (subacute stroke) AND (immersive virtual reality OR hmd) AND (upper extremity OR arm OR hand) |
| Embase | (subacute stroke) AND (immersive virtual reality OR hmd) AND (upper extremity OR arm OR hand) |
| Web of Science | (subacute stroke) AND (immersive virtual reality or hmd) AND (upper extremity OR arm OR hand) |
| PEDro | Subacute stroke AND hmd AND arm Subacute stroke AND immersive virtual reality AND arm Subacute stroke AND hmd AND hand Subacute stroke AND immersive virtual reality AND hand Subacute stroke AND hmd AND upper extremity Subacute stroke AND immersive virtual reality AND upper extremity |
| Cochrane Library | (subacute stroke) AND (immersive virtual reality OR hmd) AND (upper extremity OR arm OR hand) |
| Google Scholar | (subacute stroke) AND (immersive virtual reality OR hmd) AND (upper extremity OR arm OR hand) |
| Authors | Eligibility Criteria | Random Assignment | Concealed Allocation | Baseline Comparability | Subject Blinding | Clinician Blinding | Assessor Blinding | Adequate Follow-Up (>85%) | Intention-to-Treat Analysis | Between-Group Comparison | Point Measures and Measures of Variability | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mekbib et al. [23] | yes | x | x | x | x | x | x | 6/10 | ||||
| Huang et al. [24] | yes | x | x | x | x | x | x | x | 7/10 | |||
| Mekbib et al. [25] | yes | x | x | 2/10 | ||||||||
| Long et al. [26] | yes | x | x | x | 3/10 |
| Author, Year | Type of Study | Sample Size | Gender (M/F) | Age (Mean and Ds) | Days from Stroke (Mean and Ds) | Affected Limb (r/L) | Exclusion Criteria | Intervention | Main Outcome and Assessment Scale | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Mekbib et al. 2021 [23] | RCT | EG: 12 | 9/3 | 52 ± 13 | 36.92 ± 22.04 | 5/7 | MMSE < 16; hearing and vision not preserved | MNVR-Rehab system in LMT and ALT mode + conventional rehabilitation | Resting state (Rs) MRI + BI + FM-UE | MNVR-Rehab in LMT and ALT mode is an encouraging rehabilitation tool that may increase upper limb function in subacute stroke subjects compared to conventional rehabilitation. |
| CG: 11 | 8/3 | 61 ± 7 | 39.36 ± 18.08 | 4/7 | - | Conventional rehabilitation | Rs-MRI + BI + FM-UE | |||
| Huang et al. 2022 [24] * | RCT | EG: 20 | 13/7 | 63 ± 14 | 18.80 ± 8.44 | 10/10 | History of TIA; failure of critical organs; history of neurosurgery or epilepsy; severe cognitive impairments or aphasia | Conventional rehabilitation + immersive virtual reality with HMD | Rs-MRI + BI + FM-UE | An immersive environment is a promising rehabilitation tool for improving upper limb recovery in subacute stroke subjects. This improvement is associated with a cerebral reorganization which occurs not only immediately after the VR intervention but also in later phases. |
| CG: 20 | 11/9 | 65 ± 6 | 19.00 ± 6.64 | 7/13 | - | Conventional rehabilitation (physiotherapy and occupational therapy) | Rs-MRI + BI + FM-UE | |||
| Mekbib et al. 2020 [25] | Clinical controlled study | EG: 8 | 6/2 | 57 ± 4 | 38 | 5/3 | Moderate/severe visual, auditory, or cognitive impairments | MNVR-Rehab system in LMT+ conventional rehabilitation | Rs-MRI before and after the intervention + FM-UE | The unilateral and bilateral mirroring exercise of the upper limbs in an immersive virtual environment can increase cerebral reorganization and lead to the better functioning of the upper limbs. |
| CG: 13 healthy subjects | NR | 55 ± 7 | - | - | - | - | RS-MRI at the beginning (as a comparison) | |||
| Long et al. 2022 [26] | Clinical controlled study | EG: 10 | 5/5 | 55 ± 12 | 450 (Ds not available) | 7/3 | FM-UE score < 35; other neurological or orthopedic disease; discomfort/pain during activity | Performing unimanual tasks with the non-paretic hand and visualizing bimanual action through an HMD | EMG+ total time, reaction time, temporal asynchrony, intra-hand and inter-hand measurements | Coordination skills can be transferred to the paretic hand after performing short-term training with the non-paretic hand, collecting sensory feedback as if both hands were drawing. This would contribute to increasing the efficiency of the cortical neural network in both healthy subjects and those with stroke. |
| CG: 10 healthy subjects | 7/3 | 23 ± 3 | - | - | - | As above | As above | |||
| Sànchez-Cuesta et al. 2021 [27] | Study protocol | EG: 21 | NR | >18 years | >90 (Ds not available) | - | History of seizure or brain aneurysm; pacemakers, pumps, metal implants in the head; clinical instability; MAS > 3; other pre-existing neurological disease or cerebrovascular accidents with sequelae; neglect, aphasia, visual problems; Brunnstrom stage = 1; FM-UE score < 25 | Conventional rehabilitation + bilateral rTMS + immersive multimodal BCI-VR training system NeuRow | MI + FM-UE + Dynamometry + SIS | Not yet available. |
| CG: 21 | ND | >18 years | >90 (Ds not available) | - | - | Conventional rehabilitation + bilateral rTMS | MI + FM-UE + Dynamometry + SIS |
| Author and Year | Experimental Group | Control Group | ||
|---|---|---|---|---|
| Description | Duration | Description | Duration | |
| Mekbib et al. 2021 [23] | This group received both conventional rehabilitation and MNVR therapy. The MNVR-Rehab system includes the following elements: (1) a head-mounted display (HMD) to fully immerse the patient in the virtual environment (VE); (2) two base stations (HTC Vive tracking stations) to track the patient’s exact position in 3D; (3) Leap Motion to track the patient’s upper limb movements and transfer them onto the virtual limbs; and (4) a high-performance PC running the software system to generate the virtual environment and record the patient’s actions. The subject was seated in a chair wearing the HMD, through which they saw a virtual table. The task was to grasp balls and release them into a basket. The therapist could choose the difficulty of the task and the training mode. (1) LMT (limb mirror therapy) modes:
| 1 h rehabilitation with MNVR + 1 h conventional rehabilitation a day, for 4 days a week, for 2 weeks (total: 8 h of MNVR and 8 h of conventional rehabilitation). | This group received only conventional rehabilitation, which included daily living activities, balance control, gait training, weight shift, and distal and proximal UE functional movements. | 2 h a day, for 4 days a week, for 2 weeks. |
| Huang et al. 2022 [24] | This group received both conventional rehabilitation and VR rehabilitation. The devices used were an HMD connected to HTC Vive-VR stations. Subjects had to complete 6 programs: (1) frying food in a kitchen; (2) popping balloons with a sword in a virtual fencing room; (3) punching dolls in a virtual boxing arena; (4) playing basketball on a virtual court; (5) putting eggs in a basket; and (6) tidying up a desk in a virtual office. All tasks were to be performed with the affected limb. In the early stages of rehabilitation, subjects could perform the task with the help of the unaffected limb. | 30 min of conventional rehabilitation + 30 min of VR rehabilitation, for 5 days a week, for 3 weeks. | This group only received conventional rehabilitation (physiotherapy and occupational therapy), including grips and selective finger movements, gross movement, strength training, stretching, and training in activities of daily life. | 1 h a day, for 5 days a week, for 3 weeks. |
| Mekbib et al. 2020 [25] | This group received both conventional rehabilitation and MNVR therapy. The MNVR-Rehab system includes the same elements described in the study by Mekbib et al. from 2021 [23], cited above. The task was to grasp balls and release them into a basket. The therapist could choose the difficulty of the task and the training mode based on the patient’s characteristics. The mode was the LMT:
| 1 h rehabilitation with MNVR + 1 h conventional rehabilitation a day, for 4 days a week, for 2 weeks (total: 8 h of MNVR and 8 h of conventional rehabilitation). | Since they were healthy subjects, they did not receive any treatment. | - |
| Long et al. 2021 [26] | This group received VR therapy. An HTC Vive head-mounted display (HMD) was used, along with Hi5 VR gloves to record hand movements and 32 electrodes placed on the head to record EEG data. The subject was seated in front of a table wearing the HMD, through which they could see a virtual table. The experiment involved the patient tracing three-sided squares drawn on the table’s surface with their fingers (one on the right, one on the left), and it consisted of 3 phases: (1) the subject had to use both hands simultaneously to trace the squares; (2) the subject had to use only the unaffected limb, but in the virtual environment, both limbs were displayed; and (3) the subject had to use both hands again to complete the task. | 32 trials (with different combinations of three-sided squares) for each phase. | Healthy subjects received the same VR treatment. The data were used in a comparison with those of stroke patients. | 32 trials (with different combinations of three-sided squares) for each phase. |
| Sànchez-Cuesta et al. 2021 [27] | This group will undergo conventional rehabilitation + bilateral repetitive transcranial magnetic stimulation (rTMS), and immediately after, they will receive treatment with immersive virtual reality (NeuRow). The NeuRow treatment is based on motor imagery theory. In the first phase, the subject will be asked to imagine rowing a boat, and their brain activity will be recorded using electrodes applied to the head (EEG). EEG data will be used to distinguish the imagined movements of the right hand from those of the left hand. Then, the subject will wear an HMD, and they will see their upper limbs and two oars. In their hands, they will have two controllers that will make them perceive tactile stimuli. The subject will be asked to imagine the movement of the corresponding hand based on the stimuli presented on the screen (for example, if they see the left hand moving on the screen, the patient will have to imagine moving their left hand). The aim of this task is to perform as many correct motor imagery sequences as possible within a certain period of time. | rTSM: 5 times a week for 2 weeks (total of 10 sessions). NeuRow: 30 min, 3 times a week, for 4 weeks (total of 12 sessions). | This group will receive bilateral rTMS. | 5 times a week for 2 weeks (total of 10 sessions). |
| Feature | Immersive VR (HMD) | Semi-Immersive VR (Projection Screens) | Non-Immersive VR (Desktop/Gaming) |
|---|---|---|---|
| Level of Immersion | High | Moderate | Low |
| Sensorimotor Feedback | High—Real-time interaction | Moderate—Limited interaction | Low—Interaction via controllers |
| Cognitive Load | High | Moderate | Low |
| Risk of Cybersickness | High | Moderate | Low |
| Equipment Cost | Expensive | Moderate | Affordable |
| Suitability for Severe Cases | High—Effective for intensive motor retraining | Moderate—Suitable for structured therapy | Low—Limited effects on severe cases |
| Suitability for Mild Cases | Moderate—May be excessive for mild cases | High—Good balance between engagement and feasibility | High—Suitable for simple exercises |
| Feasibility in Clinical Settings | Moderate—Requires dedicated space and equipment | High—Easier integration into clinics | High—Widely available and easy to use |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donati, D.; Pinotti, E.; Mantovani, M.; Casarotti, S.; Fini, A.; Tedeschi, R.; Caselli, S. The Role of Immersive Virtual Reality in Upper Limb Rehabilitation for Subacute Stroke: A Review. J. Clin. Med. 2025, 14, 1903. https://doi.org/10.3390/jcm14061903
Donati D, Pinotti E, Mantovani M, Casarotti S, Fini A, Tedeschi R, Caselli S. The Role of Immersive Virtual Reality in Upper Limb Rehabilitation for Subacute Stroke: A Review. Journal of Clinical Medicine. 2025; 14(6):1903. https://doi.org/10.3390/jcm14061903
Chicago/Turabian StyleDonati, Danilo, Elena Pinotti, Monica Mantovani, Silvia Casarotti, Annalisa Fini, Roberto Tedeschi, and Serena Caselli. 2025. "The Role of Immersive Virtual Reality in Upper Limb Rehabilitation for Subacute Stroke: A Review" Journal of Clinical Medicine 14, no. 6: 1903. https://doi.org/10.3390/jcm14061903
APA StyleDonati, D., Pinotti, E., Mantovani, M., Casarotti, S., Fini, A., Tedeschi, R., & Caselli, S. (2025). The Role of Immersive Virtual Reality in Upper Limb Rehabilitation for Subacute Stroke: A Review. Journal of Clinical Medicine, 14(6), 1903. https://doi.org/10.3390/jcm14061903





