Hair Longevity—Evidence for a Multifactorial Holistic Approach to Managing Hair Aging Changes
Abstract
1. Introduction
2. Methods
3. Results
3.1. Aging and the Hair Cycle
3.2. Cellular Aging and Relevance to Follicle Processes
3.3. The Case for a Multifactorial ‘Hair Longevity’ Strategy
3.4. Challenges in Generating and Communicating Clinical Proof of Efficacy
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5αR | 5-α reductase |
| AGA | Androgenetic alopecia |
| DAMPs | Damage-associated molecular patterns |
| DHT | Dihydrotestosterone |
| DP | Dermal papilla |
| ECM | Extracellular matrix |
| FPHL | Female pattern hair loss |
| MPHL | Male pattern hair loss |
| PRP | Platelet-rich plasma |
| RCT | Randomized control trial |
| ROS | Reactive oxygen species |
| SASP | Senescence-associated secretory phenotype |
| UVR | Ultraviolet radiation |
References
- Owecka, B.; Tomaszewska, A.; Dobrzeniecki, K.; Owecki, M. The Hormonal Background of Hair Loss in Non-Scarring Alopecias. Biomedicines 2024, 12, 513. [Google Scholar] [CrossRef] [PubMed]
- Starace, M.; Orlando, G.; Alessandrini, A.; Piraccini, B.M. Female Androgenetic Alopecia: An Update on Diagnosis and Management. Am. J. Clin. Dermatol. 2020, 21, 69–84. [Google Scholar] [CrossRef]
- Messenger, A.G.; Sinclair, R. Follicular miniaturization in female pattern hair loss: Clinicopathological correlations. Br. J. Dermatol. 2006, 155, 926–930. [Google Scholar] [CrossRef]
- Gupta, A.K.; Mays, R.R.; Dotzert, M.S.; Versteeg, S.G.; Shear, N.H.; Piguet, V. Efficacy of non-surgical treatments for androgenetic alopecia: A systematic review and network meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2112–2125. [Google Scholar] [CrossRef] [PubMed]
- Rebora, A.; Guarrera, M.; Drago, F. Postpartum telogen effluvium. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 518. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Blume-Peytavi, U.; Kosmadaki, M.; Roó, E.; Vexiau-Robert, D.; Kerob, D.; Goldstein, S.R. Skin, hair and beyond: The impact of menopause. Climacteric 2022, 25, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Randall, V.A.; Ebling, F.J. Seasonal changes in human hair growth. Br. J. Dermatol. 1991, 124, 146–151. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhou, X.; Fu, S.; Luo, S.; Li, Y. Systematic Review and Meta-analysis of the Association Between Metabolic Syndrome and Androgenetic Alopecia. Acta Derm. Venereol. 2022, 102, adv00645. [Google Scholar] [CrossRef] [PubMed]
- Deloche, C.; Bastien, P.; Chadoutaud, S.; Galan, P.; Bertrais, S.; Hercberg, S.; De Lacharrière, O. Low iron stores: A risk factor for excessive hair loss in non-menopausal women. Eur. J. Dermatol. 2007, 17, 507–512. [Google Scholar]
- Moeinvaziri, M.; Mansoori, P.; Holakooee, K.; Naraghi, Z.S.; Abbasi, A. Iron status in diffuse telogen hair loss among women. Acta Dermatovenerol. Croat 2009, 17, 279–284. [Google Scholar]
- Rushton, D.H.; Dover, R.; Norris, M.J.; Gilkes, J.J. Iron and hair loss in women; what is deficiency? This is the real question! J. Am. Acad. Dermatol. 2007, 56, 518–519. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Sangwan, A. Dietary Protein Deficit and Deregulated Autophagy: A New Clinico-diagnostic Perspective in Pathogenesis of Early Aging, Skin, and Hair Disorders. Indian Dermatol Online J. 2019, 10, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Kealey, T.; Williams, R.; Philpott, M.P. The human hair follicle engages in glutaminolysis and aerobic glycolysis: Implications for skin, splanchnic and neoplastic metabolism. Skin Pharmacol. 1994, 7, 41–46. [Google Scholar] [CrossRef]
- Williams, R.; Philpott, M.P.; Kealey, T. Metabolism of freshly isolated human hair follicles capable of hair elongation: A glutaminolytic, aerobic glycolytic tissue. J. Invest Dermatol. 1993, 100, 834–840. [Google Scholar] [CrossRef]
- Miller, C.R.; Abedian, S.; Lipner, R.S. Shedding low yield testing for telogen effluvium: A cross-sectional study of 16,381 laboratory results from newly diagnosed patients. J. Am. Acad. Dermatol. 2023, 89, 623–626. [Google Scholar] [CrossRef]
- Hugh Rushton, D.; Norris, M.J.; Van Neste, D. Hair regrowth in male and female pattern hair loss does not involve the conversion of vellus hair to terminal hair. Exp. Dermatol. 2016, 25, 482–484. [Google Scholar] [CrossRef] [PubMed]
- Bushwereb, R.; Srivastava, G. Exploring Janus kinase inhibitors for alopecia areata: A comprehensive review. Ital. J. Dermatol. Venerol. 2024, 159, 380–389. [Google Scholar] [CrossRef]
- Kaufman, K.D.; Olsen, E.A.; Whiting, D.; Savin, R.; DeVillez, R.; Bergfeld, W.; Price, V.H.; Van Neste, D.; Roberts, J.L.; Hordinsky, M.; et al. Finasteride in the treatment of men with androgenetic alopecia. J. Am. Acad. Dermatol. 1998, 39, 578–589. [Google Scholar] [CrossRef]
- Feaster, B.; Onamusi, T.; Cooley, J.E.; McMichael, A.J. Oral minoxidil use in androgenetic alopecia and telogen effluvium. Arch. Dermatol. Res. 2023, 315, 201–205. [Google Scholar] [CrossRef]
- Jimenez-Cauhe, J.; Vaño-Galvan, S.; Mehta, N.; Hermosa-Gelbard, A.; Ortega-Quijano, D.; Buendia-Castaño, D.; Fernández-Nieto, D.; Porriño-Bustamante, M.; Saceda-Corralo, D.; Pindado-Ortega, C.; et al. Hair follicle sulfotransferase activity and effectiveness of oral minoxidil in androgenetic alopecia. J. Cosmet. Dermatol. 2024, 23, 3767–3773. [Google Scholar] [CrossRef]
- Van Neste, D. Placebo-controlled dose-effect studies with topical minoxidil 2% or 5% in male-patterned hair loss treated with oral finasteride employing an analytical and exhaustive study protocol. Skin Res. Technol. 2020, 26, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Shorter, K.; Farjo, N.P.; Picksley, S.M.; Randall1, V.A. Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. Faseb. J. 2008, 22, 1725–1736. [Google Scholar] [CrossRef]
- Mozafarpoor, S.; Faghihi, G.; Asilian, A.; Mokhtari, F.; Esfahani, A.A.; Bafandeh, B.; Nouraei, S.; Nilforoushzadeh, M.A.; Hosseini, S.M. The effectiveness of adding low-level light therapy to minoxidil 5% solution in the treatment of patients with androgenetic alopecia. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Tantiyavarong, J.; Charoensuksira, S.; Meephansan, J.; Hanvivattanakul, S.; Rayanasukha, Y.; Boonkoom, T.; Tantisantisom, K. Red and Green LED Light Therapy: A Comparative Study in Androgenetic Alopecia. Photodermatol. Photoimmunol. Photomed. 2024, 40, e13004. [Google Scholar] [CrossRef]
- Starace, M.; Cedirian, S.; Rapparini, L.; Quadrelli, F.; Pampaloni, F.; Bruni, F.; Piraccini, B.M. Enhanced Insights into Frontal Fibrosing Alopecia: Advancements in Pathogenesis Understanding and Management Strategies. Dermatol. Ther. 2024, 14, 1457–1477. [Google Scholar] [CrossRef]
- Cummins, D.M.; Chaudhry, I.H.; Harries, M. Scarring Alopecias: Pathology and an Update on Digital Developments. Biomedicines 2021, 9, 1755. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.; Castro, P.; Ribeiro, A.B.; Pereira, C.F.; Casanova, F.; Vilarinho, R.; Moreira, J.; Ramos, Ó.L. Bleached Hair as Standard Template to Insight the Performance of Commercial Hair Repair Products. Cosmetics 2024, 11, 150. [Google Scholar] [CrossRef]
- Sinclair, R.D. Healthy Hair: What Is it? J. Investig. Dermatol. Symp. Proc. 2007, 12, 2–5. [Google Scholar] [CrossRef]
- Lama, S.B.C.; Pérez-González, L.A.; Kosoglu, M.A.; Dennis, R.; Ortega-Quijano, D. Physical Treatments and Therapies for Androgenetic Alopecia. J. Clin. Med. 2024, 13, 4534. [Google Scholar] [CrossRef]
- Van Neste, D.; Tobin, D.J. Hair cycle and hair pigmentation: Dynamic interactions and changes associated with aging. Micron 2004, 35, 193–200. [Google Scholar] [CrossRef]
- Van Neste, D.J.; Rushton, D.H. Gender differences in scalp hair growth rates are maintained but reduced in pattern hair loss compared to controls. Skin Res. Technol. 2016, 22, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Van Neste, D.; Leroy, T.; Sandraps, E. Validation and clinical relevance of a novel scalp coverage scoring method. Skin Res. Technol. 2003, 9, 64–72. [Google Scholar] [CrossRef]
- Bittencourt, C.; Ferraro, D.A.; Soares, T.C.B.; Moraes, A.M.; Cintra, M.L. Chronic telogen effluvium and female pattern hair loss are separate and distinct forms of alopecia: A histomorphometric and immunohistochemical analysis. Clin. Exp. Dermatol. 2014, 39, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, C.; Teixeira, F.; Ferraro, D.A.; Soares, T.C.; Moraes, A.M.; Cintra, M.L. Non-invasive method distinguishes chronic telogen effluvium from mild female pattern hair loss: Clinicopathological correlation. Int. J. Dermatol. 2016, 55, e373–e379. [Google Scholar] [CrossRef]
- Fabbrocini, G.; Cantelli, M.; Masarà, A.; Annunziata, M.; Marasca, C.; Cacciapuoti, S. Female pattern hair loss: A clinical, pathophysiologic, and therapeutic review. Int. J. Womens Dermatol. 2018, 4, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.; Tosti, A. Female pattern alopecia and telogen effluvium: Figuring out diffuse alopecia. Semin. Cutan. Med. Surg. 2015, 34, 67–71. [Google Scholar] [CrossRef]
- Villani, A.; Ferrillo, M.; Fabbrocini, G.; Ocampo-Garza, S.S.; Scalvenzi, M.; Ruggiero, A. Hair Aging and Hair Disorders in Elderly Patients. Int. J. Trichol. 2022, 14, 191–196. [Google Scholar] [CrossRef]
- Hordinsky, M.; Sawaya, M.; Roberts, J.L. Hair loss and hirsutism in the elderly. Clin. Geriatr. Med. 2002, 18, 121–133, vii. [Google Scholar] [CrossRef]
- Whiting, D.A. How real is senescent alopecia? A histopathologic approach. Clin. Dermatol. 2011, 29, 49–53. [Google Scholar] [CrossRef]
- Hao, T.; Guo, J.; Liu, J.; Wang, J.; Liu, Z.; Cheng, X.; Li, J.; Ren, J.; Li, Z.; Yan, J.; et al. Predicting human age by detecting DNA methylation status in hair. Electrophoresis 2021, 42, 1255–1261. [Google Scholar] [CrossRef]
- Fukumoto, T.; Shimosawa, T.; Yakabe, M.; Yoshida, S.; Yoshida, Y. Recent advances in biomarkers for senescence: Bridging basic research to clinic. Geriatr. Gerontol. Int. 2025, 25, 139–147. [Google Scholar] [CrossRef]
- Dorf, N.; Maciejczyk, M. Skin senescence-from basic research to clinical practice. Front. Med. 2024, 11, 1484345. [Google Scholar] [CrossRef]
- Vidali, S.; Feichtinger, R.G.; Emberger, M.; Brunner, S.M.; Gaisbauer, S.; Blatt, T.; Smiles, W.J.; Kreutzer, C.; Weise, J.M.; Kofler, B. Ageing is associated with a reduction in markers of mitochondrial energy metabolism in the human epidermis. Exp. Dermatol. 2023, 32, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Westgate, G.E.; Pawlus, A.D.; Sikkink, S.K.; Thornton, M.J. Age-Related Changes in Female Scalp Dermal Sheath and Dermal Fibroblasts: How the Hair Follicle Environment Impacts Hair Aging. J. Investig. Dermatol. 2021, 141, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Premature senescence of balding dermal papilla cells in vitro is associated with p16(INK4a) expression. J. Investig. Dermatol. 2008, 128, 1088–1094. [Google Scholar] [CrossRef]
- Chew, E.G.Y.; Tan, J.H.J.; Bahta, A.W.; Ho, B.S.-Y.; Liu, X.; Lim, T.C.; Sia, Y.Y.; Bigliardi, P.L.; Heilmann, S.; Wan, A.C.A.; et al. Differential Expression between Human Dermal Papilla Cells from Balding and Non-Balding Scalps Reveals New Candidate Genes for Androgenetic Alopecia. J. Investig. Dermatol. 2016, 136, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.G.Y.; Lim, T.C.; Leong, M.F.; Liu, X.; Sia, Y.Y.; Leong, S.T.; Yan-Jiang, B.C.; Stoecklin, C.; Borhan, R.; Heilmann-Heimbach, S.; et al. Observations that suggest a contribution of altered dermal papilla mitochondrial function to androgenetic alopecia. Exp. Dermatol. 2022, 31, 906–917. [Google Scholar] [CrossRef]
- Vincent, M.; Yogiraj, K. A Descriptive Study of Alopecia Patterns and their Relation to Thyroid Dysfunction. Int. J. Trichol. 2013, 5, 57–60. [Google Scholar] [CrossRef]
- Thom, E. Stress and the Hair Growth Cycle: Cortisol-Induced Hair Growth Disruption. J. Drugs Dermatol. 2016, 15, 1001–1004. [Google Scholar]
- Hardman, J.A.; Nicu, C.; Tai, C.; Harries, M.; Mironov, A.; Purba, T.S.; Paus, R. Does dysfunctional autophagy contribute to immune privilege collapse and alopecia areata pathogenesis? J. Dermatol. Sci. 2020, 100, 75–78. [Google Scholar] [CrossRef]
- Suzuki, T.; Chéret, J.; Scala, F.D.; Rajabi-Estarabadi, A.; Akhundlu, A.; Demetrius, D.-L.; Gherardini, J.; Keren, A.; Harries, M.; Rodriguez-Feliz, J.; et al. Interleukin-15 is a hair follicle immune privilege guardian. J. Autoimmun. 2024, 145, 103217. [Google Scholar] [CrossRef]
- Piccini, I.; Sousa, M.; Altendorf, S.; Jimenez, F.; Rossi, A.; Funk, W.; Bíró, T.; Paus, R.; Seibel, J.; Jakobs, M.; et al. Intermediate Hair Follicles from Patients with Female Pattern Hair Loss Are Associated with Nutrient Insufficiency and a Quiescent Metabolic Phenotype. Nutrients 2022, 14, 3357. [Google Scholar] [CrossRef] [PubMed]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef]
- Pye, D.; Scholey, R.; Ung, S.; Dawson, M.; Shahmalak, A.; Purba, T.S. Activation of the integrated stress response in human hair follicles. PLoS ONE 2024, 19, e0303742. [Google Scholar] [CrossRef]
- Perera, E.; Sinclair, R. Treatment of chronic telogen effluvium with oral minoxidil: A retrospective study. F1000Research 2017, 6, 1650. [Google Scholar] [CrossRef] [PubMed]
- Wall, D.; Meah, N.; Fagan, N.; York, K.; Sinclair, R. Advances in hair growth. Fac. Rev. 2022, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- York, K.; Meah, N.; Bhoyrul, B.; Sinclair, R. A review of the treatment of male pattern hair loss. Expert. Opin. Pharmacother. 2020, 21, 603–612. [Google Scholar] [CrossRef]
- Egger, A.; Tomic-Canic, M.; Tosti, A. Advances in Stem Cell-Based Therapy for Hair Loss. CellR4 Repair Replace. Regen. Reprogram. 2020, 8, e2894. [Google Scholar]
- Ersan, M.; Ozer, E.; Akin, O.; Tasli, P.N.; Sahin, F. Effectiveness of Exosome Treatment in Androgenetic Alopecia: Outcomes of a Prospective Study. Aesthetic Plast. Surg. 2024, 48, 4262–4271. [Google Scholar] [CrossRef]
- Shah, M.; Dukharan, V.; Broughton, L.; Stegura, C.; Schur, N.; Samman, L.; Schlesinger, T. Exosomes for Aesthetic Dermatology: A Comprehensive Literature Review and Update. J. Cosmet. Dermatol. 2025, 24, e16766. [Google Scholar] [CrossRef]
- Daniels, G.; Akram, S.; Westgate, G.E.; Tamburic, S. Can plant-derived phytochemicals provide symptom relief for hair loss? A critical review. Int. J. Cosmet. Sci. 2019, 41, 332–345. [Google Scholar] [CrossRef]
- Feldman, P.R.; Feldman, O.J.; Guevara-Aguirre, J.; Fiebig, K.M. Sex differences in clinical trials of ALRV5XR treatment of androgenetic alopecia and telogen effluvium. Front. Med. 2022, 9, 918058. [Google Scholar] [CrossRef] [PubMed]
- Feldman, P.R.; Fiebig, K.M.; Piwko, C.; Mints, B.M.; Brown, D.; Cahan, D.J.; Guevara-Aguirre, J. Safety and efficacy of ALRV5XR in women with androgenetic alopecia or telogen effluvium: A randomised, double-blinded, placebo-controlled clinical trial. EClinicalMedicine 2021, 37, 100978. [Google Scholar] [CrossRef]
- Wang, R.; Lin, J.; Liu, Q.; Wu, W.; Wu, J.; Liu, X. Micronutrients and Androgenetic Alopecia: A Systematic Review. Mol. Nutr. Food Res. 2024, 68, e2400652. [Google Scholar] [CrossRef]
- Ablon, G.; Kogan, S. A Six-Month, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Safety and Efficacy of a Nutraceutical Supplement for Promoting Hair Growth in Women with Self-Perceived Thinning Hair. J. Drugs Dermatol. 2018, 17, 558–565. [Google Scholar] [PubMed]
- Pekmezci, E.; Dundar, C.; Turkoglu, M. A proprietary herbal extract against hair loss in androgenetic alopecia and telogen effluvium: A placebo-controlled, single-blind, clinical-instrumental study. Acta Dermatovenerol. Alp. Pannonica Adriat. 2018, 27, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Ablon, G.; Farris, P.K.; Hazan, A.; Raymond, I. A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety and Efficacy of a Nutraceutical Supplement with Standardized Botanicals in Males with Thinning Hair. J. Cosmet. Dermatol. 2025, 24, e16778. [Google Scholar] [CrossRef]
- Sivamani, R.K.; Ablon, G.; Nong, Y.; Maloh, J.; Hazan, A.; Raymond, I. A Prospective, Multi-Center Study to Evaluate the Safety and Efficacy of a Vegan Nutraceutical to Improve Hair Growth and Quality in Females Following a Plant-Based Diet. J. Drugs Dermatol. 2024, 23, 661–668. [Google Scholar] [CrossRef]
- Turkoglu, I.N.D.; Turkoglu, A.K.; Soylu, S.; Gencer, G.; Duman, R. A comprehensive investigation of biochemical status in patients with telogen effluvium: Analysis of Hb, ferritin, vitamin B12, vitamin D, thyroid function tests, zinc, copper, biotin, and selenium levels. J. Cosmet. Dermatol. 2024, 23, 4277–4284. [Google Scholar] [CrossRef]
- Rushton, D.H.; Dover, R.; Norris, M.J. Is there really no clear association between low serum ferritin and chronic diffuse telogen hair loss? Br. J. Dermatol. 2003, 148, 1282–1284. [Google Scholar] [CrossRef]
- Sinclair, R. There is no clear association between low serum ferritin and chronic diffuse telogen hair loss. Br. J. Dermatol. 2002, 147, 982–984. [Google Scholar] [CrossRef]
- Olsen, E.A.; Reed, K.B.; Cacchio, P.B.; Caudill, L. Iron deficiency in female pattern hair loss, chronic telogen effluvium, and control groups. J. Am. Acad. Dermatol. 2010, 63, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Tamer, F.; Yuksel, M.E.; Karabag, Y. Serum ferritin and vitamin D levels should be evaluated in patients with diffuse hair loss prior to treatment. Postep. Dermatol. Alergol. 2020, 37, 407–411. [Google Scholar] [CrossRef]
- Mirmirani, P. Hormones and clocks: Do they disrupt the locks? Fluctuating estrogen levels during menopausal transition may influence clock genes and trigger chronic telogen effluvium. Dermatol. Online J. 2016, 22, 13030/qt32r353c4. [Google Scholar] [CrossRef]
- Carmina, E.; Azziz, R.; Bergfeld, W.; Escobar-Morreale, H.F.; Futterweit, W.; Huddleston, H.; Lobo, R.; Olsen, E. Female Pattern Hair Loss and Androgen Excess: A Report From the Multidisciplinary Androgen Excess and PCOS Committee. J. Clin. Endocrinol. Metab. 2019, 104, 2875–2891. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.J.; Sink, J.R.; English Iii, J.C. Vitamin and Mineral Deficiencies in Patients With Telogen Effluvium: A Retrospective Cross-Sectional Study. J. Drugs Dermatol. 2016, 15, 1235–1237. [Google Scholar] [PubMed]
- Sattar, F.; Almas, U.; Ibrahim, N.A.; Akhtar, A.; Shazad, M.K.; Akram, S.; Khan, M.S.N.; Murtaza, G. Efficacy of Oral Vitamin D(3) Therapy in Patients Suffering from Diffuse Hair Loss (Telogen Effluvium). J. Nutr. Sci. Vitaminol. 2021, 67, 68–71. [Google Scholar] [CrossRef]
- Gerkowicz, A.; Chyl-Surdacka, K.; Krasowska, D.; Chodorowska, G. The Role of Vitamin D in Non-Scarring Alopecia. Int. J. Mol. Sci. 2017, 18, 2653. [Google Scholar] [CrossRef]
- Yongpisarn, T.; Tejapira, K.; Thadanipon, K.; Suchonwanit, P. Vitamin D deficiency in non-scarring and scarring alopecias: A systematic review and meta-analysis. Front. Nutr. 2024, 11, 1479337. [Google Scholar] [CrossRef]
- Zubair, Z.; Kantamaneni, K.; Jalla, K.; Renzu, M.; Jena, R.; Jain, R.; Muralidharan, S.; Yanamala, V.L.; Alfonso, M. Prevalence of Low Serum Vitamin D Levels in Patients Presenting With Androgenetic Alopecia: A Review. Cureus 2021, 13, e20431. [Google Scholar] [CrossRef]
- Henne, S.K.; Nöthen, M.M.; Heilmann-Heimbach, S. Male-pattern hair loss: Comprehensive identification of the associated genes as a basis for understanding pathophysiology. Med. Genet 2023, 35, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, S.; Brockschmidt, F.; Hillmer, A.; Hanneken, S.; Eigelshoven, S.; Ludwig, K.; Herold, C.; Mangold, E.; Becker, T.; Kruse, R.; et al. Evidence for a polygenic contribution to androgenetic alopecia. Br. J. Dermatol. 2013, 169, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Aging of hair. J. Cosmet. Dermatol. 2005, 4, 60–72. [Google Scholar] [CrossRef]
- Deng, Y.Q.; Wang, M.; He, Y.; Liu, F.; Chen, L.; Xiong, X. Cellular Senescence: Ageing and Androgenetic Alopecia. Dermatology 2023, 239, 533–541. [Google Scholar] [CrossRef]
- Cheng, Y.; Lv, L.-J.; Cui, Y.; Han, X.-M.; Zhang, Y.; Hu, C.-X. Psychological stress impact neurotrophic factor levels in patients with androgenetic alopecia and correlated with disease progression. World J. Psychiatry 2024, 14, 1437–1447. [Google Scholar] [CrossRef]
- Arias, E.M.; Floriach, N.; Moreno-Arias, G.; Camps, A.; Arias, S.; Trüeb, R.M. Targeted Nutritional Supplementation for Telogen Effluvium: Multicenter Study on Efficacy of a Hydrolyzed Collagen, Vitamin-, and Mineral-Based Induction and Maintenance Treatment. Int. J. Trichol. 2022, 14, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Colombo, F.; Gfm-O-Trial Investigators Group: Chiara Baraldo. Efficacy and tolerability of an oral supplement containing amino acids, iron, selenium, and marine hydrolyzed collagen in subjects with hair loss (androgenetic alopecia, AGA or FAGA or telogen effluvium). A prospective, randomized, 3-month, controlled, assessor-blinded study. Skin Res. Technol. 2023, 29, e13381. [Google Scholar]
- Arnaud, J.; Beani, J.C.; E Favier, A.; Amblard, P. Zinc status in patients with telogen defluvium. Acta Derm. Venereol. 1995, 75, 248–249. [Google Scholar] [CrossRef]
- Karashima, T.; Tsuruta, D.; Hamada, T.; Ono, F.; Ishii, N.; Abe, T.; Ohyama, B.; Nakama, T.; Dainichi, T.; Hashimoto, T. Oral zinc therapy for zinc deficiency-related telogen effluvium. Dermatol. Ther. 2012, 25, 210–213. [Google Scholar] [CrossRef]
- Starace, M.; Cedirian, S.; Bruni, F.; Alessandrini, A.M.; Quadrelli, F.; Sechi, A.; Piraccini, B.M. Clinical study on the efficacy and tolerability of an oral supplement based on arginine, l-cystine, zinc and B6 vitamin (Cystiphane®) in patients with telogen effluvium. Ital. J. Dermatol. Venereol. 2023, 158, 255–261. [Google Scholar] [CrossRef]
- Abdel Rahman, S.H.; Salem, R.M.; Sabry, J.H. Biotin Deficiency in Telogen Effluvium: Fact or Fiction? J. Clin. Aesthet. Dermatol. 2020, 13, 37–40. [Google Scholar] [PubMed]
- Pakhomova, E.E.; Smirnova, I.O. Comparative Evaluation of the Clinical Efficacy of PRP-Therapy, Minoxidil, and Their Combination with Immunohistochemical Study of the Dynamics of Cell Proliferation in the Treatment of Men with Androgenetic Alopecia. Int. J. Mol. Sci. 2020, 21, 6516. [Google Scholar] [CrossRef]
- Andjelkov, K.; Eremin, I.; Korac, A. Different levels of EGF, VEGF, IL-6, MCP-1, MCP-3, IP-10, Eotaxin and MIP-1α in the adipose-derived stem cell secretome in androgenetic alopecia. Exp. Dermatol. 2022, 31, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Han, Q.; Ma, Z.; Yan, Q.; Pei, Y.; Shi, P.; Zhang, J.; Rong, K.; Ma, K.; Li, P.; et al. Injectable platelet rich fibrin facilitates hair follicle regeneration by promoting human dermal papilla cell proliferation, migration, and trichogenic inductivity. Exp. Cell. Res. 2021, 409, 112888. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, A.; Mc Mahon, H. Dietary marine-derived ingredients for stimulating hair cell cycle. Biomed. Pharmacother. 2023, 163, 114838. [Google Scholar] [CrossRef]
- Brotzu, G.; Fadda, A.M.; Manca, M.L.; Manca, T.; Marongiu, F.; Campisi, M.; Consolaro, F. A liposome-based formulation containing equol, dihomo-γ-linolenic acid and propionyl-l-carnitine to prevent and treat hair loss: A prospective investigation. Dermatol. Ther. 2019, 32, e12778. [Google Scholar] [CrossRef]
- Bacqueville, D.; Lévêque, M.; Mas, C.; Haure, M.; Noustens, A.; Mengeaud, V.; Carrère, S.; Bessou-Touya, S.; Duplan, H.; Rizzi, N.C.; et al. New Plant Extracts Exert Complementary Anti-Hair Loss Properties in Human In Vitro and Ex Vivo Models. J. Cosmet. Dermatol. 2024, 23 (Suppl. S5), 1–11. [Google Scholar] [CrossRef]
- Sudeep, H.V.; Rashmi, S.; Jestin, T.V.; Richards, A.; Gouthamchandra, K.; Shyamprasad, K. Oral and Topical Administration of a Standardized Saw Palmetto Oil Reduces Hair Fall and Improves the Hair Growth in Androgenetic Alopecia Subjects–A 16-Week Randomized, Placebo-Controlled Study. Clin. Cosmet. Investig. Dermatol. 2023, 16, 3251. [Google Scholar] [CrossRef]
- Prager, N.; Bickett, K.; French, N.; Marcovici, G. A randomized, double-blind, placebo-controlled trial to determine the effectiveness of botanically derived inhibitors of 5-alpha-reductase in the treatment of androgenetic alopecia. J. Altern Complement. Med. 2002, 8, 143–152. [Google Scholar] [CrossRef]
- Narda, M.; Aladren, S.; Cestone, E.; Nobile, V. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. A prospective, randomized, double-blinded, controlled clinical trial. J. Cosmo Trichol. 2017, 3, 2. [Google Scholar] [CrossRef]
- Ibrahim, I.M.; Hasan, M.S.; Elsabaa, K.I.; Elsaie, M.L. Pumpkin seed oil vs. minoxidil 5% topical foam for the treatment of female pattern hair loss: A randomized comparative trial. J. Cosmet. Dermatol. 2021, 20, 2867–2873. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.H.; Jo, K.-J.; Park, Y.-S.; Kawk, H.W.; Yoo, J.-G.; Jang, J.D.; Kang, S.M.; Kim, S.-Y.; Kim, Y.-M. Bacillus/Trapa japonica Fruit Extract Ferment Filtrate enhances human hair follicle dermal papilla cell proliferation via the Akt/ERK/GSK-3β signaling pathway. BMC Complement. Altern Med. 2019, 19, 104. [Google Scholar] [CrossRef]
- Davis, M.G.; Piliang, M.P.; Bergfeld, W.F.; Caterino, T.L.; Fisher, B.K.; Sacha, J.P.; Carr, G.J.; Moulton, L.T.; Whittenbarger, D.J.; Schwartz, J.R. Scalp application of antioxidants improves scalp condition and reduces hair shedding in a 24-week randomized, double-blind, placebo-controlled clinical trial. Int. J. Cosmet. Sci. 2021, 43 (Suppl. S1), S14–S25. [Google Scholar] [CrossRef]
- Tursi, F.; Nobile, V.; Cestone, E.; De Ponti, I.; Lepoudere, A.; Sergheraert, R.; Soulard, J. The Effects of an Oral Supplementation of a Natural Keratin Hydrolysate on Skin Aging: A Randomized, Double-Blind, Placebo-Controlled Clinical Study in Healthy Women. J. Cosmet. Dermatol. 2025, 24, e16626. [Google Scholar] [CrossRef]
- Iwabuchi, T.; Takeda, S.; Yamanishi, H.; Ideta, R.; Ehama, R.; Tsuruda, A.; Shibata, H.; Ito, T.; Komatsu, N.; Terai, K.; et al. The topical penta-peptide Gly-Pro-Ile-Gly-Ser increases the proportion of thick hair in Japanese men with androgenetic alopecia. J. Cosmet. Dermatol. 2016, 15, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Grothe, T.; Wandrey, F.; Schuerch, C. Clinical evaluation of pea sprout extract in the treatment of hair loss. Phytother. Res. 2020, 34, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Almohanna, H.M.; Ahmed, A.A.; Tsatalis, J.P.; Tosti, A. The Role of Vitamins and Minerals in Hair Loss: A Review. Dermatol. Ther. 2019, 9, 51–70. [Google Scholar] [CrossRef]
- Oura, H.; Iino, M.; Nakazawa, Y.; Tajima, M.; Ideta, R.; Nakaya, Y.; Arase, S.; Kishimoto, J. Adenosine increases anagen hair growth and thick hairs in Japanese women with female pattern hair loss: A pilot, double-blind, randomized, placebo-controlled trial. J. Dermatol. 2008, 35, 763–767. [Google Scholar] [CrossRef]
- Kim, J.; Shin, J.Y.; Choi, Y.-H.; Kang, N.G.; Lee, S. Anti-Hair Loss Effect of Adenosine Is Exerted by cAMP Mediated Wnt/β-Catenin Pathway Stimulation via Modulation of Gsk3β Activity in Cultured Human Dermal Papilla Cells. Molecules 2022, 27, 2184. [Google Scholar] [CrossRef]
- Chen, D.; Yu, F.; Wang, C.; Chen, H.; Tan, J.; Shi, Q.; He, X.; Liu, X.; Wang, F.; Zhao, H. Anti-hair loss effect of a shampoo containing caffeine and adenosine. J. Cosmet. Dermatol. 2024, 23, 2927–2933. [Google Scholar] [CrossRef]
- Rinaldi, F.; Marzani, B.; Pinto, D.; Ramot, Y. A spermidine-based nutritional supplement prolongs the anagen phase of hair follicles in humans: A randomized, placebo-controlled, double-blind study. Dermatol. Pract. Concept 2017, 7, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Tiede, S.; Bíró, T.; Abu Bakar, M.H.; Sugawara, K.; Philpott, M.P.; Harrison, W.; Pietilä, M.; Paus, R. Spermidine promotes human hair growth and is a novel modulator of human epithelial stem cell functions. PLoS ONE 2011, 6, e22564. [Google Scholar] [CrossRef]
- Guerra-Tapia, A.; González-Guerra, E.; Caturla, J.M. A New Paradigm in the Management of Scalp Pruritus: Findings From the SCALP-PR Trial. Actas Dermosifiliogr. 2025, 116, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Limbu, S.L.; Purba, T.S.; Harries, M.; Kundu, R.; Bhogal, R.K.; Paus, R. Dandruff lesional scalp skin exhibits epidermal T cell infiltration and a weakened hair follicle immune privilege. Int. J. Cosmet. Sci. 2024, 46, 717–733. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar]
- Carrington, A.E.; Maloh, J.; Nong, Y.; Agbai, O.N.; A Bodemer, A.; Sivamani, R.K. The Gut and Skin Microbiome in Alopecia: Associations and Interventions. J. Clin. Aesthet. Dermatol. 2023, 16, 59–64. [Google Scholar]
- Lousada, M.B.; Edelkamp, J.; Lachnit, T.; Fehrholz, M.; Pastar, I.; Jimenez, F.; Erdmann, H.; Bosch, T.C.; Paus, R. Spatial Distribution and Functional Impact of Human Scalp Hair Follicle Microbiota. J. Invest. Dermatol. 2024, 144, 1353–1367.e15. [Google Scholar] [CrossRef]
- Edelkamp, J.; Lousada, M.B.; Pinto, D.; Chéret, J.; Calabrese, F.M.; Jiménez, F.; Erdmann, H.; Wessel, J.; Phillip, B.; De Angelis, M.; et al. Management of the human hair follicle microbiome by a synthetic odorant. J. Dermatol. Sci. 2023, 112, 99–108. [Google Scholar] [CrossRef]
- Ho, S.-Y.B.; Ho, E.X.P.; Chu, C.W.; Ramasamy, S.; Bigliardi-Qi, M.; de Sessions, P.F.; Bigliardi, P.L. Microbiome in the hair follicle of androgenetic alopecia patients. PLoS ONE 2019, 14, e0216330. [Google Scholar] [CrossRef]
- Duvel, L.; Chun, H.; Deppa, D.; Wertz, P.W. Analysis of hair lipids and tensile properties as a function of distance from scalp. Int. J. Cosmet. Sci. 2005, 27, 193–197. [Google Scholar] [CrossRef]
- Martí, M.; Barba, C.; Manich, A.M.; Rubio, L.; Alonso, C.; Coderch, L. The influence of hair lipids in ethnic hair properties. Int. J. Cosmet. Sci. 2016, 38, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Wills, J.; Dolphin, S.; Albiston, L.; Parmar, P.; Westgate, G.E.; Harrap, G.J. Free internal lipids in hair from pre- and post-menopausal women. Int. J. Cosmet. Sci. 2005, 27, 142. [Google Scholar] [CrossRef]
- Hawkshaw, N.; Hardman, J.; Alam, M.; Jimenez, F.; Paus, R. Deciphering the molecular morphology of the human hair cycle: Wnt signalling during the telogen-anagen transformation. Br. J. Dermatol. 2020, 182, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Philpott, M. A new model of human telogen-anagen transformation helps decipher Wnt signalling in the human hair cycle. Br. J. Dermatol. 2020, 182, 1084. [Google Scholar] [CrossRef]
- Butcher, N.J.; Monsour, A.; Mew, E.J.; Chan, A.-W.; Moher, D.; Mayo-Wilson, E.; Terwee, C.B.; Chee-A-Tow, A.; Baba, A.; Gavin, F.; et al. Guidelines for Reporting Outcomes in Trial Reports: The CONSORT-Outcomes 2022 Extension. JAMA 2022, 328, 2252–2264. [Google Scholar] [CrossRef]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef]
- Sinclair, R. Hair shedding in women: How much is too much? Br. J. Dermatol. 2015, 173, 846–848. [Google Scholar] [CrossRef]
- Thadanipon, K.; Suchonwanit, P. Measuring Patient Quality of Life Following Treatment for Alopecia. Patient Prefer. Adherence 2021, 15, 1601–1610. [Google Scholar] [CrossRef]
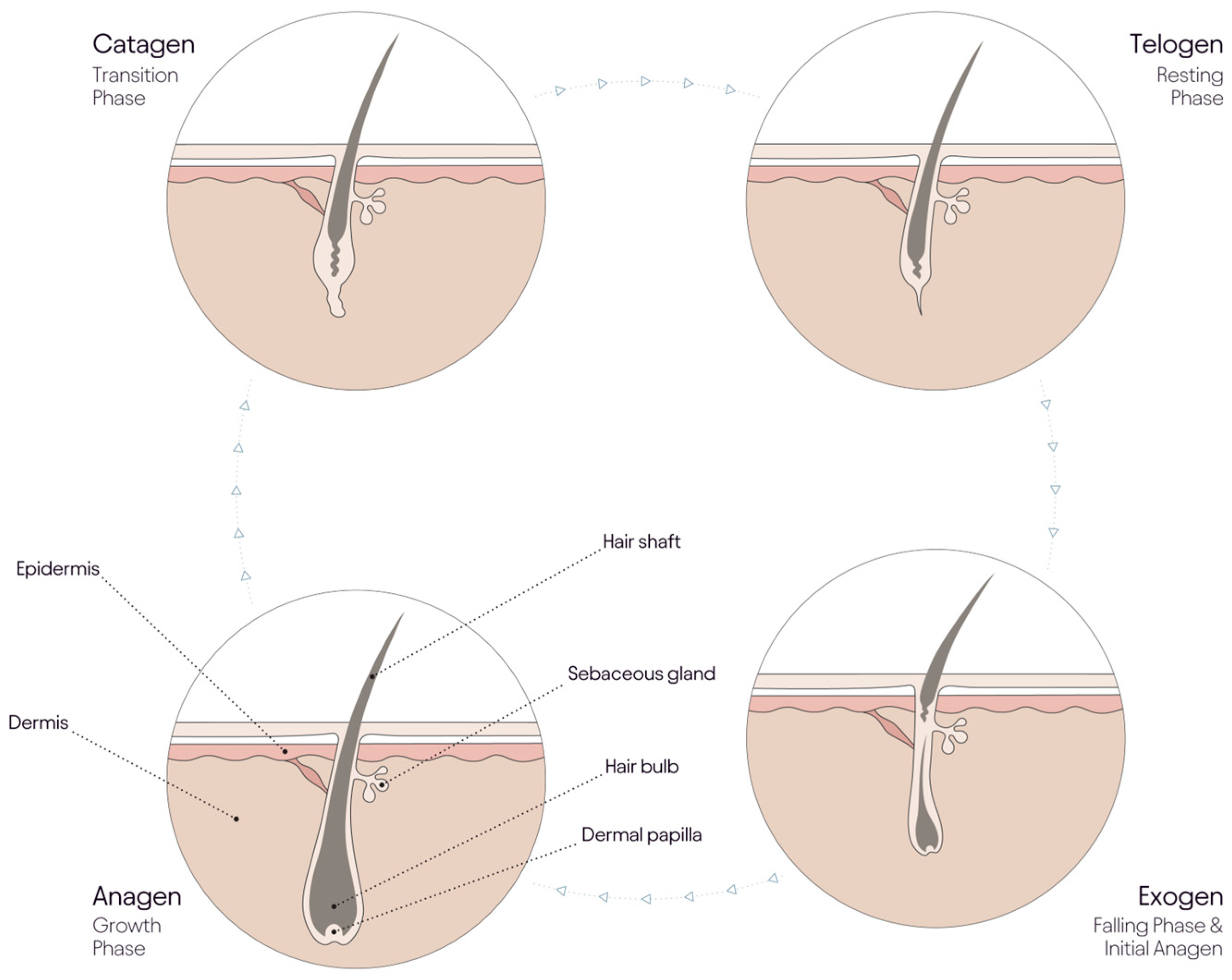
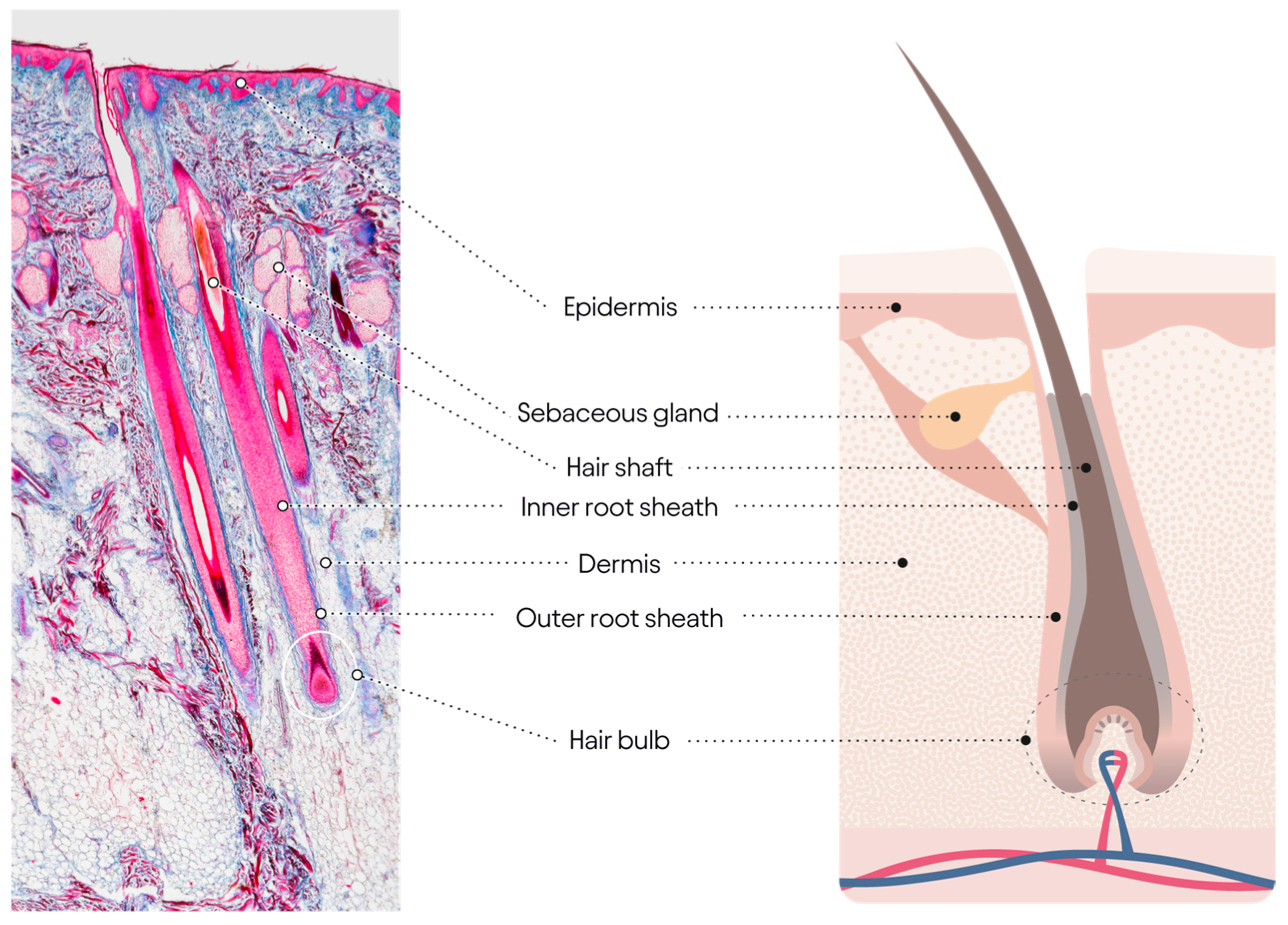
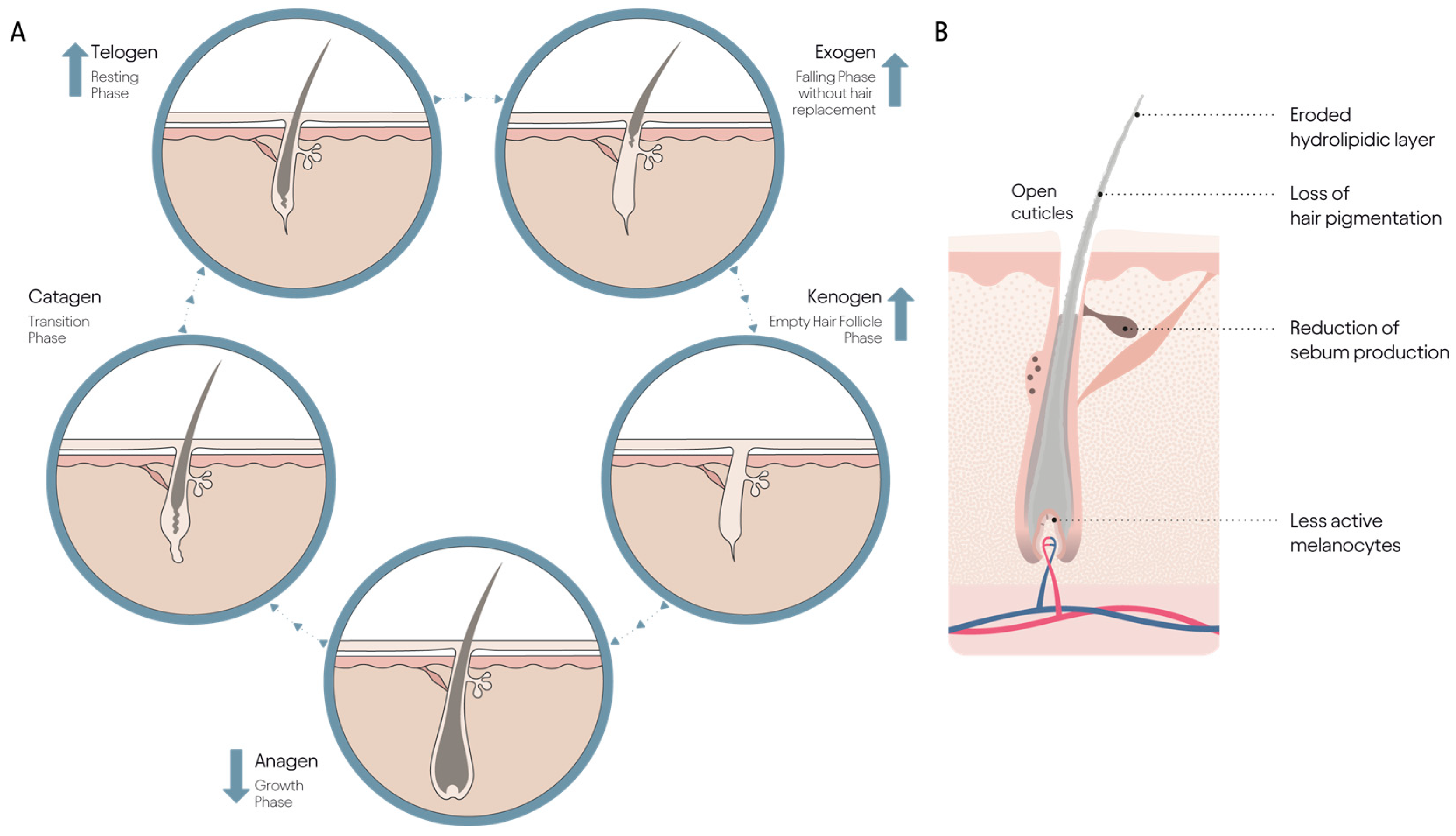
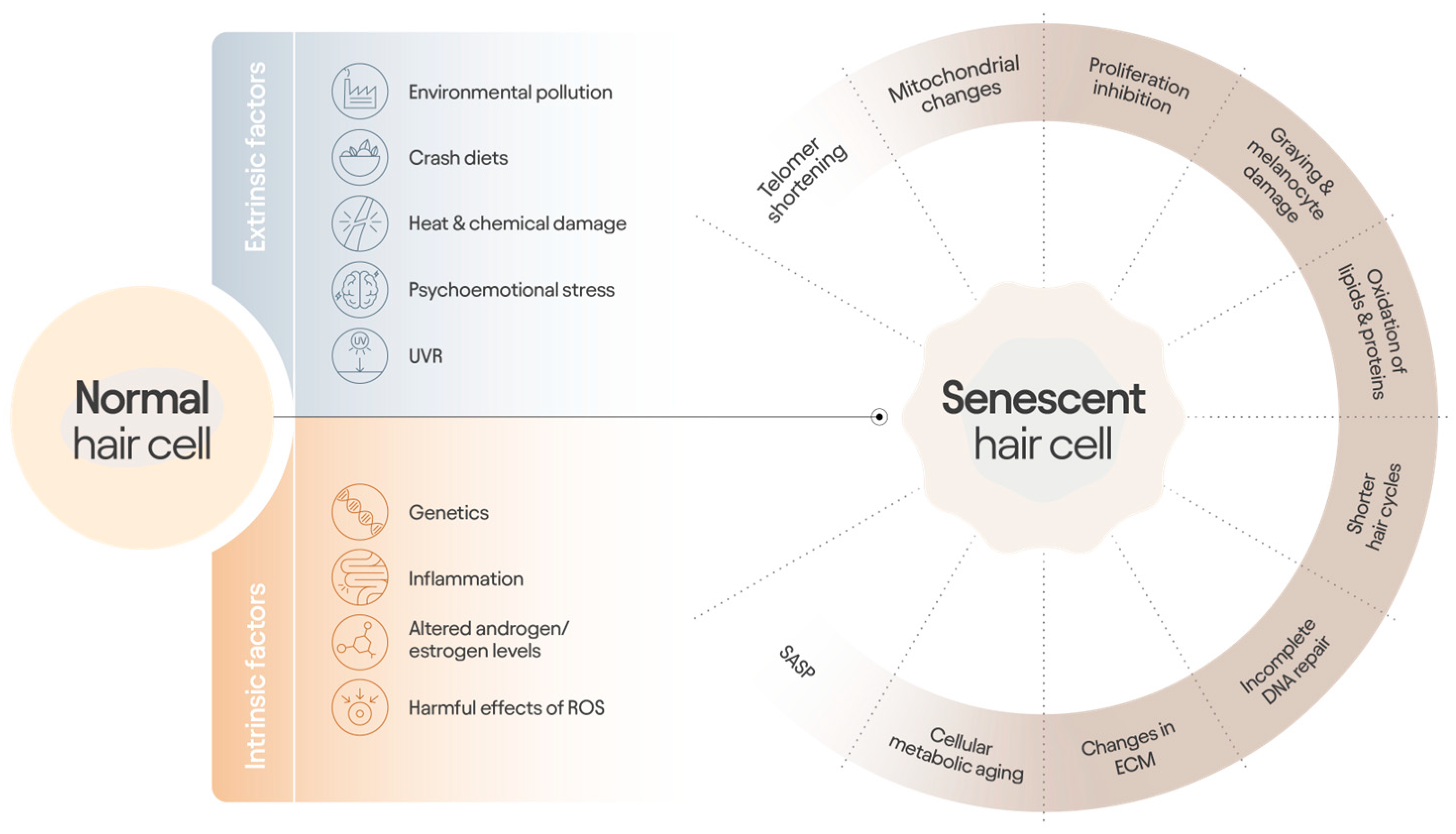
| Co-Morbid Disorders with Alopecia | Symptom | References |
|---|---|---|
| Thyroid dysfunction (hyper/hypo) | Excess hair shedding | [69] |
| Low iron status (Ferritin < 70 ng/mL) | Hair thinning | [9,10,11,69,70,71,72,73] |
| Hormonal disturbances, e.g., Menopause and PCOS | Hair thinning/pattern hair loss, hirsutism | [6,65,74,75] |
| Vitamin D deficiency | Hair thinning | [76,77,78,79,80] |
| Genetic predisposition | Pattern hair loss | [81,82] |
| Age | Senile hair changes | [37,83,84] |
| Stress—psychoemotional | Excess hair shedding | [85] |
| Complimentary Therapy Classes and Ingredients |
|---|
| Cell-derived; Stem and DP cell stimulants |
Marine based extracts
|
Phytochemicals
|
Anti-androgens
|
Anti-inflammatories
|
Antioxidants
|
| Peptides and amino acids |
Vitamins and minerals
|
| Small molecules |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westgate, G.E.; Grohmann, D.; Sáez Moya, M. Hair Longevity—Evidence for a Multifactorial Holistic Approach to Managing Hair Aging Changes. J. Clin. Med. 2025, 14, 1894. https://doi.org/10.3390/jcm14061894
Westgate GE, Grohmann D, Sáez Moya M. Hair Longevity—Evidence for a Multifactorial Holistic Approach to Managing Hair Aging Changes. Journal of Clinical Medicine. 2025; 14(6):1894. https://doi.org/10.3390/jcm14061894
Chicago/Turabian StyleWestgate, Gillian E., Daniela Grohmann, and Manuel Sáez Moya. 2025. "Hair Longevity—Evidence for a Multifactorial Holistic Approach to Managing Hair Aging Changes" Journal of Clinical Medicine 14, no. 6: 1894. https://doi.org/10.3390/jcm14061894
APA StyleWestgate, G. E., Grohmann, D., & Sáez Moya, M. (2025). Hair Longevity—Evidence for a Multifactorial Holistic Approach to Managing Hair Aging Changes. Journal of Clinical Medicine, 14(6), 1894. https://doi.org/10.3390/jcm14061894







