The MD Anderson Algorithm for Lymphedema Management
Abstract
1. Introduction
2. Lymphedema Prevention
2.1. Upper Extremity
2.1.1. Immediate Lymphatic Reconstruction (ILR)
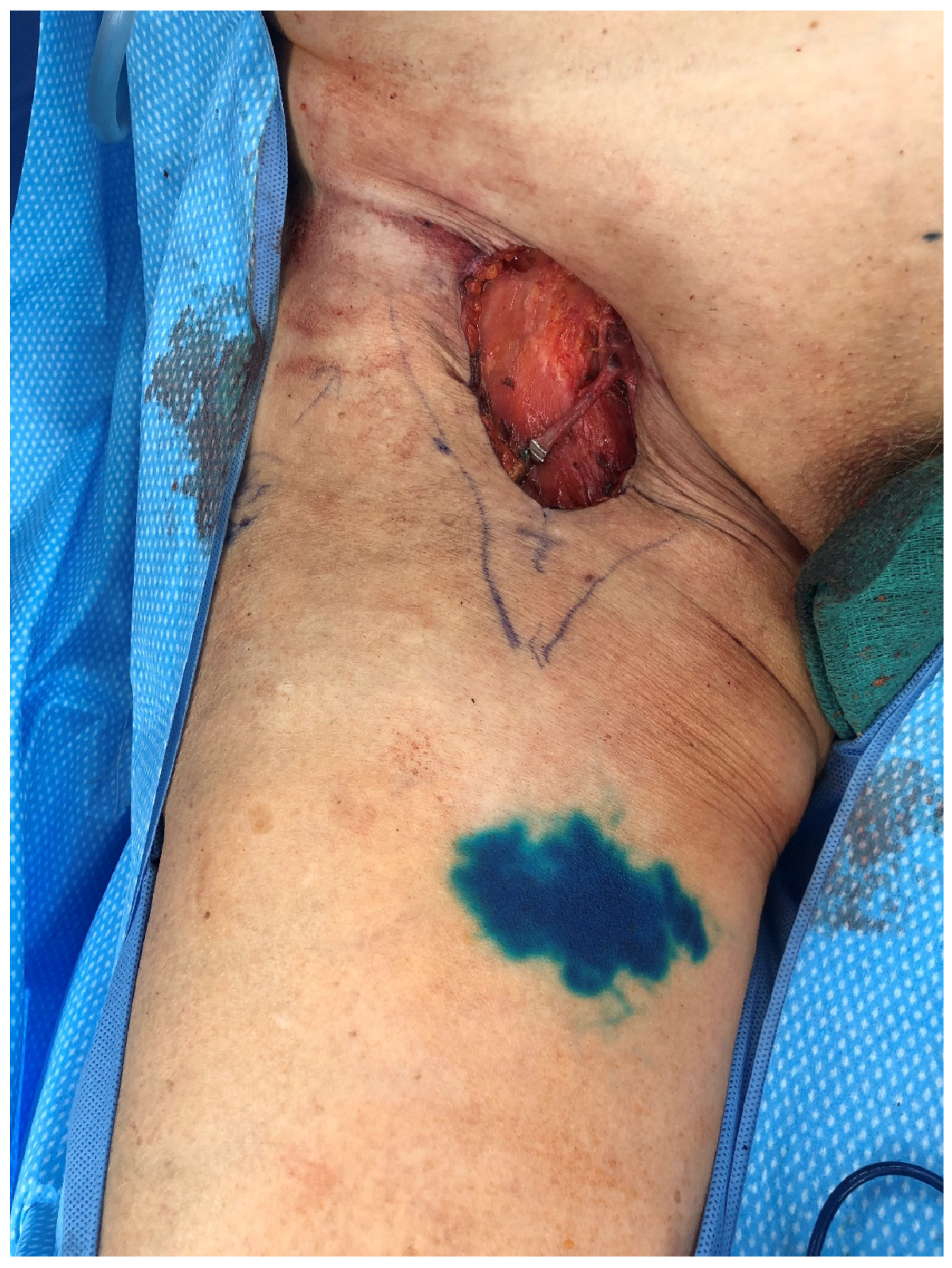
2.1.2. Vascularized Lymph Node Transfer (VLNT)
2.2. Lower Extremity
Immediate Lymphatic Reconstruction
3. Lymphedema Treatment
3.1. Upper Extremity
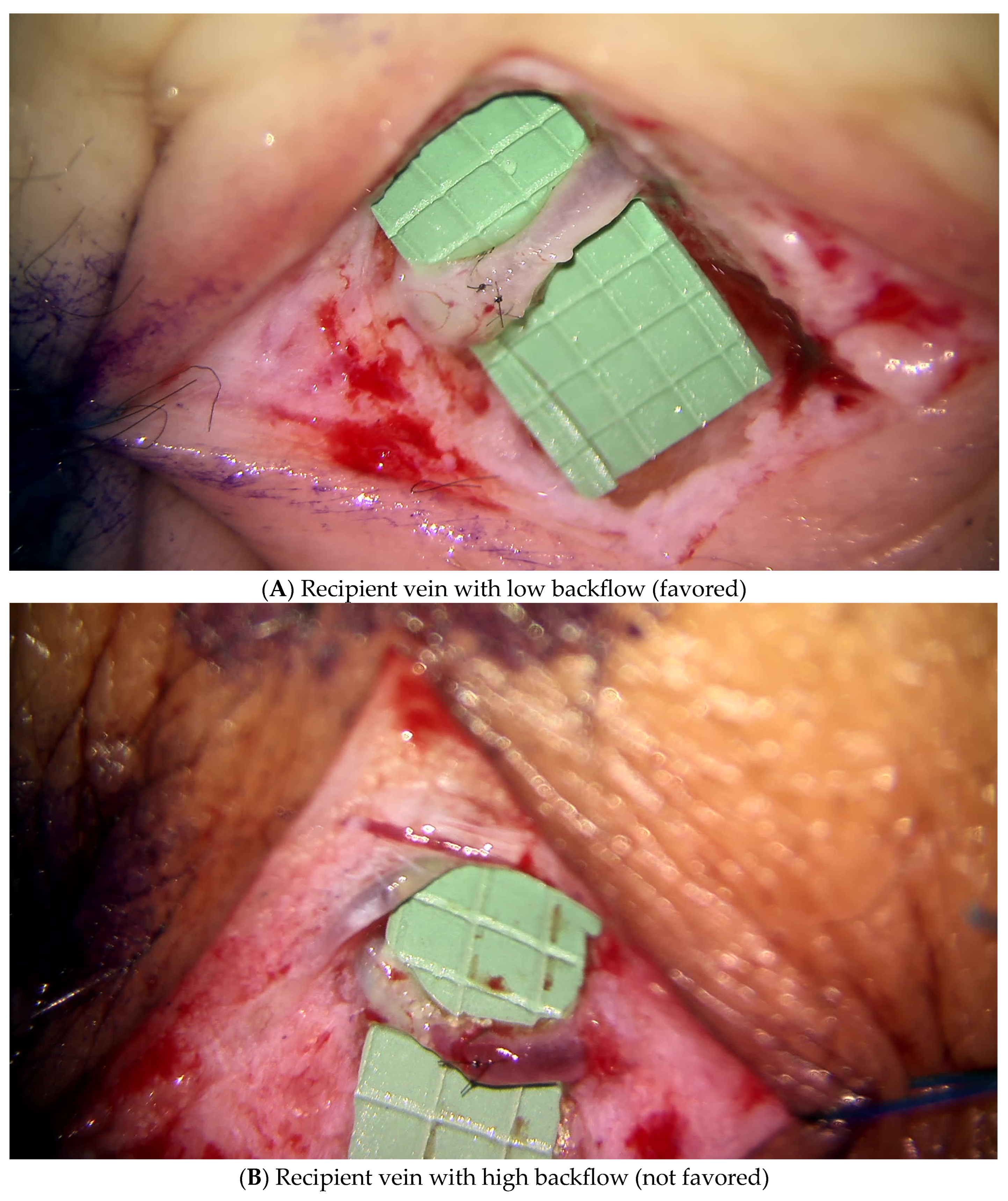
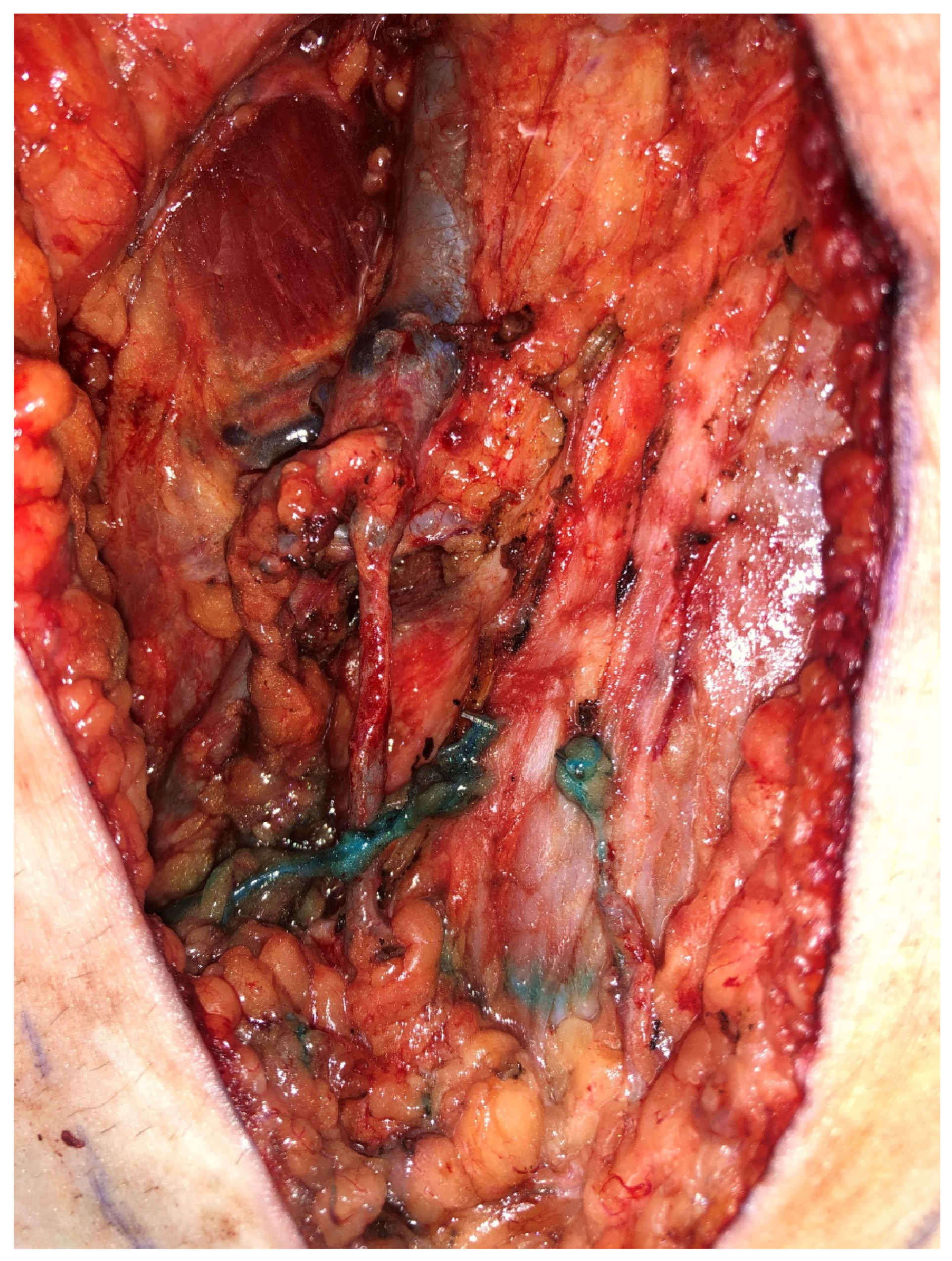
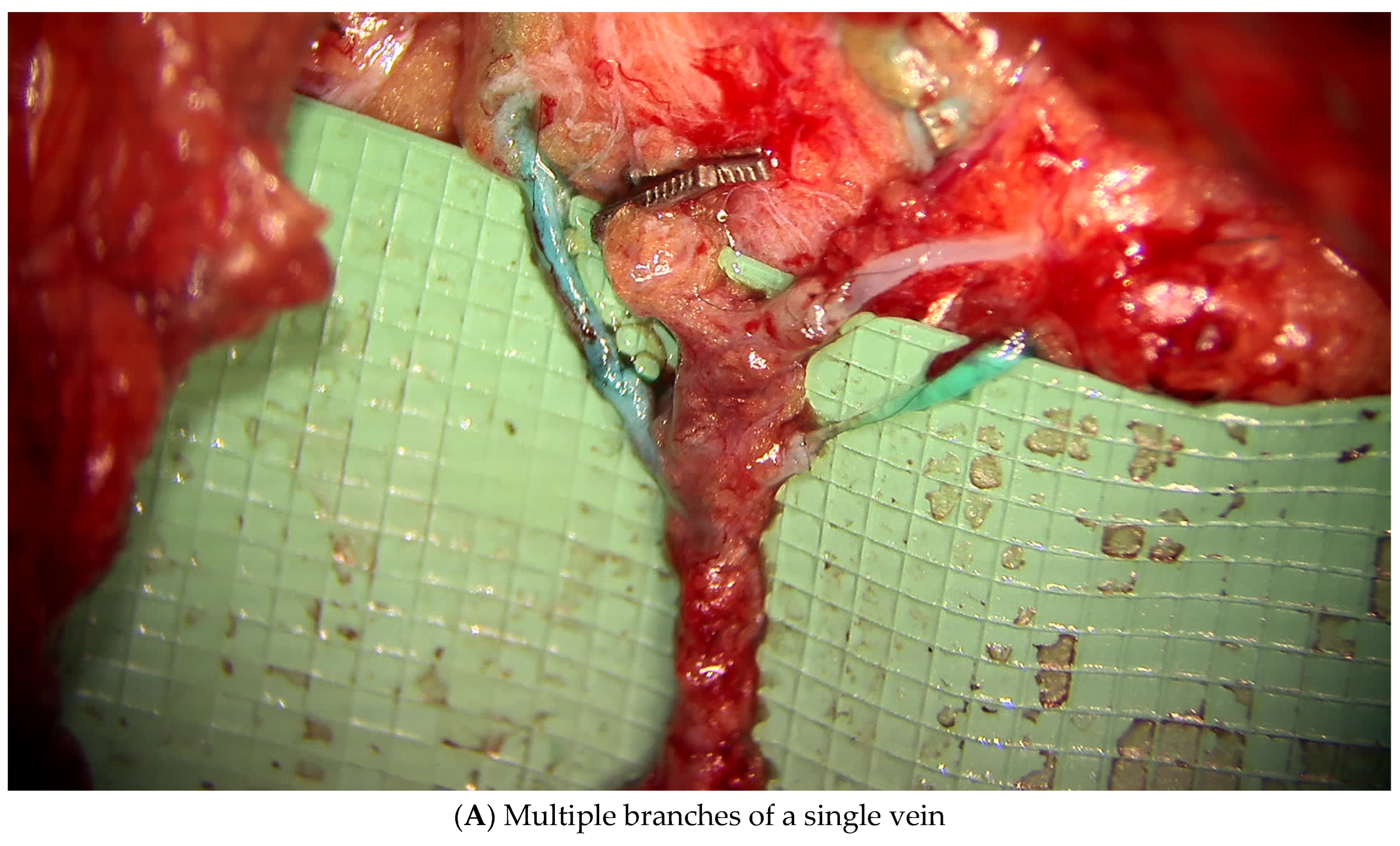
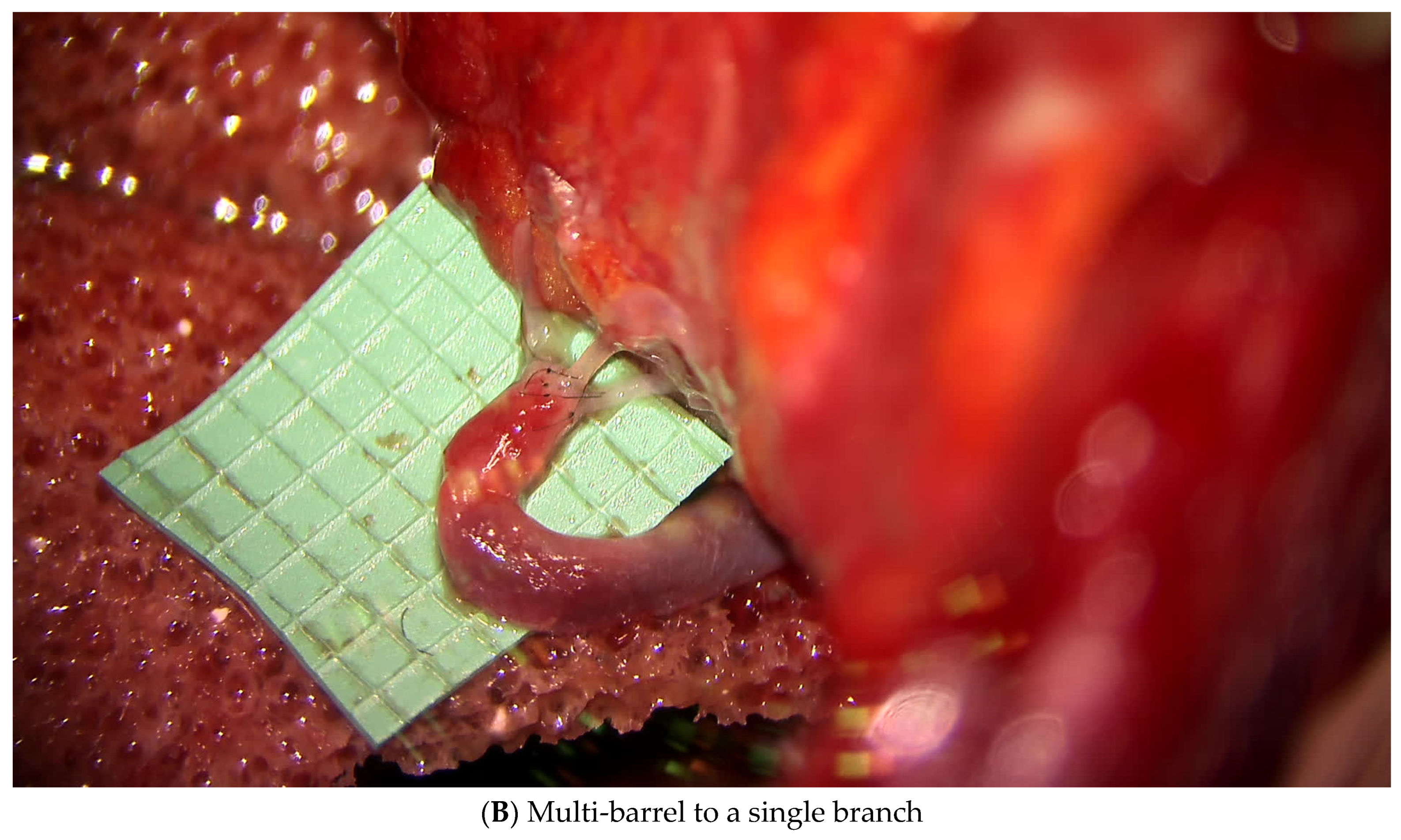
3.1.1. Lymphatic Mapping and Lymphovenous Bypass (LVB)
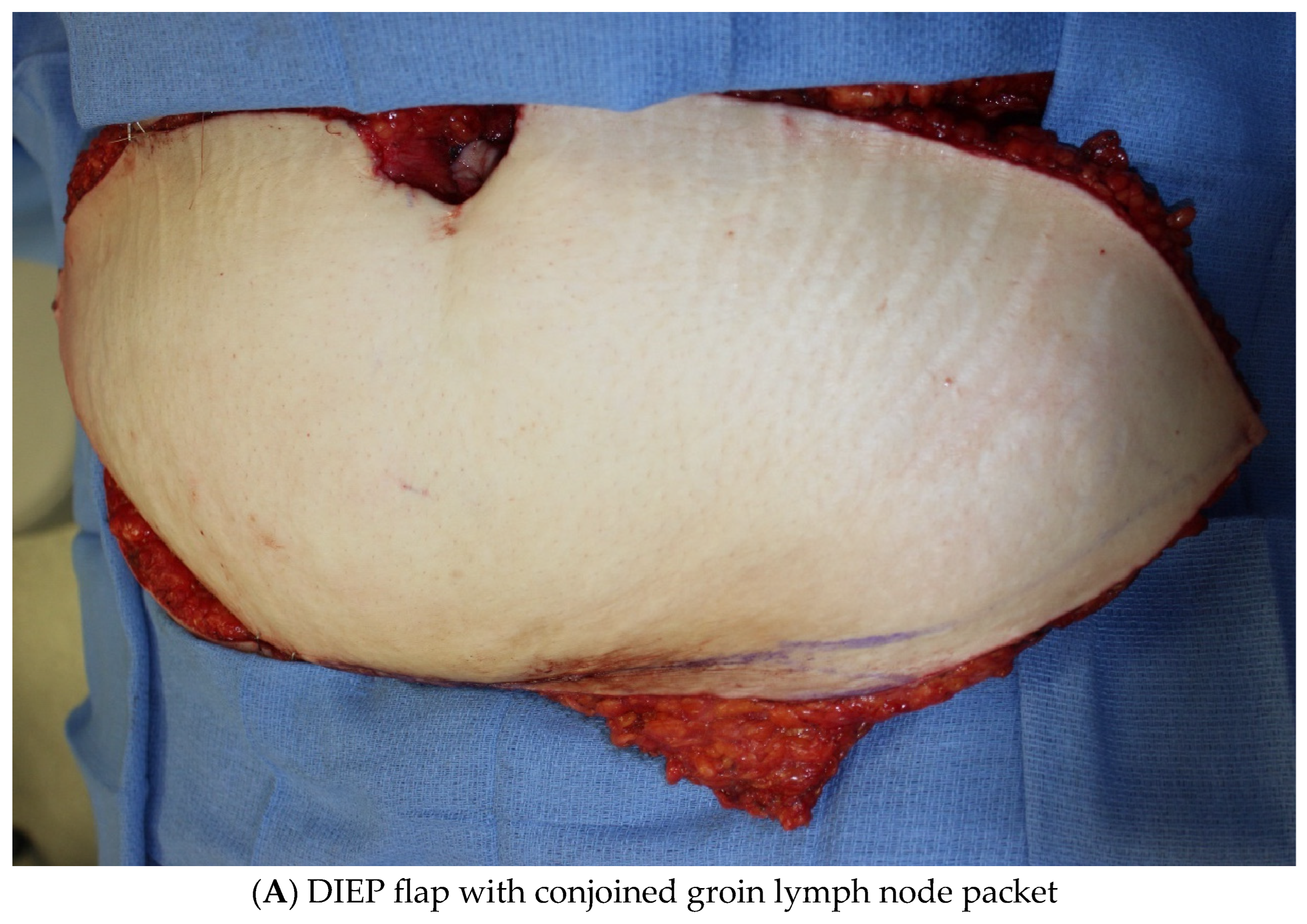
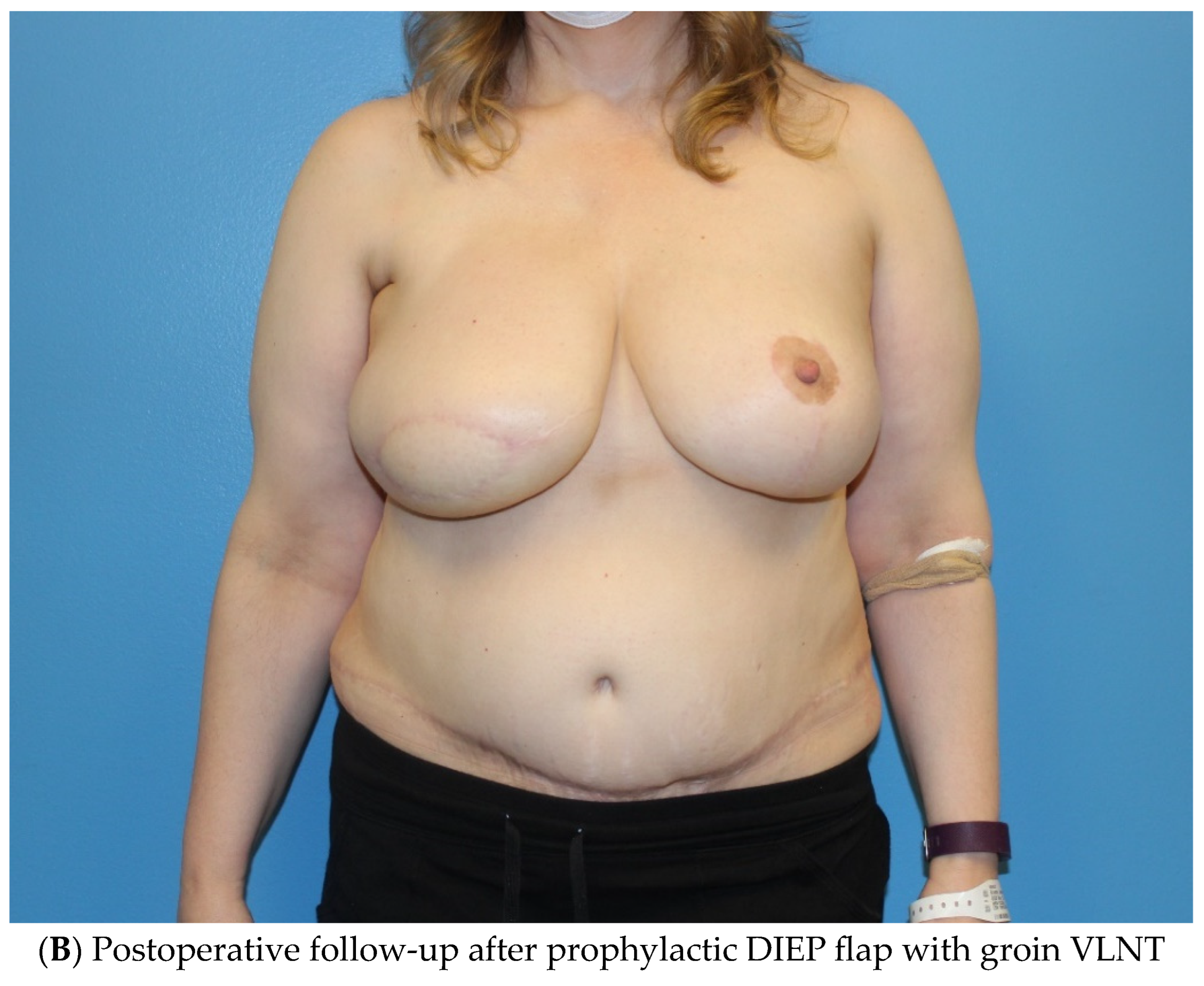
3.1.2. Vascularized Lymph Node Transfer (VLNT)
3.2. Lower Extremity
3.2.1. Lymphatic Mapping and Lymphovenous Bypass (LVB)
3.2.2. Vascularized Lymph Node Transfer (VLNT)
3.3. Adjunctive Procedures
3.3.1. Suction-Assisted Lipectomy
3.3.2. Excisional Procedures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rockson, S.G.; Rivera, K.K. Estimating the population burden of lymphedema. Ann. N. Y. Acad. Sci. 2008, 1131, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, C.J.; Franks, P.J.; Doherty, D.C.; Williams, A.F.; Badger, C.; Jeffs, E.; Bosanquet, N.; Mortimer, P.S. Lymphoedema: An underestimated health problem. QJM 2003, 96, 731–738. [Google Scholar] [CrossRef]
- Cormier, J.N.; Askew, R.L.; Mungovan, K.S.; Xing, Y.; Ross, M.I.; Armer, J.M. Lymphedema beyond breast cancer: A systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer 2010, 116, 5138–5149. [Google Scholar] [CrossRef]
- DiSipio, T.; Rye, S.; Newman, B.; Hayes, S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef]
- McDuff, S.G.R.; Mina, A.I.; Brunelle, C.L.; Salama, L.; Warren, L.E.G.; Abouegylah, M.; Swaroop, M.; Skolny, M.N.; Asdourian, M.; Gillespie, T.; et al. Timing of Lymphedema After Treatment for Breast Cancer: When Are Patients Most At Risk? Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Shaitelman, S.F.; Chiang, Y.J.; Griffin, K.D.; DeSnyder, S.M.; Smith, B.D.; Schaverien, M.V.; Woodward, W.A.; Cormier, J.N. Radiation therapy targets and the risk of breast cancer-related lymphedema: A systematic review and network meta-analysis. Breast Cancer Res. Treat. 2017, 162, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Erickson, V.S.; Pearson, M.L.; Ganz, P.A.; Adams, J.; Kahn, K.L. Arm edema in breast cancer patients. J. Natl. Cancer Inst. 2001, 93, 96–111. [Google Scholar] [CrossRef]
- Armer, J.M.; Stewart, B.R. Post-breast cancer lymphedema: Incidence increases from 12 to 30 to 60 months. Lymphology 2010, 43, 118–127. [Google Scholar]
- Passik, S.D.; McDonald, M.V. Psychosocial aspects of upper extremity lymphedema in women treated for breast carcinoma. Cancer 1998, 83, 2817–2820. [Google Scholar] [CrossRef]
- Fu, M.R.; Ridner, S.H.; Hu, S.H.; Stewart, B.R.; Cormier, J.N.; Armer, J.M. Psychosocial impact of lymphedema: A systematic review of literature from 2004 to 2011. Psychooncology 2013, 22, 1466–1484. [Google Scholar] [CrossRef]
- Chang, S.B.; Askew, R.L.; Xing, Y.; Weaver, S.; Gershenwald, J.E.; Lee, J.E.; Royal, R.; Lucci, A.; Ross, M.I.; Cormier, J.N. Prospective assessment of postoperative complications and associated costs following inguinal lymph node dissection (ILND) in melanoma patients. Ann. Surg. Oncol. 2010, 17, 2764–2772. [Google Scholar] [CrossRef]
- Burnett, A.F.; Stone, P.J.; Klimberg, S.V.; Gregory, J.L.; Roman, J.R. Lower extremity glandography (LEG): A new concept to identify and enhance lymphatic preservation. Int. J. Gynecol. Cancer 2011, 21, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Spillane, A.J.; Saw, R.P.; Tucker, M.; Byth, K.; Thompson, J.F. Defining lower limb lymphedema after inguinal or ilio-inguinal dissection in patients with melanoma using classification and regression tree analysis. Ann. Surg. 2008, 248, 286–293. [Google Scholar] [CrossRef]
- Che Bakri, N.A.; Kwasnicki, R.M.; Khan, N.; Ghandour, O.; Lee, A.; Grant, Y.; Dawidziuk, A.; Darzi, A.; Ashrafian, H.; Leff, D.R. Impact of Axillary Lymph Node Dissection and Sentinel Lymph Node Biopsy on Upper Limb Morbidity in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Ann. Surg. 2023, 277, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.W.; Sim, N.H.S.; Zhan, S.J.; Huang, J.J. The efficacy of immediate lymphatic reconstruction after axillary lymph node dissection—A meta-analysis. Eur. J. Surg. Oncol. 2025, 51, 109377. [Google Scholar] [CrossRef] [PubMed]
- Voss, R.K.; Cromwell, K.D.; Chiang, Y.J.; Armer, J.M.; Ross, M.I.; Lee, J.E.; Gershenwald, J.E.; Stewart, B.R.; Shaitelman, S.F.; Cormier, J.N. The long-term risk of upper-extremity lymphedema is two-fold higher in breast cancer patients than in melanoma patients. J. Surg. Oncol. 2015, 112, 834–840. [Google Scholar] [CrossRef]
- Boccardo, F.; Casabona, F.; De Cian, F.; Friedman, D.; Villa, G.; Bogliolo, S.; Ferrero, S.; Murelli, F.; Campisi, C. Lymphedema microsurgical preventive healing approach: A new technique for primary prevention of arm lymphedema after mastectomy. Ann. Surg. Oncol. 2009, 16, 703–708. [Google Scholar] [CrossRef]
- Chun, M.J.; Saeg, F.; Meade, A.; Kumar, T.; Toraih, E.A.; Chaffin, A.E.; Homsy, C. Immediate Lymphatic Reconstruction for Prevention of Secondary Lymphedema: A Meta-Analysis. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 1130–1141. [Google Scholar] [CrossRef]
- Levy, A.S.; Murphy, A.I.; Ishtihar, S.; Peysakhovich, A.; Taback, B.; Grant, R.T.; Ascherman, J.A.; Feldman, S.; Rohde, C.H. Lymphatic Microsurgical Preventive Healing Approach for the Primary Prevention of Lymphedema: A 4-Year Follow-Up. Plast. Reconstr. Surg. 2023, 151, 413–420. [Google Scholar] [CrossRef]
- Thompson, M.; Korourian, S.; Henry-Tillman, R.; Adkins, L.; Mumford, S.; Westbrook, K.C.; Klimberg, V.S. Axillary reverse mapping (ARM): A new concept to identify and enhance lymphatic preservation. Ann. Surg. Oncol. 2007, 14, 1890–1895. [Google Scholar] [CrossRef]
- De Brucker, B.; Zeltzer, A.; Seidenstuecker, K.; Hendrickx, B.; Adriaenssens, N.; Hamdi, M. Breast Cancer-Related Lymphedema: Quality of Life after Lymph Node Transfer. Plast. Reconstr. Surg. 2016, 137, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Chang, E.I.; Suami, H.; Chang, D.W. An algorithmic approach to simultaneous vascularized lymph node transfer with microvascular breast reconstruction. Ann. Surg. Oncol. 2015, 22, 2919–2924. [Google Scholar] [CrossRef]
- Saaristo, A.M.; Niemi, T.S.; Viitanen, T.P.; Tervala, T.V.; Hartiala, P.; Suominen, E.A. Microvascular breast reconstruction and lymph node transfer for postmastectomy lymphedema patients. Ann. Surg. 2012, 255, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Swan, M.C.; Furniss, D.; Cassell, O.C. Surgical management of metastatic inguinal lymphadenopathy. BMJ 2004, 329, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Morotti, M.; Menada, M.V.; Boccardo, F.; Ferrero, S.; Casabona, F.; Villa, G.; Campisi, C.; Papadia, A. Lymphedema microsurgical preventive healing approach for primary prevention of lower limb lymphedema after inguinofemoral lymphadenectomy for vulvar cancer. Int. J. Gynecol. Cancer 2013, 23, 769–774. [Google Scholar] [CrossRef]
- Cakmakoglu, C.; Kwiecien, G.J.; Schwarz, G.S.; Gastman, B. Lymphaticovenous Bypass for Immediate Lymphatic Reconstruction in Locoregional Advanced Melanoma Patients. J. Reconstr. Microsurg. 2020, 36, 247–252. [Google Scholar] [CrossRef]
- Boccardo, F.; Valenzano, M.; Costantini, S.; Casabona, F.; Morotti, M.; Sala, P.; De Cian, F.; Molinari, L.; Spinaci, S.; Dessalvi, S.; et al. LYMPHA Technique to Prevent Secondary Lower Limb Lymphedema. Ann. Surg. Oncol. 2016, 23, 3558–3563. [Google Scholar] [CrossRef]
- Torres Lacomba, M.; Yuste Sanchez, M.J.; Zapico Goni, A.; Prieto Merino, D.; Mayoral del Moral, O.; Cerezo Tellez, E.; Minayo Mogollon, E. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: Randomised, single blinded, clinical trial. BMJ 2010, 340, b5396. [Google Scholar] [CrossRef]
- Engel, H.; Lin, C.Y.; Huang, J.J.; Cheng, M.H. Outcomes of Lymphedema Microsurgery for Breast Cancer-related Lymphedema With or Without Microvascular Breast Reconstruction. Ann. Surg. 2018, 268, 1076–1083. [Google Scholar] [CrossRef]
- Jeong, H.H.; Yoon, I.A.; Al-Shomer, F.M.; Suh, H.P.; Pak, C.J.; Neligan, P.; Hong, J.P. Decompression of Axillary Vein: An Essential Adjunct for Advanced Lymphedema. Plast. Reconstr. Surg. 2024, 154, 218–226. [Google Scholar] [CrossRef]
- Baumann, D.P.; Crosby, M.A.; Selber, J.C.; Garvey, P.B.; Sacks, J.M.; Adelman, D.M.; Villa, M.T.; Feng, L.; Robb, G.L. Optimal timing of delayed free lower abdominal flap breast reconstruction after postmastectomy radiation therapy. Plast. Reconstr. Surg. 2011, 127, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Ramaut, L.; De Baerdemaeker, R.; Zeltzer, A. Decreasing donor site morbidity after groin vascularized lymph node transfer with lessons learned from a 12-year experience and review of the literature. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.I.; Chu, C.K.; Hanson, S.E.; Selber, J.C.; Hanasono, M.M.; Schaverien, M.V. Comprehensive Overview of Available Donor Sites for Vascularized Lymph Node Transfer. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2675. [Google Scholar] [CrossRef]
- Scaglioni, M.F.; Arvanitakis, M.; Chen, Y.C.; Giovanoli, P.; Chia-Shen Yang, J.; Chang, E.I. Comprehensive review of vascularized lymph node transfers for lymphedema: Outcomes and complications. Microsurgery 2018, 38, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, T.; Mackie, H.; Koelmeyer, L.; Heydon-White, A.; Ricketts, R.; Toyer, K.; Boyages, J.; Brorson, H.; Lam, T. Liposuction for Advanced Lymphedema in a Multidisciplinary Team Setting in Australia: 5-Year Follow-Up. Plast. Reconstr. Surg. 2024, 153, 482–491. [Google Scholar] [CrossRef]
- Chen, W.F.; Pandey, S.K.; Lensing, J.N. Does Liposuction for Lymphedema Worsen Lymphatic Injury? Lymphology 2023, 56, 3–12. [Google Scholar] [CrossRef]
- Hassan, K.; Chang, D.W. The Charles Procedure as Part of the Modern Armamentarium Against Lymphedema. Ann. Plast. Surg. 2020, 85, e37–e43. [Google Scholar] [CrossRef]
- Losco, L.; Bolletta, A.; de Sire, A.; Chen, S.H.; Sert, G.; Aksoyler, D.; Velazquez-Mujica, J.; Invernizzi, M.; Cigna, E.; Chen, H.C. The Combination of Lymph Node Transfer and Excisional Procedures in Bilateral Lower Extremity Lymphedema: Clinical Outcomes and Quality of Life Assessment with Long-Term Follow-Up. J. Clin. Med. 2022, 11, 570. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francis, A.M.; Kopplin, N.G.; Chang, E.I. The MD Anderson Algorithm for Lymphedema Management. J. Clin. Med. 2025, 14, 1851. https://doi.org/10.3390/jcm14061851
Francis AM, Kopplin NG, Chang EI. The MD Anderson Algorithm for Lymphedema Management. Journal of Clinical Medicine. 2025; 14(6):1851. https://doi.org/10.3390/jcm14061851
Chicago/Turabian StyleFrancis, Ashleigh M., Noa G. Kopplin, and Edward I. Chang. 2025. "The MD Anderson Algorithm for Lymphedema Management" Journal of Clinical Medicine 14, no. 6: 1851. https://doi.org/10.3390/jcm14061851
APA StyleFrancis, A. M., Kopplin, N. G., & Chang, E. I. (2025). The MD Anderson Algorithm for Lymphedema Management. Journal of Clinical Medicine, 14(6), 1851. https://doi.org/10.3390/jcm14061851




