A Cross-Sectoral Telemedicine Network (sekTOR-HF) for Patients with Heart Failure
Abstract
1. Introduction
2. Methods
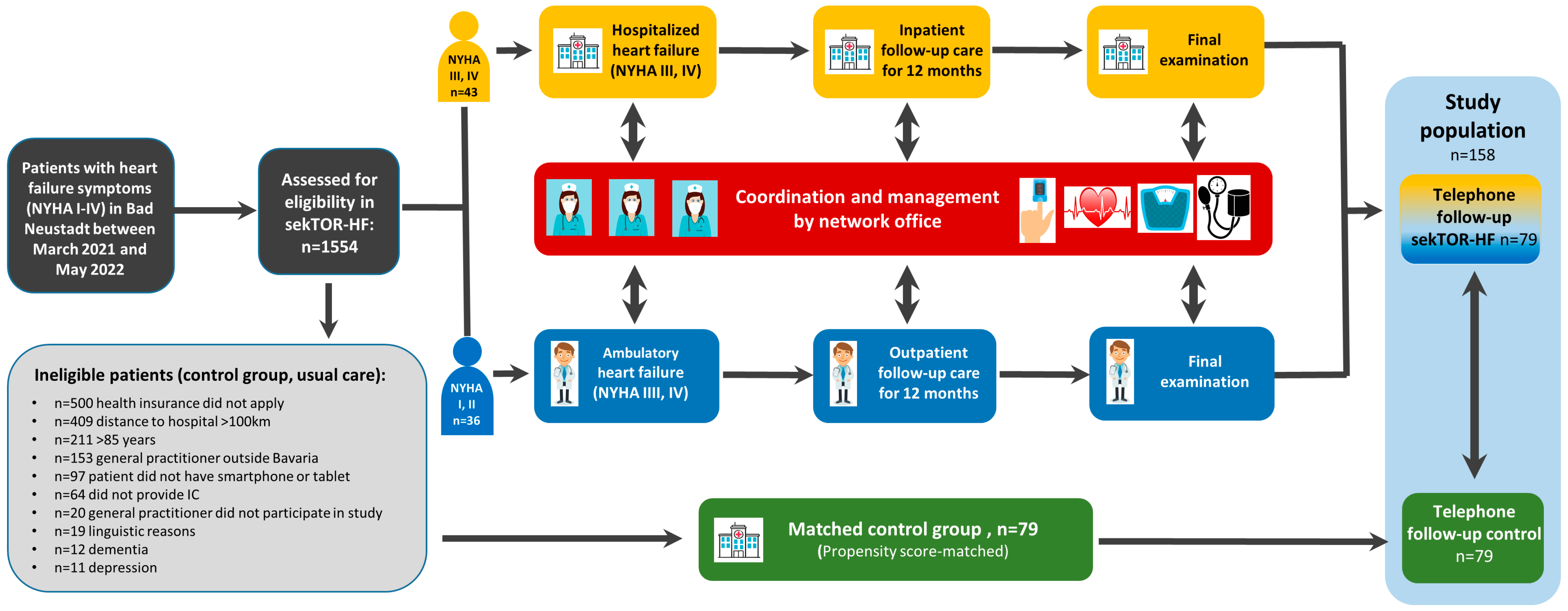
2.1. Study Population and Design of sekTOR-HF
2.2. Study Endpoints
2.3. Statistical Analysis
2.4. Propensity Score Matching
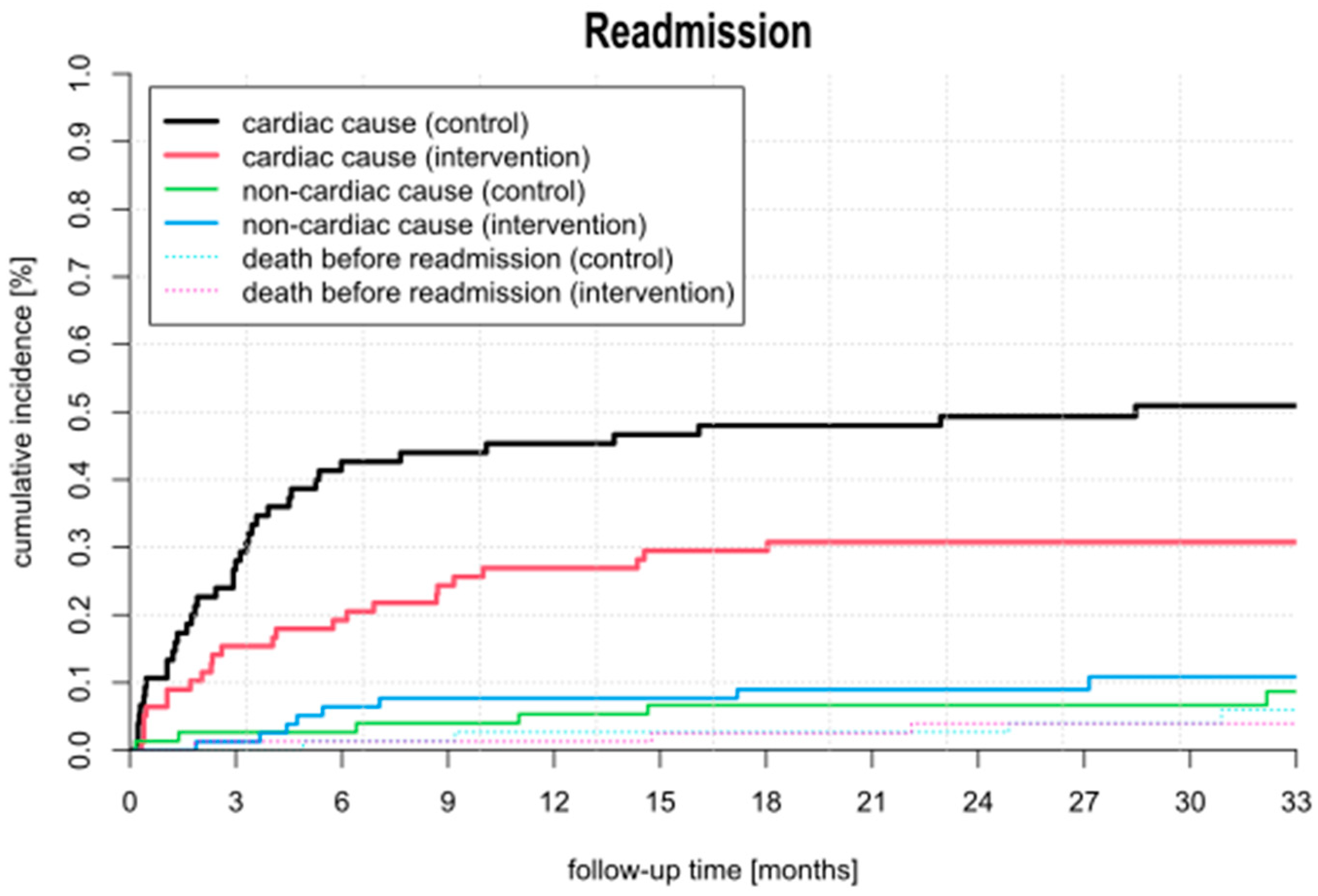
2.5. Cumulative Incidence
3. Results
3.1. Baseline Characteristics and Medical History
3.2. Medication
3.3. Compliance
3.4. Follow-Up and Outcome
4. Discussion
4.1. Rehospitalization
4.2. All-Cause Mortality
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Urbich, M.; Globe, G.; Pantiri, K.; Heisen, M.; Bennison, C.; Wirtz, H.S.; Di Tanna, G.L. A systematic review of medical costs associated with heart failure in the USA (2014–2020). PharmacoEconomics 2020, 38, 1219–1236. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV) and Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF). Nationale Versorgungsleitlinie Chronische Herzinsuffizienz—Kurzfassung, 2. Auflage. Version 1. 2017. Available online: https://register.awmf.org/de/leitlinien/detail/nvl-0064.0 (accessed on 1 December 2009).
- Bekfani, T.; Fudim, M.; Cleland, J.G.; Jorbenadze, A.; von Haehling, S.; Lorber, A.; Rothman, A.M.; Stein, K.; Abraham, W.T.; Sievert, H.; et al. A current and future outlook on upcoming technologies in remote monitoring of patients with heart failure. Eur. J. Heart Fail. 2021, 23, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Inglis, S.C.; Clark, R.A.; McAlister, F.A.; Stewart, S.; Cleland, J.G. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: Abridged Cochrane review. Eur J Heart Fail. 2011, 13, 1028–1040. [Google Scholar]
- Jonkman, N.H.; Westland, H.; Groenwold, R.H.; Ågren, S.; Anguita, M.; Blue, L.; de la Porte, P.W.B.-A.; DeWalt, D.A.; Hebert, P.L.; Heisler, M.; et al. What are effective program characteristics of selfmanagement interventions in patients with heart failure? An individual patient data meta-analysis. J. Card. Fail. 2016, 22, 861–871. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, X.; Xu, C. Effectiveness of telemedicine systems for adults with heart failure: A metaanalysis of randomized controlled trials. Heart Fail. Rev. 2020, 25, 231–243. [Google Scholar] [CrossRef]
- Ertl, G.; Angermann, C.E.; Bekeredjian, R.; Beyersdorf, F.; Güder, G.; Gummert, J.; Katus, H.A.; Kindermann, I.; Pauschinger, P.; Perings, S. Structure and organization of heart failure networks (HF-NETs) and heart failure units (HFUs) to optimize treatment of acute and chronic heart failure. Jt. Recomm. DGK DGTHG Treat. Heart Fail. Kardiol. 2016, 10, 222–235. [Google Scholar]
- Störk, S.; Handrock, R.; Jacob, J.; Walker, J.; Calado, F.; Lahoz, R.; Hupfer, S.; Klebs, S. Treatment of chronic heart failure in Germany: A retrospective database study. Clinl Res. Cardiol. 2017, 106, 923–932. [Google Scholar] [CrossRef]
- Sundmacher, L.; Fischbach, D.; Schuettig, W.; Naumann, C.; Augustin, U.; Faisst, C. Which hospitalisations are ambulatory care-sensitive, to what degree, and how could the rates be reduced? Results of a group consensus study in Germany. Health Policy 2015, 119, 1415–1423. [Google Scholar] [CrossRef]
- McAlister, F.A.; Youngson, E.; Bakal, J.A.; Kaul, P.; Ezekowitz, J.; van Walraven, C. Impact of physician continuity on death or urgent readmission after discharge among patients with heart failure. CMAJ 2013, 185, E681–E689. [Google Scholar] [CrossRef]
- Kuss, O. The z-difference can be used to measure covariate balance in matched propensity score analyses. J. Clin. Epidemiol. 2013, 66, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.J. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann. Stat. 1988, 16, 1141–1154. [Google Scholar]
- Statistisches Bundesamt der Bundesrepublik Deutschland, Sterbetafel 2017/2019. Available online: https://www.mdpi.com/authors/references (accessed on 17 February 2025).
- Angermann, C.E.; Störk, S.; Gelbrich, G.; Faller, H.; Jahns, R.; Frantz, S.; Loeffler, M.; Georg Ertl, M.D. Mode of action and effects of standardized collaborative disease management on mortality and morbidity in patients with systolic heart failure: The Interdisciplinary Network for Heart Failure (INH) study. Circ. Heart Fail. 2012, 5, 25–35. [Google Scholar] [CrossRef] [PubMed]
- DeVore, A.D.; Granger, B.B.; Fonarow, G.C.; Al-Khalidi, H.R.; Albert, N.M.; Lewis, E.F.; Butler, J.; Piña, I.L.; Allen, L.A.; Yancy, C.W. Effect of a Hospital and Postdischarge Quality Improvement Intervention on Clinical Outcomes and Quality of Care for Patients With Heart Failure With Reduced Ejection Fraction: The CONNECT-HF Randomized Clinical Trial. JAMA 2021, 326, 314–323. [Google Scholar] [CrossRef]
- Angermann, C.E.; Sehner, S.; Faller, H.; Güder, G.; Morbach, C.; Frantz, S.; Wegscheider, K.; Ertl, G.; Störk, S. Longer-Term Effects of Remote Patient Management Following Hospital Discharge After Acute Systolic Heart Failure: The Randomized E-INH Trial. JACC Heart Fail. 2023, 11, 191–206. [Google Scholar] [CrossRef]
- Cleland, J.G.; Louis, A.A.; Rigby, A.S.; Janssens, U.; Balk, A.H. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: The Trans-European Network- Home-Care Management System (TEN-HMS) study. J. Am. Coll. Cardiol. 2005, 45, 1654–1664. [Google Scholar] [CrossRef]
- Fiuzat, M.; Ezekowitz, J.; Alemayehu, W.; Westerhout, C.M.; Sbolli, M.; Cani, D.; Whellan, D.J.; Ahmad, T.; Adams, K.; Piña, I.L.; et al. Assessment of limitations to optimization of guideline-directed medical therapy in heart failure from the GUIDE-IT trial: A secondary analysis of a randomized clinical trial. JAMA Cardiol. 2020, 5, 757–764. [Google Scholar] [CrossRef]
- Greene, S.J.; Butler, J.; Albert, N.M.; DeVore, A.D.; Sharma, P.P.; Duffy, C.I.; Hill, C.L.; McCague, K.; Mi, X.; Patterson, J.H.; et al. Medical therapy for heart failure with reduced ejection fraction: The CHAMP-HF registry. J. Am. Coll. Cardiol. 2018, 72, 351–366. [Google Scholar] [CrossRef]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.-A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel- roup, unmasked trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef]
- Mariani, M.V.; Pierucci, N.; Forleo, G.B.; Schiavone, M.; Bernardini, A.; Gasperetti, A.; Mitacchione, G.; Mei, M.; Giunta, G.; Piro, A.; et al. The Feasibility, Effectiveness and Acceptance of Virtual Visits as Compared to In-Person Visits among Clinical Electrophysiology Patients during the COVID-19 Pandemic. J. Clin. Med. 2023, 12, 620. [Google Scholar] [CrossRef]
- Koehler, F.; Koehler, K.; Prescher, S.; Kirwan, B.A.; Wegscheider, K.; Vettorazzi, E.; Lezius, S.; Winkler, S.; Moeller, V.; Fiss, G.; et al. Mortality and morbidity 1 year after stopping a remote patient management intervention: Extended follow-up results from the elemedical interventional management in patients with heart failure II (TIM-HF2) randomised trial. Lancet Digit. Health. 2020, 2, E16–E24. [Google Scholar] [CrossRef]
- Frederix, I.; Vanderlinden, L.; Verboven, A.-S.; Welten, M.; Wouters, D.; De Keulenaer, G.; Ector, B.; Elegeert, I.; Troisfontaines, P.; Weytjens, C.; et al. Long-term impact of a six-month telemedical care programme on mortality, heart failure readmissions and healthcare costs in patients with chronic heart failure. J. Telemed. Telecare 2019, 25, 286–293. [Google Scholar] [CrossRef]


| Total (n = 158) | Control (n = 79) | Intervention (n = 79) | Squared Z-Differences | |
|---|---|---|---|---|
| Demographic | ||||
| Age, y | 65 ± 13 | 65 ± 12 | 0.0193 | |
| Female | 56 (35.4) | 28 (35.4) | 28 (35.4) | 0.0000 |
| Health Insurance | ||||
| AOK | 98 (62.0) | 49 (62.0) | 49 (62.0) | 0.0000 |
| DAK | 26 (16.5) | 13 (16.5) | 13 (16.5) | 0.0000 |
| TKK | 34 (21.5) | 17 (21.5) | 17 (21.5) | 0.0000 |
| Marital Status | ||||
| single | 18 (11.4) | 9 (11.4) | 9 (11.4) | 0.0000 |
| married | 113 (71.5) | 57 (72.2) | 56 (70.9) | −0.1763 |
| widowed | 25 (15.8) | 13 (16.5) | 12 (15.2) | |
| divorced | 2 (1.3) | 0 (0.0) | 2 (2.5) | |
| Distance to the clinic (km) | 47.1 ± 47.3 | 50.0 ± 37.3 | 0.4157 | |
| Heart Failure Symptoms | 1.0146 | |||
| NYHA functional Class I | 5 (3.2) | 2 (2.5) | 3 (3.8) | |
| NYHA functional Class III | 62 (39.2) | 29 (36.7) | 33 (41.8) | |
| NYHA functional Class III | 80 (50.6) | 41 (51.9) | 39 (49.4) | |
| NYHA functional Class IV | 11 (7.0) | 7 (8.9) | 4 (5.1) | |
| Comorbidities and Medical History | ||||
| Coronary artery disease | 91 (57.6) | 43 (54.4) | 48 (60.8) | 0.8066 |
| PCI | 54 (34.2) | 26 (32.9) | 28 (35.4) | |
| Cardiac Surgery | ||||
| CABG | 11 (7.0) | 2 (2.5) | 9 (11.5) | |
| Heart Valve Surgery | 7 (4.5) | 2 (2.5) | 5 (6.4) | |
| Combined Surgery (CABG + Valve) | 4 (2.5) | 3 (3.8) | 1 (1.3) | |
| LVAD | 1 (0.6) | 0 (0.0) | 1 (1.3) | |
| M-TEER | 2 (1.3) | 2 (2.5) | 0 (0.0) | |
| Peripheral vascular disease | 12 (7.6) | 6 (7.6) | 6 (7.6) | 0.0000 |
| Hypertension | 154 (97.5) | 76 (96.2) | 78 (98.7) | 1.0162 |
| Atrial Fibrillation | 65 (41.1) | 34 (43.0) | 31 (39.2) | −0.4854 |
| Atrial Flutter | 8 (5.1) | 1 (1.3) | 7 (9.1) | 2.2298 |
| Supraventricular Extrasystoles | 2 (2.5) | 0 (0.0) | 2 (1.3) | 1.4325 |
| Ventricular Extrasystoles | 27 (17.2) | 6 (7.6) | 21 (26.9) | 3.3095 |
| Pacemaker and/or ICD | 54 (34.2) | 26 (32.9) | 28 (35.4) | 0.3356 |
| COPD | 18 (11.4) | 6 (7.6) | 12 (15.2) | 1.5132 |
| Hypercholesterolemia | 128 (81.0) | 61 (77.2) | 67 (84.8) | 1.2228 |
| Diabetes mellitus | 45 (28.5) | 22 (27.8) | 23 (29.1) | −0.1763 |
| Dietary therapy | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Oral Hypoglycemic Agents | 21 (13.3) | 13 (16.5) | 12 (15.2) | |
| Insulin | 25 (15.8) | 14 (17.7) | 11 (13.9) | 0.4789 |
| Depression | 6 (3.8) | 3 (3.8) | 3 (3.8) | 0.0000 |
| Family history of premature CHD | 22 (13.9) | 12 (15.2) | 10 (12.7) | |
| Smoker (last 2 month) | 20 (12.7) | 10 (12.7) | 10 (12.7) | |
| Former smoker | 24 (15.3) | 13 (16.5) | 11 (14.1) | |
| Alcohol Abuse | 3 (1.9) | 0 (0.0) | 3 (3.8) | 1.7659 |
| Myocarditis | 9 (5.7) | 4 (5.1) | 5 (6.3) | |
| Stroke | 12 (7.6) | 8 (10.1) | 4 (5.1) | |
| ICM | 81 (51.3) | 41 (51.9) | 40 (50.6) | |
| DCM | 69 (43.7) | 25 (31.6) | 44 (55.7) | |
| HCM | 2 (1.3) | 2 (2.5) | 0 (0.0) | |
| HOCM | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Toxic CM | 4 (2.5) | 1 (1.3) | 3 (3.8) | |
| Tachycardiomyopthy | 64 (40.5) | 40 (50.6) | 24 (30.4) | |
| Measurements | ||||
| EuroSCORE II | 7.7 ± 10 | 5.5 ± 9 | 9.9 ± 14 | |
| BMI, kg/m2 | 31.1 ± 10 | 30.6 ± 6 | 31.6 ± 15 | 0.5810 |
| LVEF, % | 39.5 ± 14 | 41 ± 15 | 38 ± 13 | −1.2405 |
| LVESD | 46 ± 11 | 44 ± 13 | 46 ± 11 | |
| LVEDD | 55 ± 9 | 52 ± 9 | 58 ± 9 | |
| sPAP | 33 ± 12 | 31 ± 9 | 34 ± 13 | |
| Mitral Valve Regurgitation, grade ≥2 | 24 (15.5) | 10 (13.3) | 14 (17.7) | |
| Aortic Valve Stenosis, grade 3 | 11 (7.2) | 9 (12.3) | 2 (2.5) | |
| Tricuspid Valve Regurgitation, grade ≥3 | 3 (1.9) | 2 (2.7) | 1 (1.3) | |
| Laboratory values | ||||
| Creatinine, mg/dl | 1.14 ± 0.5 | 1.15 ± 0.5 | 1.13 ± 0.4 | −0.2228 |
| NT-proBNP, pg/ml | 2222 ± 3196 | 2346 ± 2995 | 2098 ± 3397 | −0.4037 |
| Glomerular Filtration Rate, ml/min/1.73m2 | 70.6 ± 30 | 73.1 ± 41 | 68.0 ± 22 | −0.9756 |
| Discharge Medication | ||||
| ACE/ARB | 79 (50.3) | 43 (55.1) | 36 (45.6) | |
| ARNI | 65 (41.4) | 27 (34.6) | 38 (48.1) | |
| Beta-blocker | 154 (98.1) | 27 (97.4) | 78 (98.7) | |
| Diuretic | 111 (70.7) | 52 (66.7) | 59 (74.7) | |
| MRA | 83 (52.9) | 39 (50.0) | 44 (55.7) | |
| SGLT2 Inhibitor | 69 (43.9) | 25 (32.1) | 44 (55.7) | |
| Calcium Antagonist | 25 (15.9) | 19 (24.4) | 6 (7.6) | |
| Statin | 115 (98.1) | 58 (74.4) | 57 (72.2) | |
| Ivabradine | 3 (1.9) | 1 (1.3) | 2 (2.5) | |
| Digitalis | 2 (1.3) | 1 (1.3) | 1 (1.3) | |
| OAC | 74 (46.8) | 37 (46.8) | 37 (46.8) | |
| Cumarine | 23 (14.6) | 8 (10.1) | 15 (19.0) | |
| DOAC | 51 (32.2) | 29 (36.7) | 22 (27.8) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barth, S.; Hautmann, M.; Reents, W.; Trajkovski, G.; Gebhard, B.; Kerber, S.; Zacher, M.; Divchev, D.; Schieffer, B. A Cross-Sectoral Telemedicine Network (sekTOR-HF) for Patients with Heart Failure. J. Clin. Med. 2025, 14, 1840. https://doi.org/10.3390/jcm14061840
Barth S, Hautmann M, Reents W, Trajkovski G, Gebhard B, Kerber S, Zacher M, Divchev D, Schieffer B. A Cross-Sectoral Telemedicine Network (sekTOR-HF) for Patients with Heart Failure. Journal of Clinical Medicine. 2025; 14(6):1840. https://doi.org/10.3390/jcm14061840
Chicago/Turabian StyleBarth, Sebastian, Martina Hautmann, Wilko Reents, Goran Trajkovski, Brigitte Gebhard, Sebastian Kerber, Michael Zacher, Dimitar Divchev, and Bernhard Schieffer. 2025. "A Cross-Sectoral Telemedicine Network (sekTOR-HF) for Patients with Heart Failure" Journal of Clinical Medicine 14, no. 6: 1840. https://doi.org/10.3390/jcm14061840
APA StyleBarth, S., Hautmann, M., Reents, W., Trajkovski, G., Gebhard, B., Kerber, S., Zacher, M., Divchev, D., & Schieffer, B. (2025). A Cross-Sectoral Telemedicine Network (sekTOR-HF) for Patients with Heart Failure. Journal of Clinical Medicine, 14(6), 1840. https://doi.org/10.3390/jcm14061840






