Is a Perioperative Opioid-Sparing Anesthesia-Analgesia Strategy Feasible in Open Thoracotomies? Findings from a Retrospective Matched Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Anesthesia-Analgesia Management
2.3. Study Outcomes
2.3.1. Pain and Opioid Consumption
2.3.2. Other Outcomes
- How would you assess your pain now, at this moment? (0–10)
- How severe was the worst pain during the past 4 weeks? (0–10)
- How severe was the pain during the past 4 weeks on average? (0–10)
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Primary Outcome
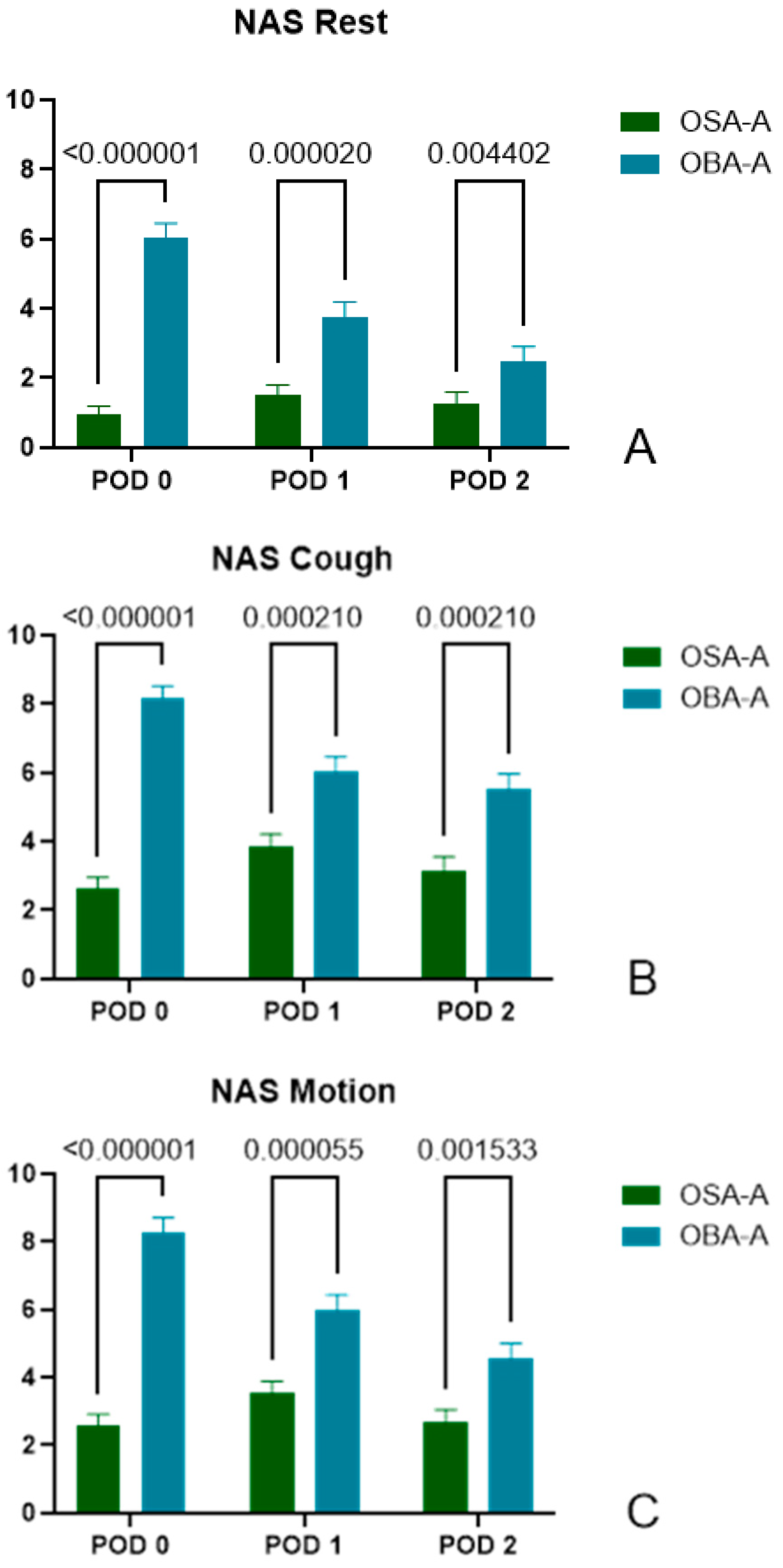
3.2.1. Pain Assessment
3.2.2. Pain Score at Rest
- POD0: (OSA-A) 0.95 ± 1.92, n = 60 vs. (OBA-A) 6.05 ± 2.56, n = 40, p < 0.000001.
- POD1: (OSA-A) 1.53 ± 2.12, n = 57 vs. (OBA-A) 3.78 ± 2.66, n = 40, p = 0.000020.
- POD2: (OSA-A) 1.28 ± 2.34, n = 54 vs. (OBA-A) 2.47 ± 2.57, n = 34, p = 0.004402.
3.2.3. Pain Score During Cough
- POD0: (OSA-A) 2.62 ± 2.67, n = 60 vs. (OBA-A) 8.18 ± 2.14, n = 39, p < 0.000001.
- POD1: (OSA-A) 3.84 ± 2.83, n = 57 vs. (OBA-A) 6.03 ± 2.78, n = 40, p = 0.000210.
- POD2: (OSA-A) 3.15 ± 2.99, n = 54 vs. (OBA-A) 5.51 ± 2.65, n = 35, p = 0.000210.
3.2.4. Pain Score During Motion
- POD0: (OSA-A) 2.58 ± 2.49, n = 60 vs. (OBA-A) 8.29 ± 2.65, n = 38, p < 0.000001.
- POD1: (OSA-A) 3.54 ± 2.61, n = 57 vs. (OBA-A) 5.98 ± 2.92, n = 40, p = 0.000055.
- POD2: (OSA-A) 2.69 ± 2.70, n = 54 vs. (OBA-A) 4.54 ± 2.76, n = 35, p = 0.001533.
3.3. Secondary Outcomes
3.3.1. PACU and Hospital Length of Stay
3.3.2. Rescue Analgesia and Morphine Consumption in PACU and on POD1 and POD2
3.3.3. Gastrointestinal Motility
3.3.4. Nausea-Vomiting
3.3.5. Chronic Pain
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OSA-A | Opioid-Sparing Anesthesia-Analgesia |
| OBA-A | Opioid-Based Anesthesia-Analgesia |
| PACU | Post Anesthesia Care Unit |
| PTPS | Post-Thoracotomy Pain Syndrome |
| MED | Morphine Equivalent Dose |
| ASA | American Society of Anesthesiology |
| RSI | Rapid Sequence Induction |
| NSAID | Non-Steroidal Anti-Inflammatory Drug |
| PCA | Patient Controlled Analgesia |
| POD | Post-Operative Day |
| NRS | Numerical Rate Scale |
| ANOVA | ANalysis Of Variance |
| FDR | False Discovery Rate |
| BMI | Body Mass Index |
| VATS | Video-Assisted Thoracoscopic Surgery |
| OFA | Opioid-Free Anesthesia |
| OSA | Opioid-Sparing Anesthesia |
| OBA | Opioid-Based Anesthesia |
| PVB | ParaVertebral Block |
| SAPB | Serratus Anterior Plane Block |
| ESPB | Erector Spinae Plane Block |
| RCT | Randomized Controlled Trial |
| NMDA | N-Methyl-D-aspartic Acid |
| CPTP | chronic post-thoracotomy pain |
| ICD | International Classification of Disease |
References
- Schwarzova, K.; Whitman, G.; Cha, S. Developments in Postoperative Analgesia in Open and Minimally Invasive Thoracic Surgery Over the Past Decade. Semin. Thorac. Cardiovasc. Surg. 2024, 36, 378–385. [Google Scholar] [CrossRef]
- Bello, M.; Oger, S.; Bedon-Carte, S.; Vielstadte, C.; Leo, F.; Zaouter, C.; Ouattara, A. Effect of Opioid-Free Anaesthesia on Postoperative Epidural Ropivacaine Requirement After Thoracic Surgery: A Retrospective Unmatched Case-Control Study. Anaesth. Crit. Care Pain Med. 2019, 38, 499–505. [Google Scholar] [CrossRef]
- Mesbah, A.; Yeung, J.; Gao, F. Pain after Thoracotomy. BJA Educ. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Arends, S.; Böhmer, A.B.; Poels, M.; Schieren, M.; Koryllos, A.; Wappler, F.; Joppich, R. Post-thoracotomy pain syndrome: Seldom severe, often neuropathic, treated unspecific, and insufficient. Pain Rep. 2020, 5, e810. [Google Scholar] [CrossRef] [PubMed]
- Bayman, E.O.; Brennan, T.J. Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: Meta-analysis. J. Pain 2014, 15, 887–897. [Google Scholar] [CrossRef]
- Andersen, C.; Ørding, H.; Licht, P.B.; Toft, P. From acute to chronic pain after thoracic surgery: The significance of different components of the acute pain response. J. Pain Res. 2018, 11, 1541–1548. [Google Scholar]
- Goto, T. What Is the Best Pain Control After Thoracic Surgery? J. Thorac. Dis. 2018, 10, 1335–1338. [Google Scholar] [CrossRef]
- D’Ercole, F.; Arora, H.; Kumar, P.A. for Thoracic Surgery. Cardiothorac. Vasc. Anesth. 2018, 32, 915–927. [Google Scholar] [CrossRef]
- Lee, L.A.; Caplan, R.A.; Stephens, L.S.; Posner, K.L.; Terman, G.W.; Voepel-Lewis, T.; Domino, K.B. Postoperative opioid-induced respiratory depression: A closed claims analysis. Anesthesiology 2015, 122, 659–665. [Google Scholar] [CrossRef]
- Xiao, J.; Nguyen, D.T.; Lichtenberg, Z.K.; Rizk, E.; Meisenbach, L.M.; Chihara, R.; Graviss, E.A.; Kim, M.P. Opioid use in thoracic surgery: A retrospective study on postoperative complications. J. Thorac. Dis. 2024, 16, 6827–6834. [Google Scholar] [CrossRef]
- Huh, J.; Hwang, W. The Role of Anesthetic Management in Lung Cancer Recurrence and Metastasis: A Comprehensive Review. J. Clin. Med. 2024, 13, 6681. [Google Scholar] [CrossRef]
- D’Amico, F.; Barucco, G.; Licheri, M.; Valsecchi, G.; Zaraca, L.; Mucchetti, M.; Zangrillo, A.; Monaco, F. Opioid Free Anesthesia in Thoracic Surgery: A Systematic Review and Meta Analysis. J. Clin. Med. 2022, 11, 6955. [Google Scholar] [CrossRef]
- Mauermann, E.; Ruppen, W.; Bandschapp, O. Different protocols used today to achieve total opioid-free general anesthesia without locoregional blocks. Best. Pract. Res. Clin. Anaesthesiol. 2017, 31, 533–545. [Google Scholar] [CrossRef]
- Şentürk, M.; Özcan, P.E.; Talu, G.K.; Kiyan, E.; Çamci, E.; Özyalçin, S.; Dilege, Ş.; Pembeci, K. The Effects of Three Different Analgesia Techniques on Long-Term Postthoracotomy Pain. Anesth. Analg. 2002, 94, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://esaic.org/wp-content/uploads/2023/12/conversion-tables-for-iv-and-oral-analgesics.pdf (accessed on 28 December 2024).
- Freynhagen, R.; Baron, R.; Gockel, U.; Tölle, T.R. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med. Res. Opin. 2006, 22, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.D.W.; Cole, C.M.W.; Lo, W.; Ura, M. Postoperative Pain in Thoracic Surgical Patients: An Analysis of Factors Associated with Acute and Chronic Pain. Heart Lung Circ. 2021, 30, 1244–1250. [Google Scholar] [CrossRef]
- Sengupta, S. Post-operative pulmonary complications after thoracotomy. Indian J. Anaesth. 2015, 59, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Patvardhan, C.; Ferrante, M. Opiate free anaesthesia and future of thoracic surgery anaesthesia. J. Vis. Surg. 2018, 4, 253. [Google Scholar] [CrossRef]
- Hah, J.M.; Bateman, B.T.; Ratliff, J.; Curtin, C.; Sun, E. Chronic Opioid Use After Surgery: Implications for Perioperative Management in the Face of the Opioid Epidemic. Anesth. Analg. 2017, 125, 1733–1740. [Google Scholar] [CrossRef]
- Wiltse Nicely, K.L.; Friend, R.; Robichaux, C.; Edwards, J.A.; Cimiotti, J.P.; Dupree Jones, K. Association Between Intra- and Postoperative Opioids in Opioid-Naïve Patients in Thoracic Surgery. Ann. Thorac. Surg. Short. Rep. 2024, 2, 865–870. [Google Scholar] [CrossRef]
- Chancellor, W.Z.; Mehaffey, J.H.; Desai, R.P.; Beller, J.; Balkrishnan, R.; Walters, D.M.; Martin, L.W. Prolonged Opioid Use Associated with Reduced Survival After Lung Cancer Resection. Ann. Thorac. Surg. 2021, 111, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Fornasari, D. Pharmacotherapy for Neuropathic Pain: A Review. Pain Ther. 2017, 6 (Suppl. S1), 25–33. [Google Scholar] [CrossRef]
- Moisset, X. Neuropathic pain: Evidence based recommendations. Presse Med. 2024, 53, 104232. [Google Scholar] [CrossRef]
- Forget, P. Opioid-free anaesthesia. Why and how? A contextual analysis. Anaesth. Crit. Care Pain Med. 2019, 38, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Guastella, V.; Mick, G.; Soriano, C.; Vallet, L.; Escande, G.; Dubray, C.; Eschalier, A. A prospective study of neuropathic pain induced by thoracotomy: Incidence, clinical description, and diagnosis. Pain 2011, 152, 74–81. [Google Scholar] [CrossRef]
- Homma, T.; Doki, Y.; Yamamoto, Y.; Ojima, T.; Shimada, Y.; Kitamura, N.; Yoshimura, N. Risk factors of neuropathic pain after thoracic surgery. J. Thorac. Dis. 2018, 10, 2898–2907. [Google Scholar] [CrossRef] [PubMed]
- Colvin, L.A.; Bull, F.; Hales, T.G. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet 2019, 393, 1558–1568. [Google Scholar] [CrossRef]
- Humble, S.R.; Dalton, A.J.; Li, L. Interventions to reduce acute and chronic post-surgical pain. EJP 2015, 19, 451–465. [Google Scholar] [CrossRef]
- Devine, G.; Cheng, M.; Martinez, G.; Patvardhan, C.; Aresu, G.; Peryt, A.; Coonar, A.S.; Roscoe, A. Opioid-Free Anesthesia for Lung Cancer Resection: A Case-Control Study. J. Cardiothorac. Vasc. Anesth. 2020, 34, 3036–3040. [Google Scholar] [CrossRef]
- Brulotte, V.; Ruel, M.M.; Lafontaine, E.; Chouinard, P.; Girard, F. Impact of Pregabalin on the occurrence of postthoracotomy pain syndrome: A randomized trial. Reg. Anesth. Pain Med. 2015, 40, 262–269. [Google Scholar] [CrossRef]
- Fabritius, M.L.; Geisler, A.; Petersen, P.L.; Nikolajsen, L.; Hansen, M.S.; Kontinen, V.; Hamunen, K.; Dahl, J.B.; Wetterslev, J.; Mathiesen, O. Gabapentin for post-operative pain management—A systematic review with meta-analyses and trial sequential analyses. Acta Anaesthesiol. Scand. 2016, 60, 1188–1208, Erratum in Acta Anaesthesiol. Scand. 2017, 61, 357–359. [Google Scholar]
- Yalşi, E.; Yakşi, O. Current treatment options for post-thoracotomy pain syndrome: A review. Curr. Thorac. Surg. 2017, 2, 103–110. [Google Scholar]
- Homma, T.; Doki, Y.; Yamamoto, Y.; Ojima, T.; Shimada, Y.; Kitamura, N.; Akemoto, Y.; Hida, Y.; Yoshimura, N. Efficacy of 50 mg pregabalin for prevention of postoperative neuropathic pain after video-assisted thoracoscopic surgery and thoracotomy: A 3-month prospective randomized controlled trial. J. Thorac. Dis. 2019, 11, 694–701. [Google Scholar] [CrossRef]
- Matsutani, N.; Kawamura, M. Successful management of postoperative pain with pregabalin after thoracotomy. Surg. Today 2014, 44, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Matsutani, N.; Dejima, H.; Takahashi, Y.; Kawamura, M. Pregabalin reduces post-surgical pain after thoracotomy: A prospective, randomized, controlled trial. Surg. Today 2015, 45, 1411–1416. [Google Scholar] [CrossRef]
- Kim, J.C.; Byun, S.; Kim, S.; Lee, S.Y.; Lee, J.H.; Ahn, S. Effect of preoperative pregabalin as an adjunct to a multimodal analgesic regimen in video-assisted thoracoscopic surgery: A randomized controlled trial. Medicine 2017, 96, e8644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, H. The efficacy of pregabalin for pain control after thoracic surgery: A meta-analysis. J. Cardiothorac. Surg. 2024, 19, 4. [Google Scholar] [CrossRef]
- Jouguelet-Lacoste, J.; La Colla, L.; Schilling, D.; Chelly, J.E. The use of intravenous infusion or single dose of low-dose ketamine for postoperative analgesia: A review of the current literature. Pain Med. 2015, 16, 383–403. [Google Scholar] [CrossRef]
- Gharaei, B.; Jafari, A.; Aghamohammadi, H.; Salimi, A. Opioid-sparing effect of preemptive bolus low-dose ketamine for moderate sedation in opioid abusers undergoing extracorporeal shock wave lithotripsy: A randomized clinical trial. Anesth. Analg. 2013, 116, 75–80. [Google Scholar] [CrossRef]
- Moyse, D.W.; Kaye, A.D.; Diaz, J.H.; Qadri, M.Y.; Lindsay, D.; Pyati, S. Perioperative Ketamine Administration for Thoracotomy Pain. Pain Physician 2017, 20, 173–184. [Google Scholar]
- Schmid, R.L.; Sandler, A.N.; Katz, J. Use and efficacy of low-dose ketamine in the management of acute postoperative pain: A review of current techniques and outcomes. Pain 1999, 82, 111–125. [Google Scholar] [CrossRef]
- Srebro, D.; Vuckovic, S.; Milovanovic, A.; Kosutic, J.; Vujovic, K.S.; Prostran, M. Magnesium in Pain Research: State of the Art. Curr. Med. Chem. 2017, 24, 424–434. [Google Scholar]
- Vanstone, R.J.; Rockett, M. Use of atypical analgesics by intravenous infusion (IV) for acute pain: Evidence base for lidocaine, ketamine and magnesium. Anesth. Intensive Care Med. 2016, 17, 460–463. [Google Scholar] [CrossRef]
- Hung, K.C.; Yang, S.H.; Liao, S.W.; Yu, C.H.; Liu, M.Y.; Chen, J.Y. Effects of perioperative magnesium on postoperative analgesia following thoracic surgery: A meta-analysis of randomised controlled trials. Magnes. Res. 2024, 36, 54–68. [Google Scholar] [CrossRef]
- Salah Abdelgalil, A.; Shoukry, A.; Kamel, M.; Heikal, A.; Ahmed, N. Analgesic Potentials of Preoperative Oral Pregabalin, Intravenous Magnesium Sulfate, and their Combination in Acute Postthoracotomy Pain. Clin. J. Pain 2019, 35, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Dexmedetomidine: Present and future directions. Korean J. Anesthesiol. 2019, 72, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Weerink, M.A.S.; Struys, M.; Hannivoort, L.N.; Barends, C.R.M.; Absalom, A.R.; Colin, P. Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin. Pharmacokinet. 2017, 56, 893–913. [Google Scholar] [CrossRef] [PubMed]
- Beloeil, H.; Garot, M.; Lebuffe, G.; Gerbaud, A.; Bila, J.; Cuvillon, P.; Dubout, E.; Oger, S.; Nadaud, J.; Becret, A.; et al. Balanced Opioid-free Anesthesia with Dexmedetomidine versus Balanced Anesthesia with Remifentanil for Major or Intermediate Noncardiac Surgery. Anesthesiology 2021, 134, 541–551. [Google Scholar] [CrossRef]
- Zhao, Y.; He, J.; Yu, N.; Jia, C.; Wang, S. Mechanisms of Dexmedetomidine in Neuropathic Pain. Front. Neurosci. 2020, 14, 330. [Google Scholar] [CrossRef]
- Wang, K.; Wu, M.; Xu, J.; Wu, C.; Zhang, B.; Wang, G.; Ma, D. Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: Systematic review and meta-analysis. Br. J. Anaesth. 2019, 123, 777–794. [Google Scholar]
- Mena, G.E.; Zorrilla-Vaca, A.; Vaporciyan, A.; Mehran, R.; Lasala, J.D.; Williams, W.; Patel, C.; Woodward, T.; Kruse, B.; Joshi, G.; et al. Intraoperative Dexmedetomidine and Ketamine Infusions in an Enhanced Recovery After Thoracic Surgery Program: A Propensity Score Matched Analysis. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Larue, A.; Jacquet-Lagreze, M.; Ruste, M.; Tronc, F.; Fellahi, J.L. Opioid-free anaesthesia for video-assisted thoracoscopic surgery: A retrospective cohort study with propensity score analysis. Anaesth. Crit. Care Pain Med. 2022, 41, 101089. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Doi, R.; Matsumoto, K. Post-thoracotomy pain syndrome in the era of minimally invasive thoracic surgery. J. Thorac. Dis. 2024, 16, 3422–3430. [Google Scholar] [CrossRef] [PubMed]
- Gerner, P. Postthoracotomy pain management problems. Anesthesiol. Clin. 2008, 26, 355–367, vii. [Google Scholar] [CrossRef]
- Hersini, K.J.; Andreasen, J.J.; Birthe Dinesen, P.G.; Arendt-Nielsen, L. Prevalence, characteristics and impact of the post-thoracotomy pain syndrome on quality of life: A cross-sectional study. J. Pain Reli 2015, 4, 1000201. [Google Scholar] [CrossRef]
- Bayman, E.O.; Parekh, K.R.; Keech, J.; Selte, A.; Brennan, T.J. A prospective study of chronic pain after thoracic surgery. Anesthesiology 2017, 126, 938–951. [Google Scholar] [CrossRef]
- Kampe, S.; Geismann, B.; Weinreich, G.; Stamatis, G.; Ebmeyer, U.; Gerbershagen, H.J. The influence of type of anesthesia, perioperative pain, and preoperative health status on chronic pain six months after thoracotomy—A prospective cohort study. Pain Med. 2016, 18, pnw230. [Google Scholar] [CrossRef]
- Hetmann, F.; Kongsgaard, U.E.; Sandvik, L.; Schou-Bredal, I. Prevalence and predictors of persistent post-surgical pain 12 months after thoracotomy. Acta Anaesthesiol. Scand. 2015, 59, 740–748. [Google Scholar] [CrossRef]
- Hetmann, F.; Kongsgaard, U.E.; Sandvik, L.; Schou-Bredal, I. Post-thoracotomy pain syndrome and sensory disturbances following thoracotomy at 6- and 12-month follow-ups. J. Pain Res. 2017, 10, 663–668. [Google Scholar] [CrossRef]
- Bayman, E.O.; Lennertz, R.; Brennan, T.J. Pain-related limitations in daily activities following thoracic surgery in a United States population. Pain Physician 2017, 20, E367–E378. [Google Scholar] [CrossRef]
- Perttunen, K.; Tasmuth, T.; Kalso, E. Chronic pain after thoracic surgery: A follow-up study. Acta Anaesthesiol. Scand. 1999, 43, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Schug, S.A.; Lavand’homme, P.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.D. The IASP classification of chronic pain for ICD-11, chronic postsurgical or posttraumatic pain. Pain 2019, 160, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Chen, D.; McNicol, E.; Sharma, L.; Varaday, G.; Sharma, A.; Wilson, E.; Wright-Yatsko, T.; Yaeger, L.; Gilron, I.; et al. Risk factors for persistent pain after breast and thoracic surgeries: A systematic literature review and meta-analysis. Pain 2022, 163, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, H.; Jensen, T.S.; Woolf, C.J. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006, 367, 1618–1625. [Google Scholar] [CrossRef]


| OBA-A Group | OSA-A Group | Comments | |
|---|---|---|---|
| Premedication Night before surgery | Anxiolysis Bromazepam 1.5 mg orally (po) | Pregabalin 25–150 mg po Amitriptyline 10 mg po | Pregabalin tailored to patients’ needs/status Low-dose Amitriptyline for its antinociceptive and anti-salivary actions, if not contraindicated due to patients’ comorbidities |
| Premedication Day of surgery | Midazolam 0.05 mg/kg intramuscularly (im) | Pregabalin 25–150 mg po Amitriptyline 10 mg po | |
| Anesthesia Induction | intravenously (iv) iv Fentanyl 150–200 mcg | iv Dexmedetomidine loading dose 1 mcg/kg administrated over 15 min | |
| iv Propofol 2–2.5 mg/kg | iv Midazolam 2–5 mg | ||
| iv Rocuronium 0.6 mg/kg (1.2 mg/kg if RSI) | iv Ketamine 0.5–1.0 mg/kg | ||
| Or | iv Propofol 1–2 mg/kg | ||
| iv Cis-Atracurium 0.2 mg/kg | iv Lidocaine 1 mg/kg | ||
| * Surgical site infiltration prior to incision with Ropivacaine (10–15 mL Ropivacaine 0.75%) | iv Rocuronium 0.6 mg/kg (1.2 mg/kg if RSI) | ||
| iv Magnesium Sulphate 30–40 mg/kg as bolus over 15 min | |||
| iv Dexamethasone 8–16 mg | |||
| * Surgical site infiltration prior to incision with Ropivacaine (10–15 mL Ropivacaine 0.75%) | |||
| Prior to incision | iv Fentanyl 50–100 mcg | iv Dexmedetomidine infusion 0.6–1.2 mcg/kg/h | |
| Or | iv Lidocaine infusion 1 mg/kg/h | ||
| iv Remifentanil infusion 0.05–0.25 mcg/kg/h | iv Paracetamol 1 gr | ||
| iv NSAID (Parecoxib 40 mg or Dexketoprofen 50 mg) | |||
| Anesthesia maintenance | Volatile anesthesia | Volatile anesthesia | |
| Intraoperative antinociception | iv Remifentanil infusion 0.05–0.25 mcg/kg/h | iv Lidocaine infusion 0.5–1 mg/kg/h | |
| Or | iv Dexmedetomidine 0.4–1 mcg/kg/h | ||
| iv boluses of Fentanyl 50–100 mcg as required | iv boluses of 20–30 mg of Ketamine as required | ||
| iv bolus of 2.5 g Magnesium sulphate | |||
| 20 min prior to surgical closure | iv NSAID if no contraindication (Parecoxib 40 mg or Dexketoprofen 50 mg) | iv bolus of 20–30 mg of Ketamine | |
| iv Paracetamol 1 g | iv Tramadol 100 mg | ||
| iv Morphine 0.05–0.15 mg/kg | |||
| PACU | iv bolus of Morphine 2 mg | iv Ketamine 30–50 mg | If patient’s pain score at rest ≥6 in the numerical rating scale score 0–10 |
| ± iv Magnesium sulphate 2.5 g | |||
| ± iv Midazolam 1–2 mg | |||
| iv Pethidine 20–30 mg | iv Pethidine 20–30 mg | If shivering | |
| iv Tramadol 100 mg | Rescue therapy | ||
| Surgical Ward 48 h after surgery | iv Paracetamol 1 gr every 8 h | iv Paracetamol 1 gr every 8 h | |
| iv PCA Morphine (Morphine solution 0.5 mg/mL) with an infusion rate 0.5–1 mg/h and possibility of bolus 1 mg every 10 min, if no contraindication, under continuous monitoring with pulse oximetry | iv Tramadol (max daily dose 300 mg) | ||
| po Pregabalin 25–150 mg daily dose, given in titrated doses | |||
| Rescue therapy: im Pethidine 50–75 mg |
| Total Daily Dose (IV) | Morphine Equivalent Dose (mg) |
|---|---|
| 1 mcg fentanyl iv | 0.066 mg morphine iv |
| 1 mg oxycodone iv | 1.5 mg morphine iv |
| 1 mg tramadol | 0.1 mg morphine iv |
| 1 mg pethidine iv | 0.13 mg morphine iv |
| Characteristic | OSA-A ( ± SD) | OBA-A ( ± SD) | p-Value | Statistical Test | |
|---|---|---|---|---|---|
| Age (y) | 62.55 ± 11.29 | 63.73 ± 12.55 | 0.6270 | Unpaired two-tailed t test | |
| Weight (kg) | 79.50 ± 15.33 | 77.43 ± 13.28 | 0.4864 | Unpaired two-tailed t test | |
| Height (m) | 1.698 ± 0.07095 | 1.678 ± 0.08057 | 0.1996 | Unpaired two-tailed t test | |
| BMI (kg ∗ m−2) | 27.56 ± 5.050 | 27.40 ± 3.751 | 0.8658 | Unpaired two-tailed t test | |
| Sex | Male | 48 | 28 | 0.3394 | Fisher’s exact test two-sided |
| Female | 12 | 12 | |||
| ASA physical status | 1 | 0 | 1 | 0.0208 | Fisher’s exact test two-sided |
| 2 | 39 | 16 | |||
| 3 | 21 | 22 | |||
| 4 | 0 | 1 | |||
| Type of surgery | Lobectomy | 39 | 23 | 0.3728 | Fisher’s exact test two-sided |
| Segmentectomy | 14 | 12 | |||
| Pneumonectomy | 3 | 0 | |||
| Other (biopsy, talc pleurodesis etc.) | 4 | 5 | |||
| Depression | Yes | 7 | 7 | 0.5576 | Fisher’s exact test two-sided |
| No | 53 | 33 | |||
| Anxiety | Yes | 20 | 11 | 0.6599 | Fisher’s exact test two-sided |
| No | 40 | 29 | |||
| Alcohol Use Disorder | Yes | 6 | 1 | 0.2375 | Fisher’s exact test two-sided |
| No | 54 | 39 | |||
| Preoperative chronic pain medication use | Yes | 13 | 3 | 0.0930 | Fisher’s exact test two-sided |
| No | 47 | 37 | |||
| Preoperative chronic opioid use | Yes | 3 | 1 | 0.6479 | Fisher’s exact test two-sided |
| No | 57 | 39 | |||
| Preoperative Steroid use (6 months) | Yes | 5 | 0 | 0.0813 | Fisher’s exact test two-sided |
| No | 55 | 40 |
| Secondary Outcome | OSA-A ( ± SD) | OBA-A ( ± SD) | p-Value | Statistical Test | |
|---|---|---|---|---|---|
| Length of stay PACU (h) | 2.030 ± 1.022 | 3.125 ± 1.036 | <0.0001 | Mann-Whitney U test | |
| Length of stay Hospital (d) | 5.767 ± 3.451 | 5.725 ± 2.050 | 0.2669 | Mann-Whitney U test | |
| Analgesics Requested PACU (POD 0) | Yes | 21 | 27 | 0.0021 | Fisher’s exact test |
| No | 39 | 13 | |||
| Analgesics Requested POD 1 | Yes | 3 | 2 | >0.9999 | Fisher’s exact test |
| No | 57 | 38 | |||
| Analgesics Requested POD 2 | Yes | 1 | 0 | >0.9999 | Fisher’s exact test |
| No | 59 | 40 | |||
| Morphine (mg) Equivalents Delivered | PACU | 1.177 ± 2.929 | 5.031 ± 5.705 | 0.000005 | Multiple Mann-Whitney U tests correcting for multiple comparisons by using the FDR method |
| POD1 | 22.863 ± 12.760 | 21.878 ± 14.288 | 0.362445 | ||
| POD2 | 16.542 ± 14.723 | 16.234 ± 16.661 | 0.631847 | ||
| Intestinal Mobilization POD 1 | Yes | 51 | 8 | <0.0001 | Fisher’s exact test |
| No | 9 | 32 | |||
| Intestinal Mobilization POD 2 | Yes | 58 | 22 | <0.0001 | Fisher’s exact test |
| No | 2 | 18 | |||
| Nausea & Vomiting POD 1 | Yes | 1 | 5 | 0.0363 | Fisher’s exact test |
| No | 59 | 35 | |||
| Nausea & Vomiting POD 2 | Yes | 0 | 4 | 0.0204 | Fisher’s exact test |
| No | 60 | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyktari, V.; Stefanakis, G.; Papastratigakis, G.; Diamantaki, E.; Koutoulaki, E.; Vasilos, P.; Giannakakis, G.; Bareka, M.; Papaioannou, A. Is a Perioperative Opioid-Sparing Anesthesia-Analgesia Strategy Feasible in Open Thoracotomies? Findings from a Retrospective Matched Cohort Study. J. Clin. Med. 2025, 14, 1820. https://doi.org/10.3390/jcm14061820
Nyktari V, Stefanakis G, Papastratigakis G, Diamantaki E, Koutoulaki E, Vasilos P, Giannakakis G, Bareka M, Papaioannou A. Is a Perioperative Opioid-Sparing Anesthesia-Analgesia Strategy Feasible in Open Thoracotomies? Findings from a Retrospective Matched Cohort Study. Journal of Clinical Medicine. 2025; 14(6):1820. https://doi.org/10.3390/jcm14061820
Chicago/Turabian StyleNyktari, Vasileia, Georgios Stefanakis, Georgios Papastratigakis, Eleni Diamantaki, Emmanouela Koutoulaki, Periklis Vasilos, Giorgos Giannakakis, Metaxia Bareka, and Alexandra Papaioannou. 2025. "Is a Perioperative Opioid-Sparing Anesthesia-Analgesia Strategy Feasible in Open Thoracotomies? Findings from a Retrospective Matched Cohort Study" Journal of Clinical Medicine 14, no. 6: 1820. https://doi.org/10.3390/jcm14061820
APA StyleNyktari, V., Stefanakis, G., Papastratigakis, G., Diamantaki, E., Koutoulaki, E., Vasilos, P., Giannakakis, G., Bareka, M., & Papaioannou, A. (2025). Is a Perioperative Opioid-Sparing Anesthesia-Analgesia Strategy Feasible in Open Thoracotomies? Findings from a Retrospective Matched Cohort Study. Journal of Clinical Medicine, 14(6), 1820. https://doi.org/10.3390/jcm14061820







