Morbidity and Mortality Outcomes After Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Treatment of Ovarian Cancer
Abstract
1. Introduction
2. Materials and Methods
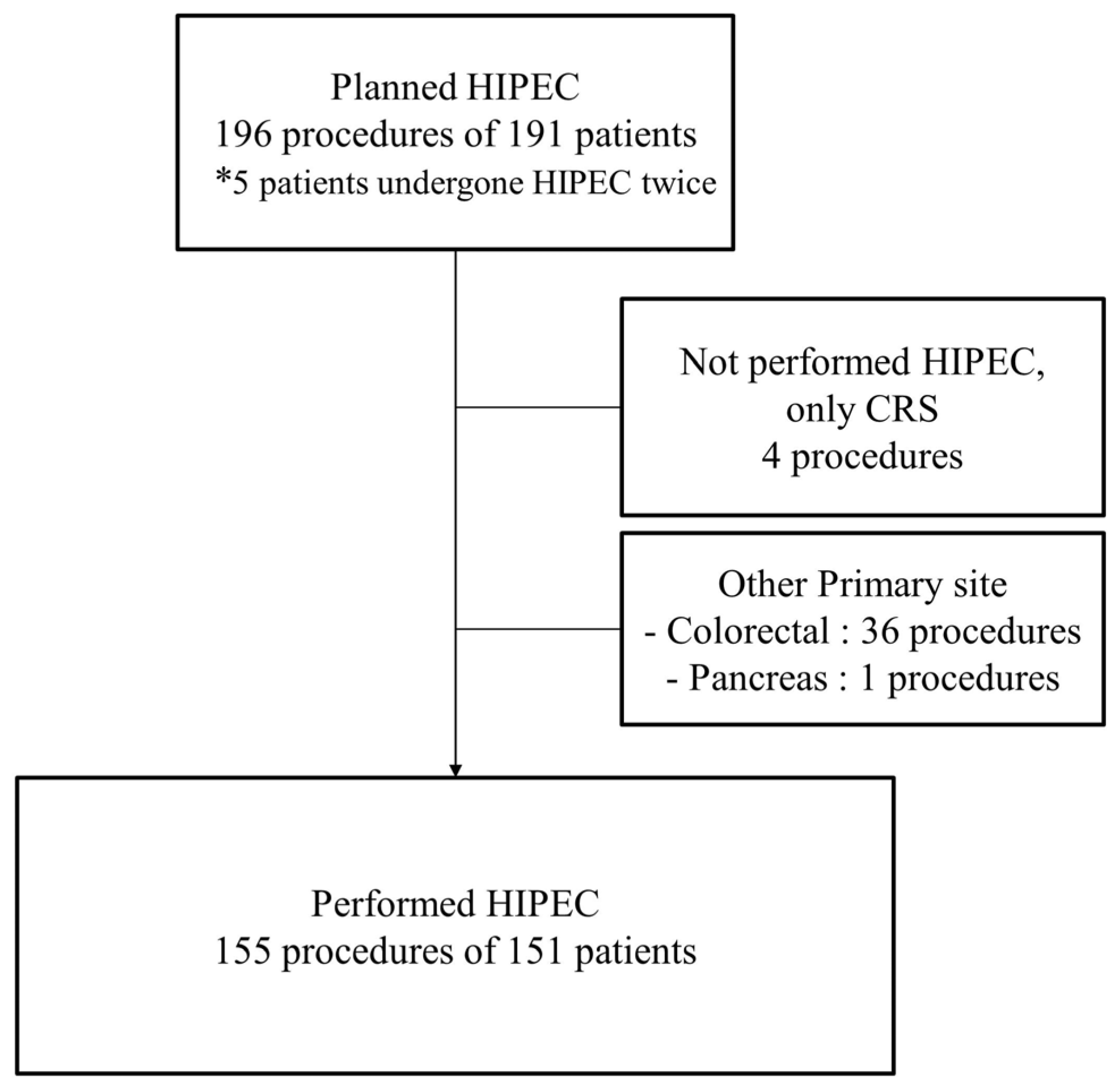
2.1. Patients
2.2. Procedures of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy
2.3. Data and Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Postoperative Morbidity and Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HIPEC | Hyperthermic intraperitoneal chemotherapy |
| CRS | Cytoreductive surgery |
| IP | Intraperitoneal |
| IDS | Interval debulking surgery |
| PDS | Primary debulking surgery |
| PCI | Peritoneal carcinomatosis index |
| CC | Completeness of the cytoreduction |
| MSKCC | The Memorial Sloan Kettering Cancer Center |
| SSE | Surgical secondary event |
| ASA | American society of Anesthesiologists |
| ECOG | Eastern Cooperative oncology Group |
| CTCAE | Common Terminology Criteria for Adverse Events |
| SCS | Surgical complexity score |
References
- Burg, L.; Timmermans, M.; van der Aa, M.; Boll, D.; Rovers, K.; de Hingh, I.; van Altena, A. Incidence and predictors of peritoneal metastases of gynecological origin: A population-based study in the Netherlands. J. Gynecol. Oncol. 2020, 31, e58. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int. J. Gynaecol. Obstet. 2021, 155, 61–85. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.J.; Walker, J.L.; Burger, R.A. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Jaaback, K.; Johnson, N.; Lawrie, T.A. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst. Rev. 2011, 11, CD005340. [Google Scholar]
- Hess, L.M.; Benham-Hutchins, M.; Herzog, T.J.; Hsu, C.H.; Malone, D.C.; Skrepnek, G.H.; Slack, M.K.; Alberts, D.S. A meta-analysis of the efficacy of intraperitoneal cisplatin for the front-line treatment of ovarian cancer. Int. J. Gynecol. Cancer 2007, 17, 561–570. [Google Scholar] [CrossRef]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; van Leeuwen, J.H.S.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Lim, M.C.; Chang, S.-J.; Park, B.; Yoo, H.J.; Yoo, C.W.; Nam, B.H.; Park, S.-Y. Survival After Hyperthermic Intraperitoneal Chemotherapy and Primary or Interval Cytoreductive Surgery in Ovarian Cancer: A Randomized Clinical Trial. JAMA Surg. 2022, 157, 374–383. [Google Scholar] [CrossRef]
- Antonio, C.C.P.; Gil Alida, G.; Elena, G.G.; Rocío, G.S.; Jerónimo, M.G.; Luis, A.R.J.; Aníbal, N.D.; Francisco, B.V.; Jesús, G.R.Á.; Pablo, R.R.; et al. Cytoreductive Surgery With or Without HIPEC After Neoadjuvant Chemotherapy in Ovarian Cancer: A Phase 3 Clinical Trial. Ann. Surg. Oncol. 2022, 29, 2617–2625. [Google Scholar] [CrossRef]
- Charo, L.M.; Jou, J.; Binder, P.; Hohmann, S.F.; Saenz, C.; McHale, M.; Eskander, R.N.; Plaxe, S. Current status of hyperthermic intraperitoneal chemotherapy (HIPEC) for ovarian cancer in the United States. Gynecol. Oncol. 2020, 159, 681–686. [Google Scholar] [CrossRef]
- Zivanovic, O.; Chi, D.S.; Filippova, O.; Randall, L.M.; Bristow, R.E.; O’Cearbhaill, R.E. It’s time to warm up to hyperthermic intraperitoneal chemotherapy for patients with ovarian cancer. Gynecol. Oncol. 2018, 151, 555–561. [Google Scholar] [CrossRef]
- Gagnière, J.; Veziant, J.; Pereira, B.; Pezet, D.; Le Roy, B.; Slim, K. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for the Elderly: Is It Reasonable? A Meta-Analysis. Ann. Surg. Oncol. 2018, 25, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Bakrin, N.; Bereder, J.; Decullier, E.; Classe, J.; Msika, S.; Lorimier, G.; Abboud, K.; Meeus, P.; Ferron, G.; Quenet, F.; et al. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: A French multicentre retrospective cohort study of 566 patients. Eur. J. Surg. Oncol. 2013, 39, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Casares, F.; Medina-Fernández, F.; Arjona-Sánchez, Á.; Casado-Adam, Á.; Sánchez-Hidalgo, J.; Rubio, M.; Ortega-Salas, R.; Muñoz-Villanueva, M.; Rufián-Peña, S.; Briceño, F. Peritonectomy procedures and HIPEC in the treatment of peritoneal carcinomatosis from ovarian cancer: Long-term outcomes and perspectives from a high-volume center. Eur. J. Surg. Oncol. 2016, 42, 224–233. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, J.-Y.; Cho, M.-S.; Nam, E.J.; Kim, S.W.; Kim, S.; Kim, Y.T. Incorporation of paclitaxel-based hyperthermic intraperitoneal chemotherapy in patients with advanced-stage ovarian cancer treated with neoadjuvant chemotherapy followed by interval debulking surgery: A protocol-based pilot study. J. Gynecol. Oncol. 2019, 30, e3. [Google Scholar] [CrossRef]
- Lee, Y.J.; Seon, K.E.; Jung, D.C.; Lee, J.-Y.; Nam, E.J.; Kim, S.W.; Kim, S.; Kim, Y.T. Interval debulking surgery with or without hyperthermic intraperitoneal chemotherapy in advanced-stage ovarian cancer: Single-institution cohort study. Front. Oncol. 2022, 12, 936099. [Google Scholar] [CrossRef]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar]
- Aletti, G.; Dowdy, S.C.; Podratz, K.C.; Cliby, W.A. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am. J. Obstet. Gynecol. 2007, 197, 676.e1–676.e7. [Google Scholar] [CrossRef]
- Aletti, G.; Dowdy, S.C.; Podratz, K.C.; Cliby, W.A. A new frontier for quality of care in gynecologic oncology surgery: Multi-institutional assessment of short-term outcomes for ovarian cancer using a risk-adjusted model. Gynecol. Oncol. 2007, 107, 99–106. [Google Scholar] [CrossRef]
- Strong, V.E.; Selby, L.V.; Sovel, M.; Disa, J.J.; Hoskins, W.; Dematteo, R.; Scardino, P.; Jaques, D.P. Development and assessment of Memorial Sloan Kettering Cancer Center’s Surgical Secondary Events grading system. Ann. Surg. Oncol. 2015, 22, 1061–1067. [Google Scholar] [CrossRef]
- Machida, H.; Tokunaga, H.; Matsuo, K.; Matsumura, N.; Kobayashi, Y.; Tabata, T.; Kaneuchi, M.; Nagase, S.; Mikami, M. Survival outcome and perioperative complication related to neoadjuvant chemotherapy with carboplatin and paclitaxel for advanced ovarian cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2020, 46, 868–875. [Google Scholar] [CrossRef]
- Kengsakul, M.; Boer, G.M.N.-D.; Udomkarnjananun, S.; Kerr, S.J.; Niehot, C.D.; van Beekhuizen, H.J. Factors predicting postoperative morbidity after cytoreductive surgery for ovarian cancer: A systematic review and meta-analysis. J. Gynecol. Oncol. 2022, 33, e53. [Google Scholar] [CrossRef] [PubMed]
- Norppa, N.; Staff, S.; Helminen, M.; Auranen, A.; Saarelainen, S. Improved survival after implementation of ultra-radical surgery in advanced epithelial ovarian cancer: Results from a tertiary referral center. Gynecol. Oncol. 2022, 165, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Zivanovic, O.; Chi, D.S.; Zhou, Q.; Iasonos, A.; Konner, J.A.; Makker, V.; Grisham, R.N.; Brown, A.K.; Nerenstone, S.; Diaz, J.P.; et al. Secondary Cytoreduction and Carboplatin Hyperthermic Intraperitoneal Chemotherapy for Platinum-Sensitive Recurrent Ovarian Cancer: An MSK Team Ovary Phase II Study. J. Clin. Oncol. 2021, 39, 2594–2604. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.V.; García, S.S.; Amo-Salas, M.; Santos, E.G.; de la Manzanara, C.L.; Alberca, A.; Padilla-Valverde, D.; Calvo, F.J.R.; Martín, J. Paclitaxel as HIPEC-Drug after Surgical Cytoreduction for Ovarian Peritoneal Metastases: A Randomized Phase III Clinical Trial (HIPECOVA). Curr. Oncol. 2024, 31, 660–671. [Google Scholar] [CrossRef]
- Somashekhar, S.; Yethadka, R.; Kumar, C.R.; Ashwin, K.; Zaveri, S.; Rauthan, A. Toxicity profile of chemotherapy agents used in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancies. Eur. J. Surg. Oncol. 2020, 46, 577–581. [Google Scholar] [CrossRef]
- Laplace, N.; Kepenekian, V.; Friggeri, A.; Vassal, O.; Ranchon, F.; Rioufol, C.; Gertych, W.; Villeneuve, L.; Glehen, O.; Bakrin, N. Sodium thiosulfate protects from renal impairement following hyperthermic intraperitoneal chemotherapy (HIPEC) with Cisplatin. Int. J. Hyperth. 2020, 37, 897–902. [Google Scholar] [CrossRef]
- Lim, P.-Q.; Han, I.-H.; Seow, K.-M.; Chen, K.-H. Hyperthermic Intraperitoneal Chemotherapy (HIPEC): An Overview of the Molecular and Cellular Mechanisms of Actions and Effects on Epithelial Ovarian Cancers. Int. J. Mol. Sci. 2022, 23, 10078. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Intraperitoneal paclitaxel: Pharmacology, clinical results and future prospects. J. Gastrointest. Oncol. 2021, 12, S231–S239. [Google Scholar] [CrossRef]
- Flood, M.; Narasimhan, V.; Waters, P.; Ramsay, R.; Michael, M.; Warrier, S.; Heriot, A. Survival after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastases: A systematic review and discussion of latest controversies. Surgeon 2021, 19, 310–320. [Google Scholar] [CrossRef]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- de Chirurgie, A.F.; Glehen, O.; Gilly, F.N.; Arvieux, C.; Cotte, E.; Boutitie, F.; Mansvelt, B.; Bereder, J.M.; Lorimier, G.; Quenet, F.; et al. Peritoneal carcinomatosis from gastric cancer: A multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann. Surg. Oncol. 2010, 17, 2370–2377. [Google Scholar]
- Glehen, O.; Gilly, F.N.; Boutitie, F.; Bereder, J.M.; Quenet, F.; Sideris, L.; Mansvelt, B.; Lorimier, G.; Msika, S.; Elias, D.; et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: A multi-institutional study of 1290 patients. Cancer 2010, 116, 5608–5618. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.C.; Kang, S.; Choi, J.; Song, Y.J.; Park, S.; Seo, S.-S.; Park, S.-Y. Hyperthermic intraperitoneal chemotherapy after extensive cytoreductive surgery in patients with primary advanced epithelial ovarian cancer: Interim analysis of a phase II study. Ann. Surg. Oncol. 2009, 16, 993–1000. [Google Scholar] [CrossRef]
- Newton, A.D.; Bartlett, E.K.; Karakousis, G.C. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: A review of factors contributing to morbidity and mortality. J. Gastrointest. Oncol. 2016, 7, 99–111. [Google Scholar]
- Franko, J.; Gusani, N.J.; Holtzman, M.P.; Ahrendt, S.A.; Jones, H.L.; Zeh, H.J.; Bartlett, D.L. Multivisceral resection does not affect morbidity and survival after cytoreductive surgery and chemoperfusion for carcinomatosis from colorectal cancer. Ann. Surg. Oncol. 2008, 15, 3065–3072. [Google Scholar] [CrossRef]
- Macrì, A.; Accarpio, F.; Arcoraci, V.; Casella, F.; De Cian, F.; De Iaco, P.; Orsenigo, E.; Roviello, F.; Scambia, G.; Saladino, E.; et al. Predictors of morbidity and mortality in patients submitted to cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for ovarian carcinomatosis: A multicenter study. Pleura Peritoneum 2021, 6, 21–30. [Google Scholar] [CrossRef]
- Cham, S.; Chen, L.; Clair, C.M.S.; Hou, J.Y.; Tergas, A.I.; Melamed, A.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L.; Wright, J.D. Development and validation of a risk-calculator for adverse perioperative outcomes for women with ovarian cancer. Am. J. Obstet. Gynecol. 2019, 220, 571.e1–571.e8. [Google Scholar] [CrossRef]
- Fagotti, A.; Ferrandina, G.; Vizzielli, G.; Fanfani, F.; Gallotta, V.; Chiantera, V.; Costantini, B.; Margariti, P.A.; Alletti, S.G.; Cosentino, F.; et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur. J. Cancer 2016, 59, 22–33. [Google Scholar] [CrossRef]
- Schneider, M.A.; Eshmuminov, D.; Lehmann, K. Major Postoperative Complications Are a Risk Factor for Impaired Survival after CRS/HIPEC. Ann. Surg. Oncol. 2017, 24, 2224–2232. [Google Scholar] [CrossRef]
- Simkens, G.A.; van Oudheusden, T.R.; Luyer, M.D.; Nienhuijs, S.W.; Nieuwenhuijzen, G.A.; Rutten, H.J.; de Hingh, I.H. Serious Postoperative Complications Affect Early Recurrence After Cytoreductive Surgery and HIPEC for Colorectal Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2015, 22, 2656–2662. [Google Scholar] [CrossRef]
| Number of Procedures = 155 | ||
|---|---|---|
| Age (years), median (range) | 55 (16–79) | |
| BMI (kg/m2), median (range) | 23.1 (15.2–34.5) | |
| Length of stay (days), median (range) | 15 (6–135) | |
| ASA class | 1 | 57 (36.8%) |
| 2 | 85 (54.8%) | |
| 3 | 13 (8.4%) | |
| ECOG performance status | 0 | 133 (85.8%) |
| 1 | 22 (14.2%) | |
| Underlying disease | HTN | 43 (27.7%) |
| DM | 22 (14.2%) | |
| Thromboembolism | 12 (7.7%) | |
| FIGO stage | III | 87 (56.1%) |
| IV | 68 (43.9%) | |
| Origin | Ovary | 138 (89.0%) |
| Fallopian tube | 3 (1.9%) | |
| Peritoneum | 14 (9.0%) | |
| Histologic type | High grade serous | 124 (80%) |
| Mucinous | 8 (5.2%) | |
| Clear cell | 8 (5.2%) | |
| Low grade serous | 4 (2.6%) | |
| Endometrioid | 1 (0.6%) | |
| Other * | 10 (6.5%) | |
| PCI score, median (range) | 8 (0–27) | |
| Timing of Surgery | Primary debulking surgery | 4 (2.6%) |
| Interval debulking surgery | 80 (47.0%) | |
| ≥2nd debulking surgery | 71 (45.8%) | |
| Chemotherapy agents | Paclitaxel | 96 (61.9%) |
| Cisplatin | 59 (38.1%) | |
| CC | CC-0 | 112 (72.3%) |
| CC-1 | 14 (9.0%) | |
| CC-2 | 29 (18.7%) | |
| Administration method | Open | 146 (94.2%) |
| Close | 9 (5.8%) | |
| SCS, median(range) | 4 (0–12) | |
| SCS group | Low (<3) | 72 (46.5%) |
| Intermediate (4–7) | 63 (40.6%) | |
| High (>8) | 20 (12.9%) | |
| EBL (mL), median (range) | 580 (10–8600) | |
| OP time (min), median (range) | 470 (195–1080) |
| Organ System | Any Grade (%) | Grade 3–5 | |
|---|---|---|---|
| (Total Procedure = 155) | |||
| General | Poor oral intake/general weakness | 1 (0.6%) | 0 |
| Cardiovascular system | Hypotension | 2 (1.3%) | 0 |
| Arrhythmia | 4 (2.6%) | 0 | |
| Head and neck | Salivary gland infection | 1 (0.6%) | 0 |
| Gastrointestinal system | Ileus | 34 (21.9%) | 0 |
| Nausea/Vomiting | 1 (0.6%) | 0 | |
| Gastrointestinal bleeding | 1 (0.6%) | 0 | |
| Anastomotic stricture | 1 (0.6%) | 1 (0.6%) | |
| Constipation | 1 (0.6%) | 0 | |
| Pancreatitis | 1 (0.6%) | 0 | |
| Small bowel obstruction | 1 (0.6%) | 0 | |
| Small bowel perforation | 1 (0.6%) | 1 (0.6%) | |
| Non infective intra-abdominal fluid collection | 2 (1.3%) | 2 (1.3%) | |
| Chylous ascites | 2 (1.3%) | 0 | |
| Liver enzyme elevation | 3 (1.9%) | 0 | |
| Lipase elevation | 1 (0.6%) | 0 | |
| Pulmonary system | Pleural effusion | 63 (40.6%) | 3 (1.9%) |
| Pneumonia | 2 (1.3%) | 0 | |
| Atelectasis | 2 (1.3%) | 0 | |
| Pneumothorax | 2 (1.3%) | 0 | |
| Hypoxia | 1 (0.6%) | 0 | |
| Pulmonary edema | 1 (0.6%) | 0 | |
| Genitourinary system | Urinary retention | 6 (3.9%) | 0 |
| Hydronephrosis | 1 (0.6%) | 0 | |
| Ureter stricture and fistula | 1 (0.6%) | 1 (0.6%) | |
| Metabolic | Electrolyte imbalance | 1 (0.6%) | 0 |
| Hematologic or Vascular system | Anemia | 79 (51.0%) | 0 |
| Thrombocytopenia | 17 (11.0%) | 0 | |
| Neutropenia | 19 (12.3%) | 0 | |
| Febrile neutropenia | 1 (0.6%) | 0 | |
| Deep vein thrombosis | 1 (0.6%) | 0 | |
| Pulmonary embolism | 3 (1.9%) | 1 (0.6%) | |
| Disseminated intravascular coagulation | 1 (0.6%) | 0 | |
| Postoperative hemorrhage | 3 (1.9%) | 3 (1.9%) | |
| Hematoma | 1 (0.6%) | 0 | |
| Musculoskeletal system | Lymphedema | 1 (0.6%) | 0 |
| Nervous system | Delirium | 9 (5.8%) | 0 |
| Vocal cord paralysis | 1 (0.6%) | 0 | |
| Pain | Abdominal pain | 5 (3.2%) | 0 |
| Wound or skin | Wound dehiscence | 10 (6.5%) | 0 |
| Sore | 1 (0.6%) | 0 | |
| Infection | Fever | 71 (45.8%) | 0 |
| Wound infection | 7 (4.5%) | 5 | |
| Catheter related infection | 2 (1.3%) | 2 (1.3%) | |
| Urinary tract infection | 1 (0.6%) | 0 | |
| Intra-abdominal infection | 1 (0.6%) | 1 (0.6%) | |
| ≤Grade 2 AE (137 Procedures) | ≥Grade 3 AE (18 Procedures) | p-Value | ||
|---|---|---|---|---|
| Age (year), median (range) | 54 (16–79) | 61 (32–70) | 0.026 | |
| Length of stay (day), median (range) | 14 (6–46) | 26 (14–135) | <0.001 | |
| Underlying disease | HTN | 36 (26.3%) | 7 (38.9%) | 0.272 |
| DM | 14 (13.1%) | 4 (22.2%) | 0.291 | |
| Thromboembolism | 11 (7.4%) | 1 (5.6%) | 1.000 | |
| ASA class | 1 | 49 (35.8%) | 8 (44.4%) | 0.236 |
| 2 | 75 (54.7%) | 10 (55.6%) | ||
| 3 | 13 (9.5%) | 0 (0%) | ||
| PCI, median (range) | 8 (0–25) | 12 (2–27) | 0.013 | |
| Timing of Surgery | Primary debulking surgery | 4 (2.9%) | 0 (0%) | 0.920 |
| Interval debulking surgery | 70 (51.1%) | 10 (55.6%) | ||
| ≥2nd debulking surgery | 63 (46.0%) | 8 (44.4%) | ||
| Operation extent | Hysterectomy | 68 (49.6%) | 9 (50.0%) | 0.977 |
| Lymph node dissection | 59 (43.1%) | 7 (38.9%) | 0.736 | |
| Peritonectomy | 84 (61.3%) | 13 (72.2%) | 0.369 | |
| Bowel resection | 25 (18.2%) | 8 (44.4%) | 0.027 | |
| Hepatectomy | 23 (16.8%) | 1 (5.6%) | 0.311 | |
| Diaphragmatic stripping | 40 (29.2%) | 7 (38.9%) | 0.400 | |
| Splenectomy | 17 (12.4) | 3 (16.7%) | 0.706 | |
| SCS | Low | 66 (48.2%) | 6 (28.6%) | 0.463 |
| Intermediate | 53 (38.7%) | 10 (55.6%) | ||
| High | 18 (13.1%) | 2 (11.1%) | ||
| CC | 0 | 103 (75.2%) | 9 (50.0%) | 0.016 |
| 1 | 12 (8.8%) | 2 (11.1%) | ||
| 2 | 22 (16.1%) | 7 (38.9%) | ||
| Chemotherapy agents | Paclitaxel | 84 (56.4%) | 12 (57.1%) | 0.660 |
| Cisplatin | 53 (35.6%) | 6 (28.6%) | ||
| OP time (min), median (range) | 460 (195–1080) | 557.5 (400–1080) | 0.007 | |
| EBL (mL), median (range) | 550 (10–8600) | 800 (200–5050) | 0.224 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age | 1.049 | 0.995–1.106 | 0.075 | |||
| PCI score | 1.106 | 1.022–1.196 | 0.012 | 1.066 | 0.971–1.170 | 0.182 |
| Bowel Resection | 3.584 | 1.285–9.997 | 0.015 | 2.109 | 0.647–6.870 | 0.216 |
| OP time | 1.003 | 1.001–1.006 | 0.011 | 1.002 | 0.998–1.005 | 0.385 |
| CC | 0.066 | |||||
| CC-1 | 1.907 | 0.368–9.879 | ||||
| CC-2 | 3.641 | 1.225–10.828 | ||||
| van Driel et al. (2018) [6] | Zivanovic et al. (2021) [23] | Antonio et al. (2022) [8] | Campos et al. (2024) [24] | Present Study | |
|---|---|---|---|---|---|
| N of HIPEC arm | 122 | 49 | 35 | 32 | 155 |
| Age (years), median (range) | 61 (IQR, 55–66) | 59 (39–74) | 56 (29–75) | 60.34 (±11.7) | 55 (16–79) |
| PCI, median(range) | - | - | 10 (2–22) | - | 8 (0–27) |
| Complete resection (%) | 84 (69%) | 40 (82%) | 33 (94.3%) | 32 (100%) | 112 (72.3%) |
| Timing of surgery | IDS | 2nd CRS | IDS | PDS, IDS, 2nd CRS | PDS, IDS, ≥2nd CRS |
| Anticancer drug | Cisplatin | Carboplatin | Cisplatin | Paclitaxel | Paclitaxel, Cisplatin |
| AE assessment system | CTCAE ver 4.0 | MSKCC SSE | CTCAE ver 3.0 | Cavien-Dindo | MSKCC SSE |
| ≥Grade 3 AE (%) | 32 (27%) | 12 (24%) | 10 (28.6%) | 5 (15.6%) | 18 (11.6%) |
| Most common AE, Grade 3–5 (%) | Abdominal pain (6%), Infection (5%), Ileus (4%) | - | Anemia (11.4%), Ileus (5.7%) | - | Wound infection (3.2%) Pleural effusion (1.9%) Postoperative hemorrhage (1.9%) |
| Mortality rate (%) | 0 | 0 | 1 (2.8%) | 2 (6.3%) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Lee, Y.J.; Seon, K.E.; Kim, S.; Lee, C.; Park, H.; Choi, M.C.; Lee, J.-Y. Morbidity and Mortality Outcomes After Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Treatment of Ovarian Cancer. J. Clin. Med. 2025, 14, 1782. https://doi.org/10.3390/jcm14051782
Kim M, Lee YJ, Seon KE, Kim S, Lee C, Park H, Choi MC, Lee J-Y. Morbidity and Mortality Outcomes After Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Treatment of Ovarian Cancer. Journal of Clinical Medicine. 2025; 14(5):1782. https://doi.org/10.3390/jcm14051782
Chicago/Turabian StyleKim, Migang, Yong Jae Lee, Ki Eun Seon, Sunghoon Kim, Chan Lee, Hyun Park, Min Chul Choi, and Jung-Yun Lee. 2025. "Morbidity and Mortality Outcomes After Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Treatment of Ovarian Cancer" Journal of Clinical Medicine 14, no. 5: 1782. https://doi.org/10.3390/jcm14051782
APA StyleKim, M., Lee, Y. J., Seon, K. E., Kim, S., Lee, C., Park, H., Choi, M. C., & Lee, J.-Y. (2025). Morbidity and Mortality Outcomes After Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Treatment of Ovarian Cancer. Journal of Clinical Medicine, 14(5), 1782. https://doi.org/10.3390/jcm14051782








