1. Introduction
One of the leading causes of disability-related loss of life years worldwide is low back pain (LBP). It therefore represents a significant global economic burden on healthcare systems [
1]. While LBP-related pain syndromes are estimated to cost USD 135 billion in the United States [
2], in Germany, where the study was conducted, they are the most common condition, with a 14% share of disability-related loss of life years [
3]. In addition, the cure rate for acute LBP (aLBP) is only one third. The remainder, i.e., the majority of all aLBP patients, suffer new episodes of pain within the next year [
4].
Besides some risk factors such as older age, higher BMI and greater physical exertion or in particular previous phases of aLBP [
5], there is increasing evidence that the thoracolumbar fascia (TLF) may play a contributory role in the development of low back pain [
6,
7,
8,
9]. The TLF is an aponeurotic, diamond-shaped structure that covers the paraspinal muscles and separates them from the abdominal muscles with a layer. The most posterior layer is dominated by the insertion of the latissimus dorsi muscle [
10]. Its rich innervation, involving in particular type A and C nociceptive nerve fibers, makes it susceptible to pathophysiological changes [
8,
11]. In recent years, a number of potential causes of nociceptor sensitization have been identified: the loss of elasticity and lubricity [
6], micro-injuries [
12], swelling due to overuse [
13], and even indications that psychosocial factors may considerably reduce the fascial ability to shear or its stiffness [
14,
15,
16].
In view of these TLF-LBP relationships, ultrasound (US) methods have been developed to quantify the sliding and deformation properties of the TLF [
6,
17,
18,
19,
20]. Two generally different approaches have been developed for these methods. One is the use of US measurements for landmarks of prominent anatomical structures [
19,
21], and the other is the use of automated methods based on speckle tracking and cross-correlation functions [
6,
17]. We have recently introduced a new, landmark-based easy-to-use method for diagnosis and treatment control in daily practice. Its intra- and inter-rater reliability in quantifying TLF deformation (TLFD) was therefore demonstrated in a comprehensive study [
21]. For a detailed theoretical framework of the method, its intra- and inter-rater reliability, and its clinical relevance, please see Brandl et al. [
21]. However, to date, it is neither clear that the absolute distance value of the TLFD US measurement (TLFD_US) corresponds to the real distance nor in which tissue layers (dermis and erector spinae muscle) the deformation occurs and in what proportion.
This work follows on from our previous reliability study, in which we described in more detail the rationale for a new ultrasound measurement method for assessing TLF properties and outlined its relevance for clinical use and further practical application. The aim of the present study was to investigate the criterion validity of the TLFD_US method in terms of a comparison between the measurements within the ultrasound images and real-world data. Therefore, the concurrent validity of the ground truth data in vitro and the speckle tracking analysis in vivo should be evaluated. In addition, the sliding of the TLF in other tissue layers should be determined as a secondary objective with the speckle tracking analysis that defines the TLFD.
2. Materials and Methods
The work presented here was a criterion validity study and part of a larger project investigating the neuromotor associations of the TLF. The project was prospectively registered with the German Registry for Clinical Trials (DRKS00027074) and was reviewed and approved for in vivo validation with human participants by the ethics committee of the Diploma University of Applied Science (No. 1014/2021). This study was conducted in accordance with the Declaration of Helsinki and written informed consent was obtained from the participants.
2.1. Ultrasound Measurement of the Thoracolumbar Fascia Deformation
The TLFD_US using the junction between the latissimus dorsi muscle and the TLF as an anatomical landmark has already been described by Brandl et al. [
21,
22,
23]. A detailed description of the ultrasound examination protocol can be found at
https://dx.doi.org/10.17504/protocols.io.eq2lyjbmwlx9/v1, accessed on 4 February 2025.
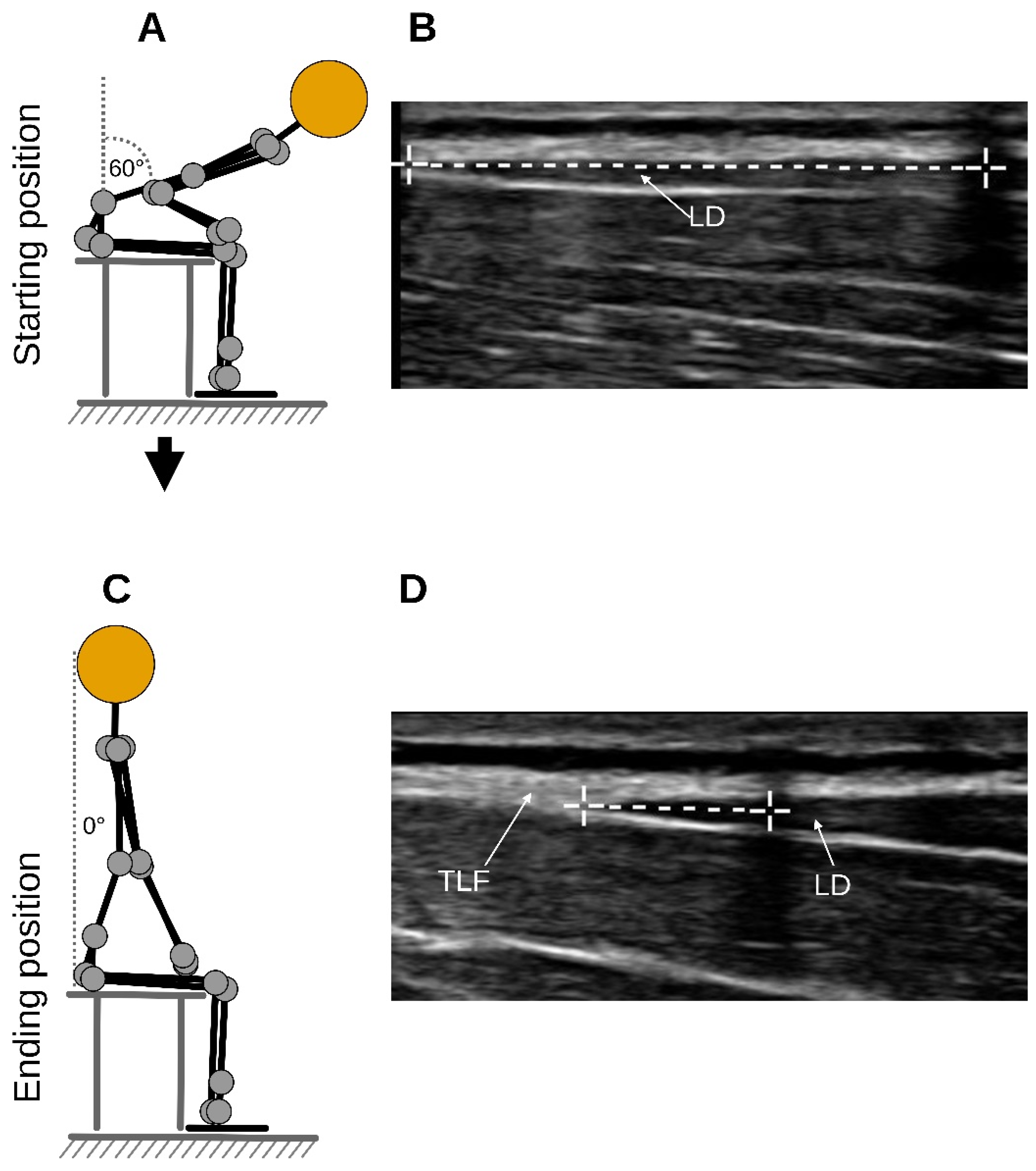
Briefly, each participant sat on a treatment table and flexed their trunk to an approximate flexion angle of 60 degrees (starting position) as determined by the examiner, and here, a static US image was acquired (Clarius L15 HD3, 5–15 MHz Linear Transducer, Vancouver, BC, Canada;
Figure 1A,B). Participants then extended their trunk to 0 degrees, which was the ending position of the trunk extension task (TET), and a static, secondary US image was acquired again (
Figure 1C,D). The measurement was the distance between the junction of the latissimus dorsi muscle with the TLF and an artificial reference created by reflective tape on the skin shown in the images (
Figure 1B,D). The difference between the distances of the starting and the ending position represents the deformation of the TLF (
Figure 1B,D).
In a comprehensive recent reliability study by our research group, intra-rater reliability was rated as excellent, with an ICC of 0.92 and a minimal detectable change of 5.54 mm (
p < 0.001). The inter-rater reliability was slightly worse, with a good ICC of 0.78 and a minimal detectable change of 8.70 mm (
p < 0.001). Furthermore, a cut-off point of 6 mm was determined to differentiate LBP patients from healthy individuals, meaning that a TLFD of less than 6 mm identified 100% of LBP patients (sensitivity) and more than 6 mm identified 93.75% of healthy individuals (specificity) [
21].
2.2. In Vitro Concurrent Validity with Ground Truth
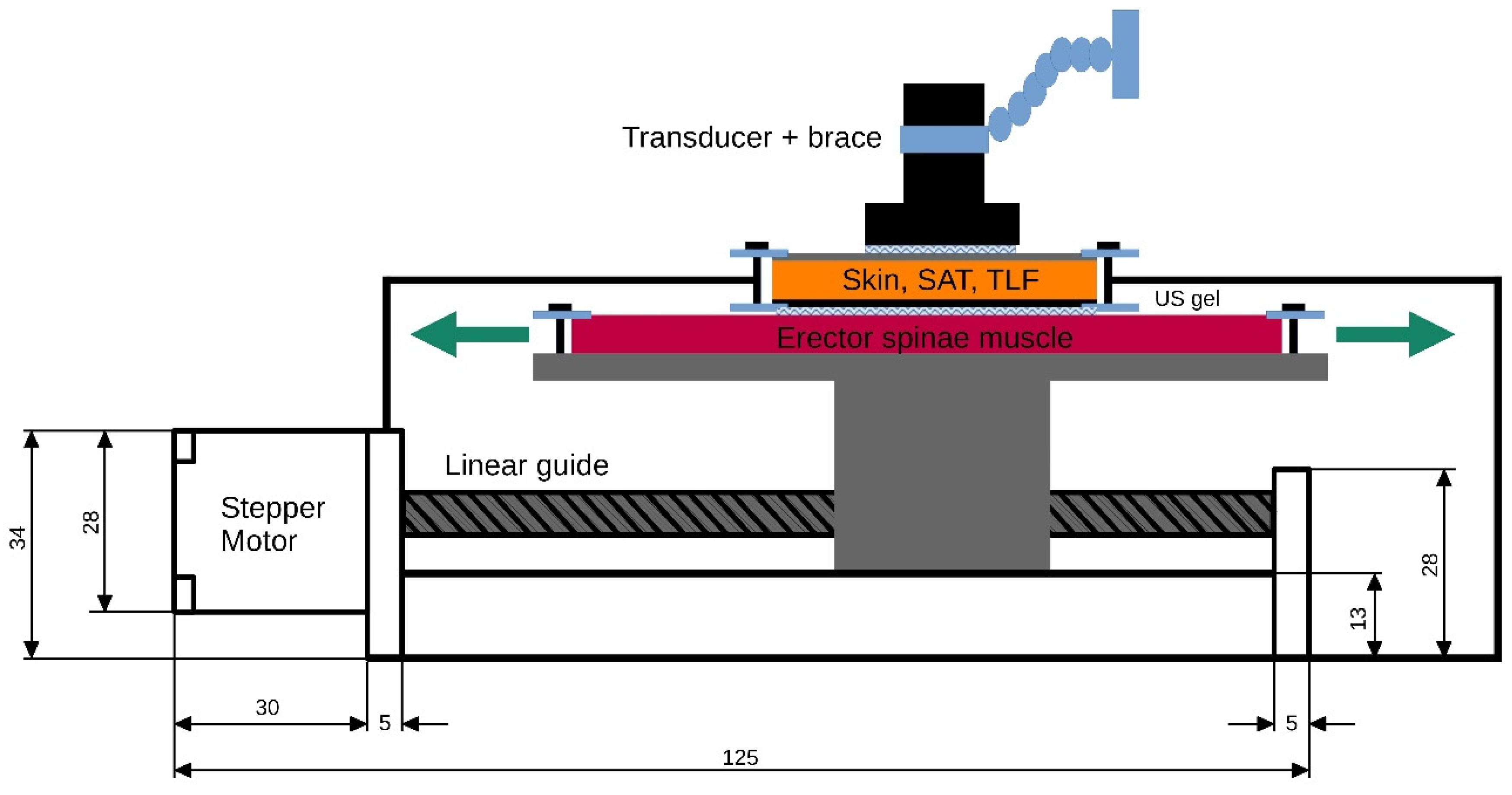
To generate ground truth data for the subsequent validation of the TLFD_US, a custom-built tissue sliding device was constructed. A polyurethane gel pad that mimics the erector spinae muscle is attached to a plateau mounted on a linear actuator (SFU1605-100 mm, Shandong Sair Mechanical Guide Co., Ltd., Gaotang, China) and driven by a high-precision stepper motor (NEMA17, ACT Motor GmbH, Bremen, Germany). This configuration allows the gel pad to move laterally under another gel pad that mimics the skin and SAT (
Figure 2).
The gel pads (21 cm × 31 cm; Technogel GmbH, Berlingrode, Germany), which represent a two-layer phantom model of the lumbar tissues with its typical thickness and stiffness, were manufactured on the basis of properties described in the literature. For a detailed description of the development, properties and application, see Bartsch et al. [
24] and Brandl et al. [
25].
Between the gel pads, ultrasound gel (DocCheck Ultrasound Gel, Gello GmbH Geltechnik, Ahaus-Wüllen, Germany) for reducing friction and enabling smooth gliding was attached. At each gel pad, a strain gauge sensor (ZD10-100, Shenzhen Lanqi Technology Co., Ltd., Shenzhen, China) was attached to measure any shear deformation during their relative movement. Equipped with a digital caliper, the stepper motor maintained constant speeds with a positioning accuracy of ± 1 µm. This ensured the reliable documentation of the displacement of each gel pad relative to the other. No shear deformation was detected within the gel pads when determining the ground truth data (<0.1 mm). The measurement uncertainty for the absolute distance between the gel pads was ±2 µm, k = 2 (95% confidence interval), in accordance with the “Guide to the expression of uncertainty in measurement” [
26].
With this setting, 36 distances between 3 and 20 mm with a constant shear rate of 3.15 mm/s were recorded. In addition, 10 different velocities from 2.41 mm/s to 5.36 mm/s were recorded for a distance of 20 mm. These distances and speeds correspond to the typical TLFD and trunk extension velocities of 200 LBP patients and healthy individuals that we previously assessed [
27]. The complete ground truth dataset and epidemiologic data as well as a detailed description of the device including the design description are available at
https://zenodo.org/records/11402043, accessed on 4 February 2025.
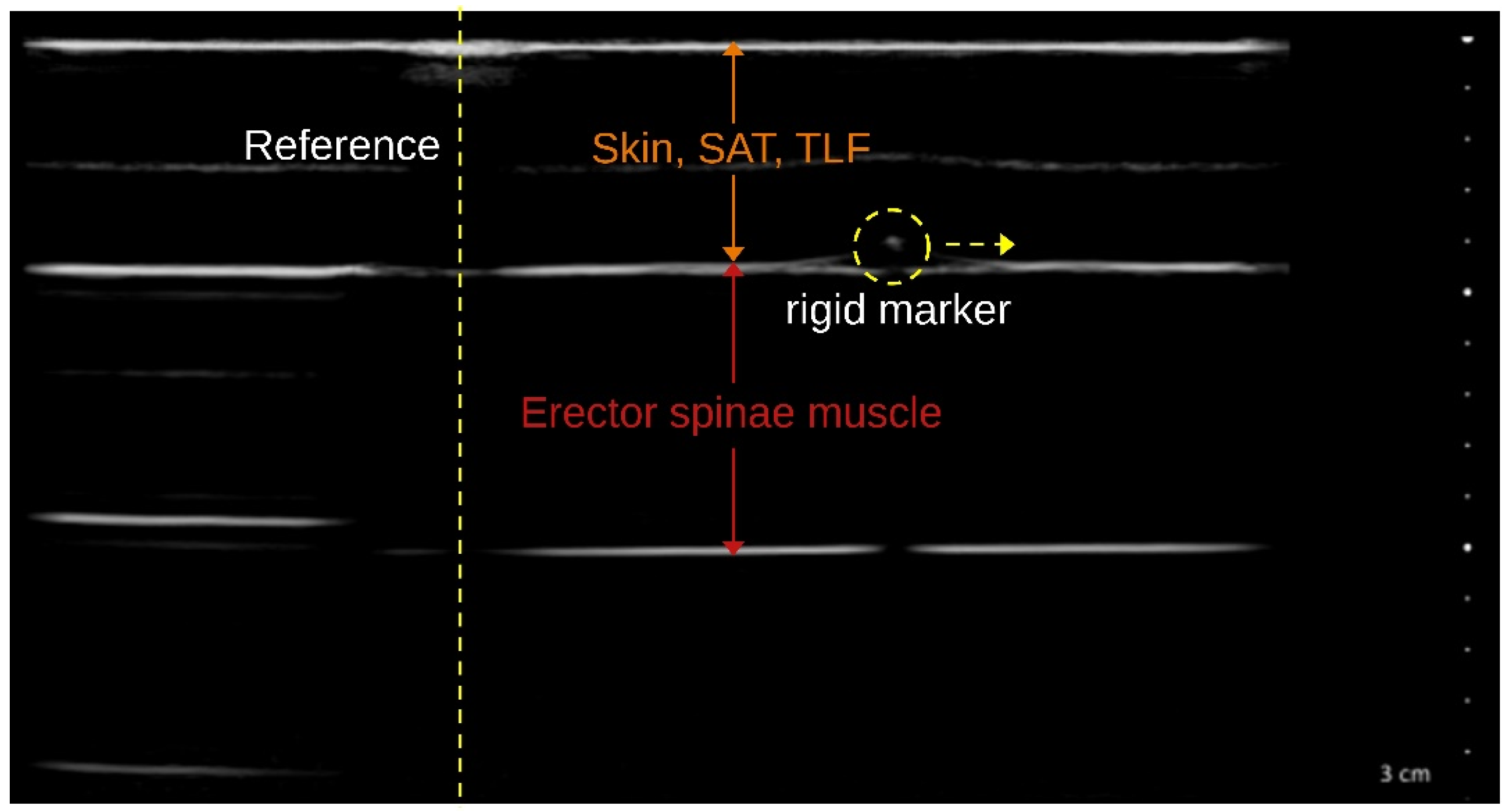
The TLFD_US of the distances corresponding to each ground truth was performed with a reference marker in each phantom tissue layer clearly visible in the US image (
Figure 3). Images were acquired simultaneously with the generation of ground truth data for all 36 distances and 10 velocities using the tissue sliding device with an attached US transducer by a blinded examiner with a total of 10 years of practice in US examinations. Subsequently, the measured distances were compared with the respective real value by an evaluator who was also blinded.
2.3. In Vivo Concurrent Validity with Speckle Tracking Analysis
To assess the concurrent validity of the manual TLFD_US and the automated speckle tracking analysis commonly used in research, 10 aLBP patients and 10 healthy individuals were studied. The reliability of speckle tracking methods for fascial shearing was previously tested by Langevin et al. (ICC = 0.98) [
6] and Tomita et al. (ICC = 0.95) [
17] for the TLF and by Ellis for the tibial nerve (ICC = 0.75) [
28].
We calculated the sample size of 20 participants based on the least acceptable correlation to define concurrent validity between the methods of r = 0.60 [
29], a significance level of 0.05, and a statistical power of 0.80 [
30]. Inclusion criteria for the aLBP group according to the European guidelines for the treatment of aLBP (<6-week pain duration) were a visual analog scale score higher than 3 and an Oswestry disability score higher than 10. Healthy individuals had no pain episodes or physician visits for LBP in the last 5 years. Exclusion criteria were age under 18 or over 60 years, operations or scars in the area of the TLF, skin changes (e.g., urticaria or neurodermatitis), medications that affect blood circulation or act as muscle relaxants, and rheumatic diseases.
The investigator, who was blinded to the group membership, measured the TLFD sonographically from each participant as described in
Section 2.1. A US video of the entire trunk extension task was then recorded from the TLF (for more details on this procedure, see Brandl et al. [
23]). The videos were then tracked in post-analysis using Kinovea (version 0.9.5; Kinovea open source project,
www.kinovea.org). The tracking pixels from the videos were tested for several scenarios, and Kinovea was recommended as a valid and reliable tool [
31,
32]. The individual speckle tracking of pixels was applied within a 4 × 7 mm rectangle representing the region of interest as described by Rodriguez et al. [
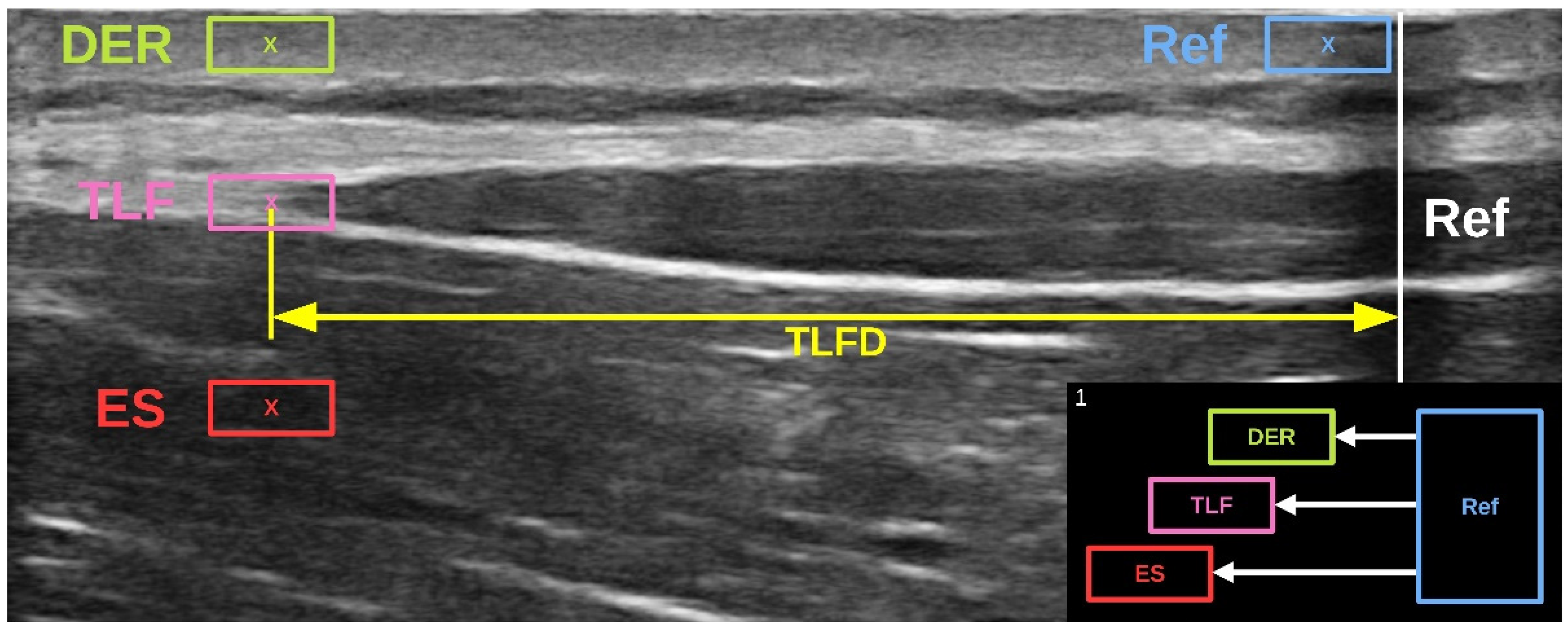
33]. The analysis involved the determination of lateral tissue displacement from the starting to the ending position (
Figure 1). The measured pixels were converted to millimeters using the scale bar in the US video and the Kinovea calibration function [
31]. The procedure was performed simultaneously by the program for the different lumbar tissue layers (dermis, SAT, TLF, and erector spinae) and the artificial reference in the US video that we had previously defined (
Figure 4).
2.4. Statistical Analysis
All data met the criteria for parametric testing. Descriptive statistics are reported as the mean, standard deviation (SD), minimum, maximum and 95% confidence interval (95% CI).
The intraclass correlation coefficient (ICC) between the in vitro TLFD_US and the distance ground truth was calculated using a 2-way random effects model (absolute agreement, ICC
(2,k), multiple raters, k = 2). The resulting values from the ICC calculation were categorized as “poor” (<0.50), “moderate” (0.50 to 0.75), “good” (0.75 to 0.90), and “excellent” (>0.90) according to Koo and Li [
34]. A Bland–Altman plot was created to provide further visual information on the limits of agreement between the manual TLFD_US and the real values. The agreement between TLFD_US and the speed ground truth was presented in a Bland–Altman difference quantile–quantile plot (Q-Q) in addition to the descriptive evaluation.
The Pearson’s product moment correlation coefficient was calculated between in vivo TLFD_US and speckle tracking analysis and interpreted according to Cohen [
35] as ”small” (0.1 to 0.3), “medium” (0.3 to 0.5) or “large” (0.5 to 1.0) correlations.
A multiple linear regression model with the speckle tracking analysis of TLF as the dependent variable and dermis as well as the erector spinae muscle sliding as independent variables was performed to predict TLFD adhesion properties to adjacent tissue layers. Effect sizes (adjusted R
2) were interpreted according to Cohen as “small” (0.01–0.08), “medium” (0.09–0.24), and “large” (>0.25) [
35].
The significance level was set at
p = 0.05. Analyses were performed using Jamovi 2.3 (The jamovi project,
https://www.jamovi.org, accessed on 4 February 2025).
3. Results
A total of 36 images for distance and 10 images for velocity were derived and analyzed to verify the concurrent validity of TLFD_US and ground truth in vitro. For in vivo validation, the concurrent validity of TLFD_US and speckle tracking analysis was performed in 20 participants.
3.1. In Vitro Concurrent Validity with Ground Truth
An excellent degree of agreement was found between TLFD_US and distance ground truth (ICC(2,2) = 0.99, 95% CI [0.998, 0.999], F(35.0, 36.0) = 1846, p < 0.001).
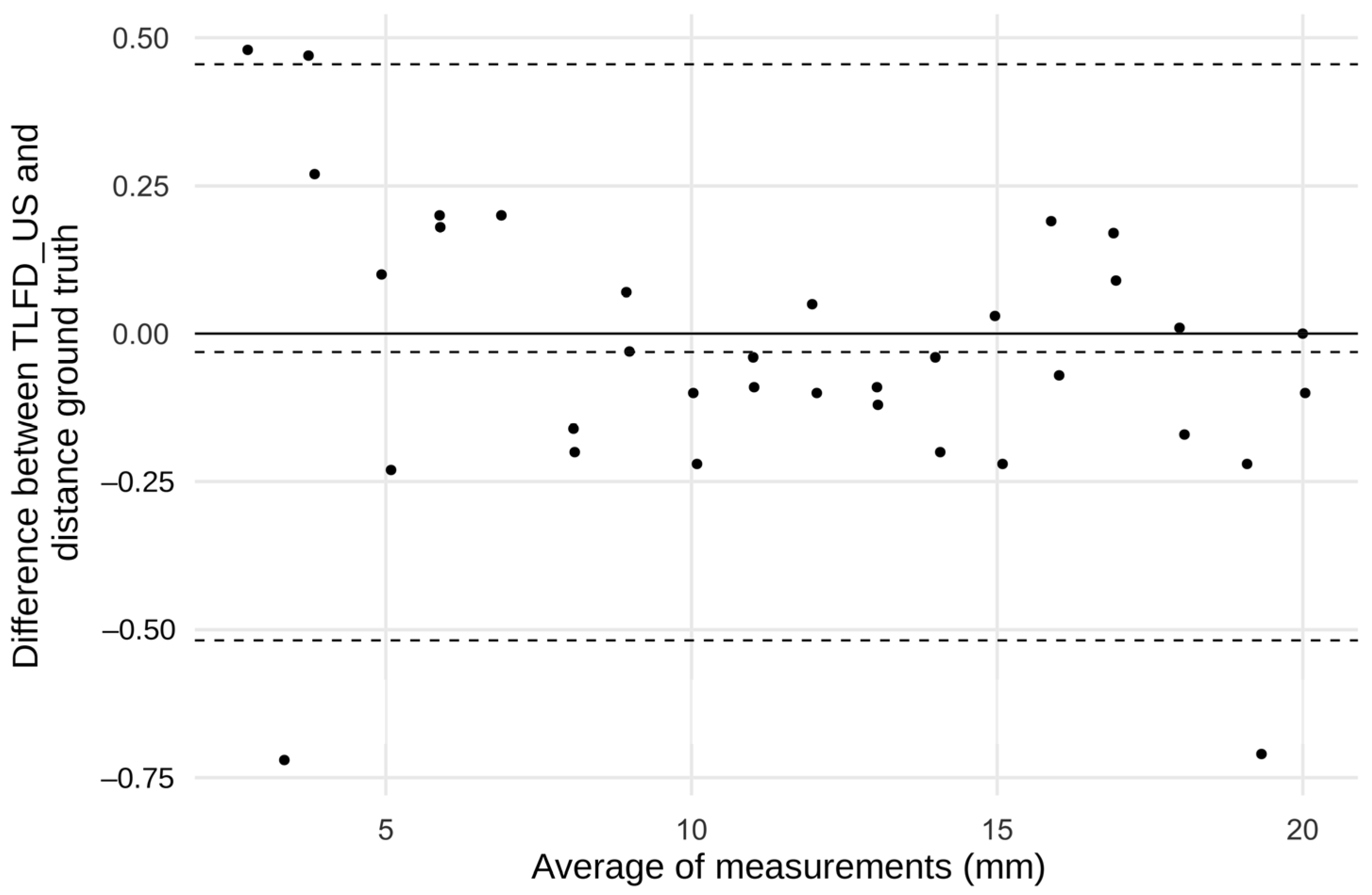
The Bland–Altman diagram showed that almost all points were within the small limits of agreement. The mean difference was less than 0.05 mm, indicating that there were no systematic errors. Outliers exceeding the limits of agreement were only observed at the absolute end of the measurement range below 3.7 and above 19.6 mm (
Figure 5).
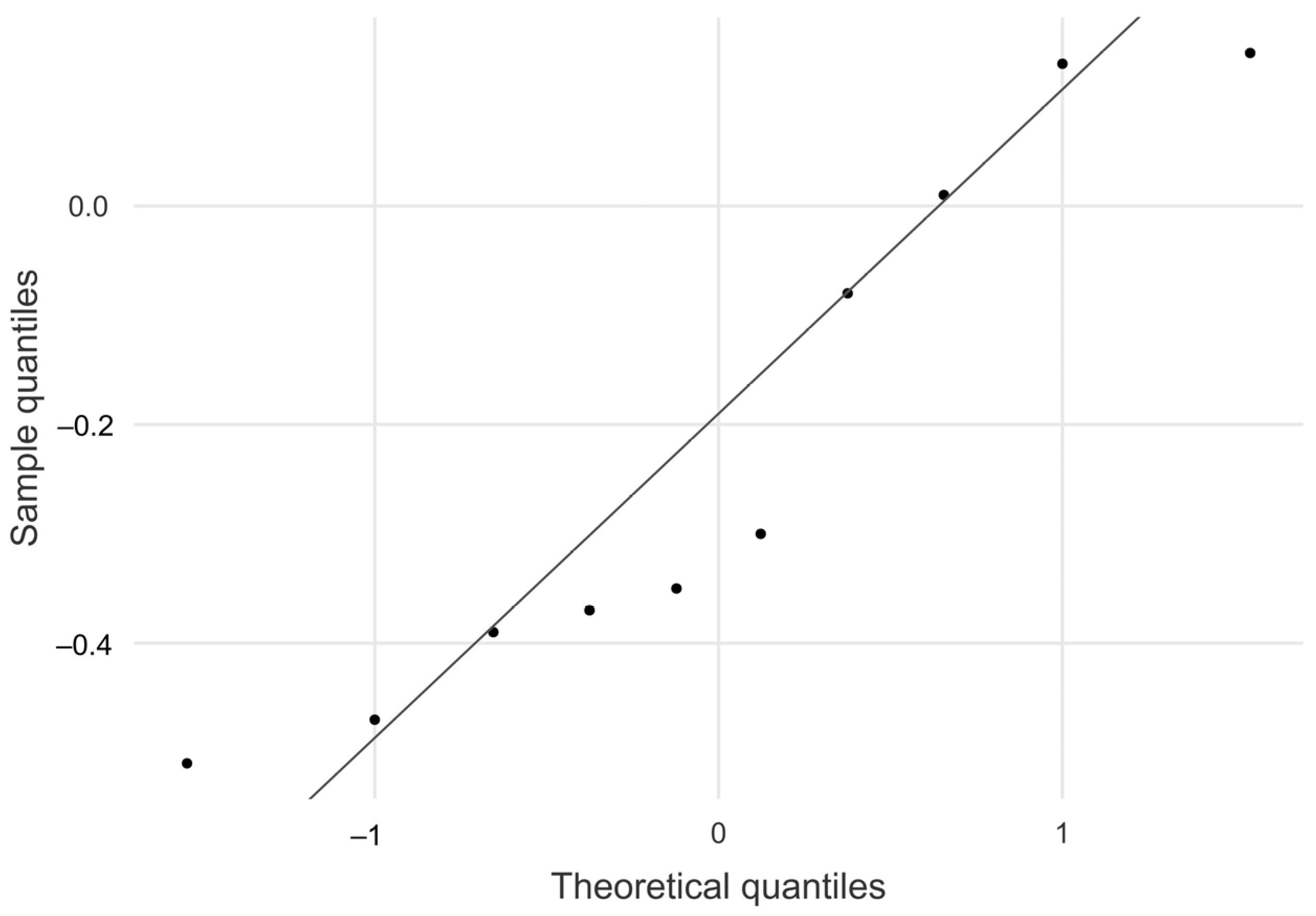
The absolute mean difference between TLFD_US and the speed ground truth was 0.215 mm, 95% CI [−0.410, −0.035]. As shown in the Q–Q plot, sample quantiles follow the theoretical quantiles and only deviate in a small range (
Figure 6). Descriptive data for this analysis are shown in
Table 1.
3.2. In Vivo Concurrent Validity with Speckle Tracking Analysis
A total of 20 participants were analyzed for the in vivo validation between TLFD_US and the speckle tracking analysis. The sample characteristics are listed in
Table 2.
Pearson’s product moment correlation coefficient was large between the TLFD_US and speckle tracking analysis (r(18) = 0.701; p < 0.001).
3.3. Thoracolumbar Fascia Deformation in Relation to Other Tissue Layers
The overall linear model fit was F(2,17) = 6.19, p = 0.01, adjusted R2 = 0.353. The model explained 35% of the TLFD variability, emphasizing the significant influence of the included predictors and a large effect. Dermis sliding as a predictor of TLFD showed a significant effect (B = 0.780, 95% CI [0.15, 0.93]; standardized B = 0.541, 95% CI [0.22, 1.34]; p = 0.009). In contrast, erector spinae muscle sliding as a predictor of TLFD showed no significant effect (B = 0.386, 95% CI [−0.04, 0.81]; standardized B = 0.356, 95% CI [−0.03, 0.75]; p = 0.071).
4. Discussion
This is, to our knowledge, the first validation study to examine a US method for quantifying the sliding and deformation properties of the TLF. Given the growing evidence of a link between TLFD and LBP and the global economic burden of LBP, an in vitro validation was performed to determine whether the US measurements of TLFD correspond to real-world values. In addition, the method was validated in vivo in concurrence with speckle tracking analysis, which is commonly used in laboratory settings. A post-analysis of speckles from US videos was then used to deduce in which tissue layer surrounding the TLF (dermis and erector spinae muscle) did deformation occur and in what proportion.
4.1. In Vitro Concurrent Validity with Ground Truth
The degree of agreement between TLFD_US and the distance truth was excellent and, apart from a small area at the end of the measurement range, the US values matched the real values exactly. The TLFD_US is based on a trunk extension task performed by the participant. For ease of use in daily practice of this protocol, the speed at which the task is performed is only given in a rough range. Therefore, the accuracy of the method at different speeds was also investigated. The absolute mean difference was small and within a low 95% CI. To summarize, the TLFD_US has proven its high precision in an in vitro environment, and it can be assumed that the measured values represent the actual values.
Many authors have performed calculations of the physical properties of the TLF based on US analyses [
6,
17,
18,
19]. However, there is an urgent need to ensure that the calculations are based on valid data. This work therefore provides a trustworthy basis. In the course of developing the in vitro procedure, we have published the entire ground truth evaluation with the tissue sliding device in an open repository (
https://zenodo.org/records/11402043, accessed on 4 February 2025). Work is currently underway with these data to validate certain speckle tracking analyses [
27], and other authors are also invited to evaluate their various US methods of TLF against ground truth as well.
4.2. In Vivo Concurrent Validity with Speckle Tracking Analysis
TLFD_US and speckle tracking analysis showed large correlations. This analysis was performed in vivo with symptomatic aLBP patients and healthy individuals to reflect the population commonly seen in a physician’s office. Speckle tracking analysis using Kinovea, version 0.9.5, software in a recent study showed a high correlation of r = 0.97 with a minimal detectable change of 0.21 mm with the ground truth data, demonstrating similar concurrent in vitro validity [
36]. The large in vivo relation of r = 0.70 between the two different methods proves the reliable use of the TLFD_US as a more practical and time-efficient measurement method for use in daily practice, which is well above the limit of the recommended minimum percentage agreement of 60% for diagnostic procedures in manual and musculoskeletal medicine [
29,
37]. In recent years, attempts have been made to characterize TLF properties using simple methods such as compressive stiffness (e.g., ultrasound elastography, myotonometry or intendometry) or TLF thickness measurements [
38,
39]. However, compressive stiffness is unlikely to detect stiffness changes in the thin TLF [
24,
25] and the measurement of TLF thickness is significantly influenced by the observer [
40]. Furthermore, these measurements only consider one dimension in space, which gives TLFD_US an advantage as an additional method (a two-dimensional measurement of tissue sliding in an XY Cartesian coordinate system) for diagnosing and monitoring LBP [
21].
4.3. Thoracolumbar Fascia Deformation in Relation to Other Tissue Layers
We performed multiple linear regression to determine which tissue layers the TLF deforms in vivo and to what extent. The overall model fit showed a large effect size in this regard, meaning that the included components of the dermis and erector spinae muscle significantly influenced TLFD. The linear model showed that mainly the dermis influenced TLFD. In total, 70% of the dermis sliding was transferred to the TLF. The 39% proportion of the erector spinae muscle was not significant in the modal but showed a trend with a p-value of 0.07.
Previous studies have primarily looked at the relationship between the TLF and the erector spinae muscle [
6,
23], just as most research on fascia has tended to focus on deeper layers of tissue [
41]. In recent years, the role of the overlying layers of the TLF, particularly the superficial fascia, also known as Scarpa’s fascia, has been studied in more detail, and there is increasing evidence of the importance of these structures for the mechanical influence on the TLF [
41,
42]. Our study results are consistent with the observation of van Amstel et al. [
43] on the direct effect of skin displacement on the range of motion of the spine, pelvis and hip. Wilke et al. [
44] have demonstrated force transmission between muscle and superficial fascia. This study provides an initial indication that the tissue layers overlying the TLF may have a greater influence on TLFD and lumbar mobility than previously considered.
Pirri et al. [
19] and Willard et al. [
10] have spoken of a kind of “frozen back” in LBP pathologies. Tomita et al. [
17] calculated the internal shear strain of the TLF based on a Lagrangian approach to solid mechanics and found greater stress within the TLF in LBP patients. This sounds plausible considering that a decrease in sliding against the surrounding tissue must increase the shear strain within the TLF during movement (
Figure 7). This could have inter-related pathological effects of various tissue overloads that lead to angiogenesis failure, hypoxia, inflammation, fibrosis, the excitation of nociceptors and others [
9,
45]. Our results of the influence of dermis on TLFD and, although not significant, a trend of the erector spinae muscle could confirm this hypothesis. However, due to the small sample size, no sub-analysis could be performed to separate the results of symptomatic and healthy participants. Further research could take this into account and use the effect sizes from our validity study in addition to the earlier study on intra- and inter-rater reliability to conduct more comprehensive follow-up work. It would be of particular interest to investigate the contribution of each tissue layer to TLFD, which should also include a heterogeneous group and appropriate subgroup analysis.
4.4. Limitations
This study has a number of limitations. First, various internal shear stresses occur during movement in vital biomaterials. This behavior can hardly be mimicked in vitro. We monitored the material deformation of the gel pad layers of the tissue sliding device and found no undesired shear stresses in the material. However, the approach is a two-dimensional simplification of real biomechanics in living subjects. Therefore, we also investigated its validity against the speckle tracking analysis in vitro with real patients and healthy individuals. The speckle tracking analysis showed strong validity against ground truth (r = 0.97) and small minimal detectable changes of 0.21 mm in a recent study [
26]. This justifies the comparison of both methods in terms of their concurrent validity in this study. Third, we thoroughly calculated the sample size for the in vivo validation with a sufficient effect size to detect both statistically and clinically significant correlations. However, this calculation did not include a subgroup analysis to analyze aLBP patients and healthy individuals separately. This should be considered in future work.
5. Conclusions
TLFD_US showed excellent in vitro validity, and it can be assumed that the measurements also apply to the real-world data. Furthermore, it proved its resistance to variable tissue velocities with a stable measurement within small deviation ranges.
There was a large correlation between TLFD_US and speckle tracking analysis in an in vivo validation involving 10 aLBP patients and 10 healthy subjects. Additionally, speckle tracking analysis showed that TLFD was primarily caused by dermis sliding, although erector spinae muscle movement also appeared to have an impact. These results are a promising starting point for further research and may support the idea that LBP patients have a “frozen back”.
In addition to its previously established reliability, the TLFD_US has demonstrated its validity. When screening for LBP patients, the technique can be suggested to capture an extra morphologic TLF parameter.
Author Contributions
Conceptualization, A.B.; methodology, A.B.; formal analyses, A.B.; investigation, A.B.; writing—original draft preparation, A.B.; writing—review and editing, A.B. and R.S.; visualization, A.B.; supervision, R.S.; project administration, A.B. and R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Diploma Hochschule, Germany (Nr. 1014/2021, 27 October 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data can be made available by the author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| aLBP | Acute low back pain |
| LBP | Low back pain |
| TLF | Thoracolumbar fascia |
| TLFD | Deformation of the thoracolumbar fascia |
| TLFD_US | Ultrasound method to measure the deformation of the thoracolumbar fascia |
| US | Ultrasound |
References
- Ferreira, M.L.; de Luca, K.; Haile, L.M.; Steinmetz, J.D.; Culbreth, G.T.; Cross, M.; Kopec, J.A.; Ferreira, P.H.; Blyth, F.M.; Buchbinder, R.; et al. Global, Regional, and National Burden of Low Back Pain, 1990–2020, Its Attributable Risk Factors, and Projections to 2050: A Systematic Analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e316–e329. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, J.L.; Cao, J.; Chapin, A.; Chen, C.; Li, Z.; Liu, A.; Horst, C.; Kaldjian, A.; Matyasz, T.; Scott, K.W.; et al. US Health Care Spending by Payer and Health Condition, 1996–2016. JAMA 2020, 323, 863. [Google Scholar] [CrossRef] [PubMed]
- Von der Lippe, E.; Krause, L.; Prost, M.; Wengler, A.; Leddin, J.; Müller, A.; Zeisler, M.-L.; Anton, A.; Rommel, A.; BURDEN 2020 Study Group. Prävalenz von Rücken- und Nackenschmerzen in Deutschland. Ergebnisse der Krankheitslast-Studie BURDEN 2020. J. Health Monit. 2021, 6, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Itz, C.J.; Geurts, J.W.; van Kleef, M.; Nelemans, P. Clinical Course of Non-Specific Low Back Pain: A Systematic Review of Prospective Cohort Studies Set in Primary Care. Eur. J. Pain 2013, 17, 5–15. [Google Scholar] [CrossRef]
- Parreira, P.; Maher, C.G.; Steffens, D.; Hancock, M.J.; Ferreira, M.L. Risk Factors for Low Back Pain and Sciatica: An Umbrella Review. Spine J. 2018, 18, 1715–1721. [Google Scholar] [CrossRef]
- Langevin, H.M.; Fox, J.R.; Koptiuch, C.; Badger, G.J.; Greenan- Naumann, A.C.; Bouffard, N.A.; Konofagou, E.E.; Lee, W.-N.; Triano, J.J.; Henry, S.M. Reduced Thoracolumbar Fascia Shear Strain in Human Chronic Low Back Pain. BMC Musculoskelet. Disord. 2011, 12, 203. [Google Scholar] [CrossRef]
- Schleip, R.; Vleeming, A.; Lehmann-Horn, F.; Klingler, W. Letter to the Editor Concerning “A Hypothesis of Chronic Back Pain: Ligament Subfailure Injuries Lead to Muscle Control Dysfunction” (M. Panjabi). Eur. Spine J. 2007, 16, 1733–1735. [Google Scholar] [CrossRef]
- Brandl, A.; Wilke, J.; Egner, C.; Schmidt, T.; Schilder, A.; Schleip, R. Pain Quality Patterns in Delayed Onset Muscle Soreness of the Lower Back Suggest Sensitization of Fascia Rather than Muscle Afferents: A Secondary Analysis Study. Pflug. Arch—Eur. J. Physiol. 2023, 476, 395–405. [Google Scholar] [CrossRef]
- Wilke, J.; Schleip, R.; Klingler, W.; Stecco, C. The Lumbodorsal Fascia as a Potential Source of Low Back Pain: A Narrative Review. BioMed Res. Int. 2017, 2017, 5349620. [Google Scholar] [CrossRef]
- Willard, F.H.; Vleeming, A.; Schuenke, M.D.; Danneels, L.; Schleip, R. The Thoracolumbar Fascia: Anatomy, Function and Clinical Considerations. J. Anat. 2012, 221, 507–536. [Google Scholar] [CrossRef]
- Schilder, A.; Hoheisel, U.; Magerl, W.; Benrath, J.; Klein, T.; Treede, R.-D. Sensory Findings after Stimulation of the Thoracolumbar Fascia with Hypertonic Saline Suggest Its Contribution to Low Back Pain. Pain 2014, 155, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Brandl, A.; Egner, C.; Reer, R.; Schmidt, T.; Schleip, R. Immediate Effects of Myofascial Release Treatment on Lumbar Microcirculation: A Randomized, Placebo-Controlled Trial. J. Clin. Med. 2023, 12, 1248. [Google Scholar] [CrossRef] [PubMed]
- Brandl, A.; Wilke, J.; Egner, C.; Schmidt, T.; Schleip, R. Effects of Maximal Eccentric Trunk Extensor Exercise on Lumbar Extramuscular Connective Tissue: A Matched-Pairs Ultrasound Study. J. Sports Sci. Med. 2023, 22, 446–453. [Google Scholar] [CrossRef]
- Brandl, A.; Engel, R.; Egner, C.; Schleip, R.; Schubert, C. Relations between Daily Stressful Events, Exertion, Heart Rate Variability, and Thoracolumbar Fascia Deformability: A Case Report. J. Med. Case Rep. 2024, 18, 589. [Google Scholar] [CrossRef]
- Michalak, J.; Aranmolate, L.; Bonn, A.; Grandin, K.; Schleip, R.; Schmiedtke, J.; Quassowsky, S.; Teismann, T. Myofascial Tissue and Depression. Cogn. Ther. Res. 2022, 46, 560–572. [Google Scholar] [CrossRef]
- Schubert, C. The Integrative Single-Case Design as a Biosemiotic-Systemic Research Tool in Psychoneuroimmunology. In Psychoneuroimmunology: Methods and Protocols; Yan, Q., Ed.; Springer: New York, NY, USA, 2024; ISBN 978-1-4939-7827-4. [Google Scholar]
- Tomita, N.; Roy-Cardinal, M.-H.; Chayer, B.; Daher, S.; Attiya, A.; Boulanger, A.; Gaudreault, N.; Cloutier, G.; Bureau, N.J. Thoracolumbar Fascia Ultrasound Shear Strain Differs between Low Back Pain and Asymptomatic Individuals: Expanding the Evidence. Insights Imaging 2025, 16, 18. [Google Scholar] [CrossRef]
- Wong, K.-K.; Chai, H.-M.; Chen, Y.-J.; Wang, C.-L.; Shau, Y.-W.; Wang, S.-F. Mechanical Deformation of Posterior Thoracolumbar Fascia after Myofascial Release in Healthy Men: A Study of Dynamic Ultrasound Imaging. Musculoskelet. Sci. Pract. 2017, 27, 124–130. [Google Scholar] [CrossRef]
- Pirri, C.; Pirri, N.; Guidolin, D.; Macchi, V.; Porzionato, A.; De Caro, R.; Stecco, C. Ultrasound Imaging of Thoracolumbar Fascia Thickness: Chronic Non-Specific Lower Back Pain versus Healthy Subjects; A Sign of a “Frozen Back”? Diagnostics 2023, 13, 1436. [Google Scholar] [CrossRef]
- Brandl, A. Associations between Thoracolumbar Fascia Deformation and Activation of Paraspinal Muscles in Patients with Acute Low Back Pain and Healthy Controls. A Matched-Pair Case-Control Study. In Proceedings of the European Fascia Research Exchange Meeting 2021, Munich, Germany, 10 December 2021. [Google Scholar]
- Brandl, A.; Wilke, J.; Horstmann, T.; Reer, R.; Egner, C.; Schmidt, T.; Schleip, R. Quantifying Thoracolumbar Fascia Deformation to Discriminate Acute Low Back Pain Patients and Healthy Individuals Using Ultrasound. Sci. Rep. 2024, 14, 20044. [Google Scholar] [CrossRef]
- Brandl, A.; Wilke, J.; Egner, C.; Reer, R.; Schmidt, T.; Schleip, R. Thoracolumbar Fascia Deformation during Deadlifting and Trunk Extension in Individuals with and without Back Pain. Front. Med. 2023, 10, 1177146. [Google Scholar] [CrossRef]
- Brandl, A.; Egner, C.; Reer, R.; Schmidt, T.; Schleip, R. Associations between Deformation of the Thoracolumbar Fascia and Activation of the Erector Spinae and Multifidus Muscle in Patients with Acute Low Back Pain and Healthy Controls: A Matched Pair Case-Control Study. Life 2022, 12, 1735. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, K.; Brandl, A.; Weber, P.; Wilke, J.; Bensamoun, S.F.; Bauermeister, W.; Klingler, W.; Schleip, R. Assessing Reliability and Validity of Different Stiffness Measurement Tools on a Multi-Layered Phantom Tissue Model. Sci. Rep. 2023, 13, 815. [Google Scholar] [CrossRef] [PubMed]
- Brandl, A.; Acikalin, E.; Bartsch, K.; Wilke, J.; Schleip, R. Reliability and Validity of an App-Assisted Tissue Compliance Meter in Measuring Tissue Stiffness on a Phantom Model. PeerJ 2024, 12, e17122. [Google Scholar] [CrossRef] [PubMed]
- ISO/IEC GUIDE 98-3: 2008; Guide to the Expression of Uncertainty in Measurement. International Organization for Standardization: Geneva, Switzerland, 2008.
- Brandl, A.; van Amstel, R. Nimal Ground Truth Data for Ultrasound Assessment of Thoracolumbar Fascia Deformation/Shearing. 2024. Available online: https://zenodo.org/records/14049288 (accessed on 4 February 2025).
- Ellis, R.; Hing, W.; Dilley, A.; McNair, P. Reliability of Measuring Sciatic and Tibial Nerve Movement with Diagnostic Ultrasound During a Neural Mobilisation Technique. Ultrasound Med. Biol. 2008, 34, 1209–1216. [Google Scholar] [CrossRef]
- Pillai, N.V.; Mohan, R. Reliability, Validity and Uni-Dimensionality: A Primer. 2020. Available online: https://mpra.ub.uni-muenchen.de/101714 (accessed on 4 February 2025).
- Arifin, W.N. Sample Size Calculator (Web). 2024. Available online: http://wnarifin.github.io (accessed on 4 February 2025).
- Puig-Diví, A.; Escalona-Marfil, C.; Padullés-Riu, J.M.; Busquets, A.; Padullés-Chando, X.; Marcos-Ruiz, D. Validity and Reliability of the Kinovea Program in Obtaining Angles and Distances Using Coordinates in 4 Perspectives. PLoS ONE 2019, 14, e0216448. [Google Scholar] [CrossRef]
- Vicente-Pina, L.; Sánchez-Rodríguez, R.; Ferrández-Laliena, L.; Heredia-Jimenez, J.; Müller-Thyssen-Uriarte, J.; Monti-Ballano, S.; Hidalgo-García, C.; Tricás-Moreno, J.M.; Lucha-López, M.O. Validity and Reliability of Kinovea® for Pelvic Kinematic Measurement in Standing Position and in Sitting Position with 45° of Hip Flexion. Sensors 2025, 25, 250. [Google Scholar] [CrossRef]
- Rodrigues Júnior, J.L.; Duarte, W.; Falqueto, H.; Andrade, A.G.P.; Morandi, R.F.; Albuquerque, M.R.; de Assis, M.G.; Serpa, T.K.F.; Pimenta, E.M. Correlation between Strength and Skin Temperature Asymmetries in the Lower Limbs of Brazilian Elite Soccer Players before and after a Competitive Season. J. Therm. Biol. 2021, 99, 102919. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, Rev. ed.; Academic Press: New York, NY, USA, 1988; ISBN 978-0-12-179060-8. [Google Scholar]
- Amstel, R.V.; Brandl, A.; Weide, G.; Bartsch, K.; Jaspers, R.T.; Pool-Goudzwaard, A.; Schleip, R. Validation of Speckle Tracking Analysis for Assessing Fascia Sliding Mobility. J. Biomech. 2025, 2025, 112580. [Google Scholar] [CrossRef]
- Patijn, J. Reproducibility Protocol for Diagnostic Procedures in Manual/Musculoskeletal Medicine. Man. Med. 2019, 57, 451–479. [Google Scholar] [CrossRef]
- Seidel, A.; Brandl, A.; Egner, C.; Schleip, R. Examination of Myofascial Stiffness and Elasticity in the Upper Trapezius Region in Patients with Unilateral Neck Pain: A Cross-Sectional Study. J. Clin. Med. 2023, 12, 6339. [Google Scholar] [CrossRef] [PubMed]
- Yerli, S.; Yinanç, S.B.; Yağcı, G.; Erbahçeci, F.; Özçakar, L. Thoracolumbar Fascia and Chronic Low Back Pain in Idiopathic Lumbar Scoliosis: An Ultrasonographic Study. Eur. Spine J. 2024, 33, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Watanabe, S. Influence of Inward Pressure of the Transducer on Lateral Abdominal Muscle Thickness During Ultrasound Imaging. J. Orthop. Sports Phys. Ther. 2012, 42, 815–818. [Google Scholar] [CrossRef]
- Fede, C.; Petrelli, L.; Pirri, C.; Neuhuber, W.; Tiengo, C.; Biz, C.; De Caro, R.; Schleip, R.; Stecco, C. Innervation of Human Superficial Fascia. Front. Neuranat. 2022, 16, 981426. [Google Scholar] [CrossRef] [PubMed]
- Fede, C.; Clair, C.; Pirri, C.; Petrelli, L.; Zhao, X.; Sun, Y.; Macchi, V.; Stecco, C. The Superficial Fascia: A Narrative Review. Int. J. Mol. Sci. 2024, 26, 1289. [Google Scholar] [CrossRef]
- van Amstel, R.N.; Jaspers, R.T.; Pool-Goudzwaard, A.L. Skin Displacement as Fascia Tissue Manipulation at the Lower Back Affects Instantaneously the Flexion-and Extension Spine, Pelvis, and Hip Range of Motion. Front. Physiol. 2022, 13, 1067816. [Google Scholar] [CrossRef]
- Wilke, J.; Tenberg, S. Semimembranosus Muscle Displacement Is Associated with Movement of the Superficial Fascia: An in Vivo Ultrasound Investigation. J. Anat. 2020, 237, 1026–1031. [Google Scholar] [CrossRef]
- Sakers, A.; Siqueira, M.K.D.; Seale, P.; Villanueva, C.J. Adipose-Tissue Plasticity in Health and Disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).