Assessment of Left Ventricular Strain Echocardiography in Individuals with Hashimoto’s Thyroiditis and Its Association with Serum TIMP-1 Concentration
Abstract
1. Introduction
2. Study Population
3. Echocardiographic Study
3.1. Conventional Echocardiography
3.2. Two-Dimensional Speckle Tracking Echocardiography
3.3. Venous Blood Sampling for TIMP-1
3.4. Statistical Analysis
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ragusa, F.; Fallahi, P.; Elia, G.; Gonnella, D.; Paparo, S.R.; Giusti, C.; Churilov, L.P.; Ferrari, S.M.; Antonelli, A. Hashimotos’ Thyroiditis: Epidemiology, Pathogenesis, Clinic and Therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101367. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.; Danzi, S. Thyroid Disease and the Heart. Circulation 2007, 116, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Barbier, P.; Mirea, O.; Cefalu, C.; Maltagliati, A.; Savioli, G.; Guglielmo, M. Reliability and Feasibility of Longitudinal AFI Global and Segmental Strain Compared with 2D Left Ventricular Volumes and Ejection Fraction: Intra-and Inter-Operator, Test–Retest, and Inter-Cycle Reproducibility. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Collier, P.; Phelan, D.; Klein, A. A Test in Context: Myocardial Strain Measured by Speckle-Tracking Echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1043–1056. [Google Scholar] [CrossRef]
- Haji, K.; Marwick, T.H. Clinical Utility of Echocardiographic Strain and Strain Rate Measurements. Curr. Cardiol. Rep. 2021, 23, 18. [Google Scholar] [CrossRef]
- Pislaru, C.; Abraham, T.P.; Belohlavek, M. Strain and Strain Rate Echocardiography. Curr. Opin. Cardiol. 2002, 17, 443–454. [Google Scholar] [CrossRef]
- Markousis-Mavrogenis, G.; Pepe, A.; Gargani, L.; Kariki, U.; Bonou, M.; Koutsogeorgopoulou, L.; Manolopoulou, D.; Tektonidou, M.G.; Vartela, V.; Kolovou, G.; et al. Myocardial Involvement in Rheumatic Disorders. Curr. Heart Fail. Rep. 2020, 17, 171–180. [Google Scholar] [CrossRef]
- Jia, F.; Li, X.; Zhang, D.; Jiang, S.; Yin, J.; Feng, X.; Zhu, Y.; Liu, Y.; Zhu, Y.; Lai, J. Predictive Value of Echocardiographic Strain for Myocardial Fibrosis and Adverse Outcomes in Autoimmune Diseases. Front. Cardiovasc. Med. 2022, 9, 836942. [Google Scholar] [CrossRef]
- Kostov, K.; Blazhev, A. Changes in Serum Levels of Matrix Metalloproteinase-1 and Tissue Inhibitor of Metalloproteinases-1 in Patients with Essential Hypertension. Bioengineering 2022, 9, 119. [Google Scholar] [CrossRef]
- Hansson, J.; Lind, L.; Hulthe, J.; Sundstrom, J. Relations of Serum MMP-9 and TIMP-1 Levels to Left Ventricular Measures and Cardiovascular Risk Factors: A Population-Based Study. Eur. J. Prev. Cardiol. 2009, 16, 297–303. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. J. Echocardiogr. 2016, 17, 1321–1360. [Google Scholar]
- Farsalinos, K.E.; Daraban, A.M.; Ünlü, S.; Thomas, J.D.; Badano, L.P.; Voigt, J.-U. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. J. Am. Soc. Echocardiogr. 2015, 28, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, A.; Pingitore, A.; Pearce, S.H.; Zaman, A.; Iervasi, G.; Razvi, S. Thyroid Hormones and Cardiovascular Disease. Nat. Rev. Cardiol. 2017, 14, 39–55. [Google Scholar] [CrossRef]
- Yamakawa, H.; Kato, T.S.; Noh, J.Y.; Yuasa, S.; Kawamura, A.; Fukuda, K.; Aizawa, Y. Thyroid Hormone Plays an Important Role in Cardiac Function: From Bench to Bedside. Front. Physiol. 2021, 12, 606931. [Google Scholar] [CrossRef]
- Kranias, E.G.; Hajjar, R.J. Modulation of Cardiac Contractility by the Phopholamban/SERCA2a Regulatome. Circ. Res. 2012, 110, 1646–1660. [Google Scholar] [CrossRef]
- Shui-Boon, S.O.H.; Tar-Choon, A.W. Laboratory Testing in Thyroid Conditions-Pitfalls and Clinical Utility. Ann. Lab. Med. 2019, 39, 3–14. [Google Scholar]
- Wémeau, J.-L.; Proust-Lemoine, E.; Ryndak, A.; Vanhove, L. Thyroid Autoimmunity and Polyglandular Endocrine Syndromes. Hormones 2013, 12, 39–45. [Google Scholar] [CrossRef]
- Guldvog, I.; Reitsma, L.C.; Johnsen, L.; Lauzike, A.; Gibbs, C.; Carlsen, E.; Lende, T.H.; Narvestad, J.K.; Omdal, R.; Kvaløy, J.T.; et al. Thyroidectomy Versus Medical Management for Euthyroid Patients With Hashimoto Disease and Persisting Symptoms: A Randomized Trial. Ann. Intern. Med. 2019, 170, 453. [Google Scholar] [CrossRef]
- Liu, J.E.; Barac, A.; Thavendiranathan, P.; Scherrer-Crosbie, M. Strain Imaging in Cardio-Oncology. JACC CardioOncology 2020, 2, 677–689. [Google Scholar] [CrossRef]
- Huttin, O.; Girerd, N.; Coiro, S.; Bozec, E.; Selton-Suty, C.; Lamiral, Z.; Frikha, Z.; Kobayashi, M.; Argulian, E.; Narula, J. Association between Layer-Specific Longitudinal Strain and Risk Factors of Heart Failure and Dyspnea: A Population-Based Study. J. Am. Soc. Echocardiogr. 2019, 32, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Azak, E.; Uçaktürk, S.A.; Çetin, İ.İ.; Gürsu, H.A.; Mengen, E.; Pamuk, U. Subclinical Myocardial Dysfunction Demonstrated by Speckle Tracking Echocardiography in Children with Euthyroid Hashimoto’s Thyroiditis. J. Clin. Res. Pediatr. Endocrinol. 2019, 11, 410. [Google Scholar] [CrossRef] [PubMed]
- Angum, F.; Khan, T.; Kaler, J.; Siddiqui, L.; Hussain, A. The Prevalence of Autoimmune Disorders in Women: A Narrative Review. Cureus 2020, 12, e8094. [Google Scholar] [CrossRef] [PubMed]
- Arata, A.; Ricci, F.; Khanji, M.Y.; Mantini, C.; Angeli, F.; Aquilani, R.; Di Baldassarre, A.; Renda, G.; Mattioli, A.V.; Nodari, S. Sex Differences in Heart Failure: What Do We Know? J. Cardiovasc. Dev. Dis. 2023, 10, 277. [Google Scholar] [CrossRef]
- Di Minno, M.N.D.; Forte, F.; Tufano, A.; Buonauro, A.; Rossi, F.W.; De Paulis, A.; Galderisi, M. Speckle Tracking Echocardiography in Patients with Systemic Lupus Erythematosus: A Meta-Analysis. Eur. J. Intern. Med. 2020, 73, 16–22. [Google Scholar] [CrossRef]
- Brahem, M.; Amor, H.I.H.; Sarraj, R.; Touil, I.; Kraiem, S.; Rouabhia, R.; Hmaier, E.; Mbarek, G.H.; Salem, A.B.; Mlouki, I. Echocardiography Coupled with Strain Method in the Screening for Cardiac Involvement in Rheumatoid Arthritis. Curr. Rheumatol. Rev. 2024, 20, 72–81. [Google Scholar] [CrossRef]
- Brunner, S.; Kim, J.-O.; Methe, H. Relation of Matrix Metalloproteinase-9/Tissue Inhibitor of Metalloproteinase-1 Ratio in Peripheral Circulating CD14+ Monocytes to Progression of Coronary Artery Disease. Am. J. Cardiol. 2010, 105, 429–434. [Google Scholar] [CrossRef]
- Sundström, J.; Evans, J.C.; Benjamin, E.J.; Levy, D.; Larson, M.G.; Sawyer, D.B.; Siwik, D.A.; Colucci, W.S.; Wilson, P.W.; Vasan, R.S. Relations of Plasma Total TIMP-1 Levels to Cardiovascular Risk Factors and Echocardiographic Measures: The Framingham Heart Study. Eur. Heart J. 2004, 25, 1509–1516. [Google Scholar] [CrossRef]
- Jublanc, C.; Beaudeux, J.L.; Aubart, F.; Raphael, M.; Chadarevian, R.; Chapman, M.J.; Bonnefont-Rousselot, D.; Bruckert, E. Serum Levels of Adhesion Molecules ICAM-1 and VCAM-1 and Tissue Inhibitor of Metalloproteinases, TIMP-1, Are Elevated in Patients with Autoimmune Thyroid Disorders: Relevance to Vascular Inflammation. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 817–822. [Google Scholar] [CrossRef]
- Ries, C. Cytokine Functions of TIMP-1. Cell. Mol. Life Sci. 2014, 71, 659–672. [Google Scholar] [CrossRef]
- Kormi, I.; Nieminen, M.T.; Havulinna, A.S.; Zeller, T.; Blankenberg, S.; Tervahartiala, T.; Sorsa, T.; Salomaa, V.; Pussinen, P.J. Matrix Metalloproteinase-8 and Tissue Inhibitor of Matrix Metalloproteinase-1 Predict Incident Cardiovascular Disease Events and All-Cause Mortality in a Population-Based Cohort. Eur. J. Prev. Cardiol. 2017, 24, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Luneva, E.B.; Vasileva, A.A.; Karelkina, E.V.; Boyarinova, M.A.; Mikhaylov, E.N.; Ryzhkov, A.V.; Babenko, A.Y.; Konradi, A.O.; Moiseeva, O.M. Simple Predictors for Cardiac Fibrosis in Patients with Type 2 Diabetes Mellitus: The Role of Circulating Biomarkers and Pulse Wave Velocity. J. Clin. Med. 2022, 11, 2843. [Google Scholar] [CrossRef] [PubMed]
- Duzen, I.V.; Tabur, S.; Ozturk, S.; Savcilioglu, M.D.; Alıc, E.; Yetisen, M.; Sanli, S.; Goksuluk, H.; Vuruskan, E.; Altunbas, G.; et al. Assessment of Subclinical Left Ventricular Dysfunction with Speckle-Tracking Echocardiography in Hyperthyroid and Euthyroid Graves’ Disease and Its Correlation with Serum TIMP-1. Acta Cardiol. 2021, 76, 177–184. [Google Scholar] [CrossRef] [PubMed]

| Total (n = 122) | Control 1 (n = 40) | Hypothyroid 2 HT (n = 40) | Euthyroid 3 HT (n = 42) | p Value | Post Hoc Comparisons’ p Values | |||
|---|---|---|---|---|---|---|---|---|
| Age, years | 28.3 ± 7.7 | 26 ± 2.5 | 28.4 ± 7.8 | 30.2 ± 9.2 | 0.154 | |||
| Female n (%) | 107 (87.7) | 34 (85) | 35 (87.5) | 38 (90.5) | 0.774 | |||
| Heart rate, bpm | 72 ± 10 | 68 ± 10 | 74 ± 8 | 0.872 | N/A | |||
| Systolic blood pressure, mmHg | 125 ± 12.5 | 115 ± 10.5 | 124 ± 11.4 | 0.895 | N/A | |||
| Diastolic blood pressure, mmHg | 73.5 ± 7.4 | 70.3 ± 6.8 | 72.8 ± 7.2 | 0.925 | N/A | |||
| Arrhythmia | 3 (0.075) | 4 (0.01) | 3 (0.071) | 0.826 | N/A | |||
| TFT | p 1,2 | p 1−3 | p 2,3 | |||||
| T3 (ng/L) | 3.2 (0.9) | 1.2 (0.4) | 3.2 (0.9) | <0.001 | <0.001 | 1.00 | <0.001 | |
| T4 (ng/L) | 1.0 (0.3) | 0.3 (0.2) | 1.0 (0.3) | <0.001 | <0.001 | 1.00 | <0.001 | |
| TSH (mU/L) | 1.9 (0.9) | 14.3 (14.3) | 1.9 (2.0) | <0.001 | <0.001 | 1.00 | <0.001 | |
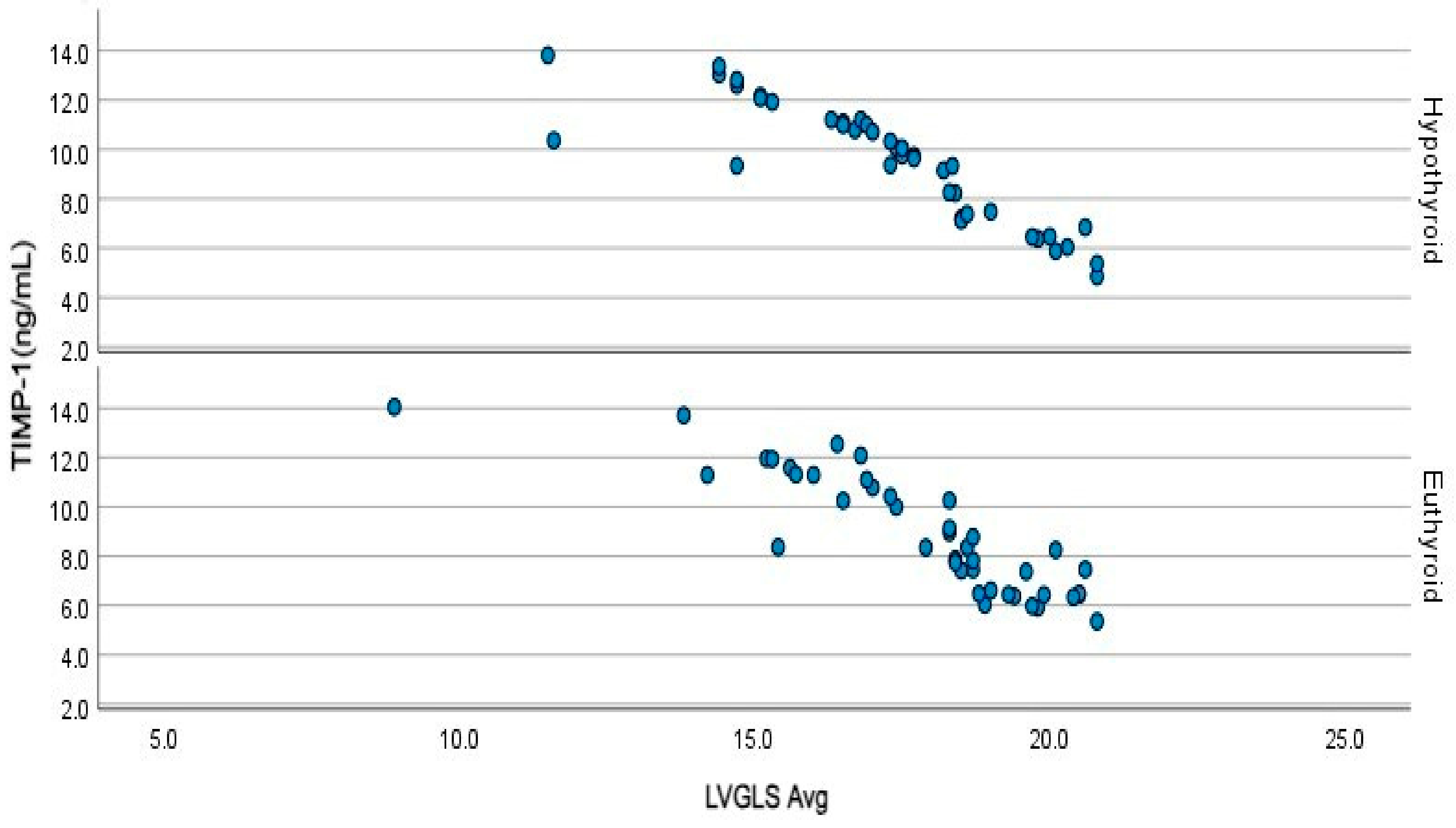
| TIMP 1 (ng/mL) | 5.4 (2.0) | 9.8 (3.9) | 8.4 (4.6) | <0.001 | <0.001 | <0.001 | 1.00 | |
| Measurement |
Controls (n = 40) |
Hypothyroid (n = 40) |
Euthyroid (n = 42) | p Value | p 1,2 | p 1–3 | p 2,3 |
|---|---|---|---|---|---|---|---|
| Left atrial diameter (mm) | 35 (3) | 36 (3) | 35 (2) | <0.001 | <0.001 | 0.011 | 0.247 |
| Left atrial volume index (ml/m2) | 31 (1) | 39 (2) | 33 (1) | <0.001 | <0.001 | 0.123 | <0.001 |
| Aortic root (mm) | 31 (1.8) | 32 (3) | 32 (2) | 0.368 | - | - | - |
| LVEDD (mm) | 44 (5) | 48 (4) | 47 (4) | <0.001 | <0.001 | 0.007 | 0.029 |
| LVESD (mm) | 33 (2) | 33 (1) | 32 (2) | <0.001 | 0.005 | 0.986 | <0.001 |
| LVEF (%) | 60 (6.5) | 58 (4.8) | 59 (4) | 0.025 | 0.027 | 0.168 | 1.00 |
| IVS. (mm) | 0.9 (0.2) | 1 (0.2) | 1 (0.1) | 0.012 | 0.063 | 0.018 | 1.00 |
| PW thickness (mm) | 0.7 (0.1) | 0.9 (0.1) | 0.8 (0.1) | <0.001 | <0.001 | 0.068 | <0.001 |
| E-wave (cm/s) | 70.5 (12) | 66 (12) | 70 (7) | 0.005 | 0.033 | 1.00 | 0.007 |
| A-wave (cm/s) | 60 (5) | 66 (5) | 65 (10) | <0.001 | <0.001 | <0.001 | 0.236 |
| E’ | 10.2 (1.1) | 7.9 (1.4) | 9.5 (1.3) | <0.001 | <0.001 | 0.200 | <0.001 |
| IVRT (ms) | 83 (9) | 123 (15) | 88 (38) | <0.001 | <0.001 | 0.020 | <0.001 |
| DT (ms) | 197 (13) | 226 (13) | 203 (22) | <0.001 | <0.001 | 0.126 | <0.001 |
| E/A | 1.2 (0.2) | 1.0 (0.1) | 1.1 (0.1) | <0.001 | <0.001 | 0.077 | <0.001 |
| E/E’ | 7.1 (1.6) | 8.2 (2.0) | 7.5 (0.9) | <0.001 | <0.001 | 0.111 | 0.069 |
| Region | Basal/Mid |
Controls (n = 40) |
Hypothyroid HT (n = 40) |
Euthyroid HT (n = 42) | p Value | p 1,2 | p 1–3 | p 2,3 |
|---|---|---|---|---|---|---|---|---|
| p | p | p | ||||||
| Posterior Septal Wall | Basal | −18 (6) | −16 (8) | −17 (6) | 0.065 | - | - | - |
| Mid | −21 (6) | −17 (7) | −17 (6) | 0.001 | 0.004 | 0.008 | 1.00 | |
| Anterior Septal Wall | Basal | −18 (5) | −14 (6) | −16 (5) | 0.002 | 0.002 | 1.00 | 0.038 |
| Mid | −20.5 (5) | −17 (5) | −18 (4) | <0.001 | <0.001 | 0.003 | 1.00 | |
| Lateral Wall | Basal | −18 (4) | −15 (6) | −17.5 (7) | 0.002 | 0.003 | 1.00 | 0.031 |
| Mid | −20 (4) | −17 (5) | −19 (6) | 0.002 | 0.002 | 0.161 | 0.361 | |
| Inferior Wall | Basal | −19 (3) | −14 (8) | −18 (6) | <0.001 | <0.001 | 0.517 | 0.016 |
| Mid | −21 (3) | −17 (5) | −20 (4) | <0.001 | <0.001 | 0.046 | 0.045 | |
| Anterior Wall | Basal | −18 (3.8) | −13.5 (5) | −14.5 (7.4) | <0.001 | <0.001 | <0.001 | 0.492 |
| Mid | −20.5 (2.8) | −14.5 (6.8) | −16 (7) | <0.001 | <0.001 | <0.001 | 1.00 | |
| Apical Regions | Anterior Apex | −26 (4) | −21 (8) | −21 (7) | <0.001 | <0.001 | <0.001 | 1.00 |
| Inferior Apex | −26 (4) | −22 (9) | −22 (7) | <0.001 | <0.001 | <0.001 | 1.00 | |
| Lateral Apex | −26 (5) | −22 (8) | −22 (7) | <0.001 | 0.002 | 0.003 | 1.00 | |
| Apical Cap | −27 (5) | −21 (7) | −21 (6) | <0.001 | <0.001 | <0.001 | 1.00 | |
| GLS Values | GLS A3C | −20.4 (2.1) | −18.6 (4.4) | −17.9 (6.1) | <0.001 | 0.003 | 0.003 | 1.00 |
| GLS A4C | −20.4 (2.3) | −17.8 (4.4) | −18.3 (3.8) | <0.001 | <0.001 | <0.001 | 1.00 | |
| GLS A2C | −21.2 (2.7) | −16.2 (3.9) | −18.7 (3) | <0.001 | <0.001 | <0.001 | 0.081 | |
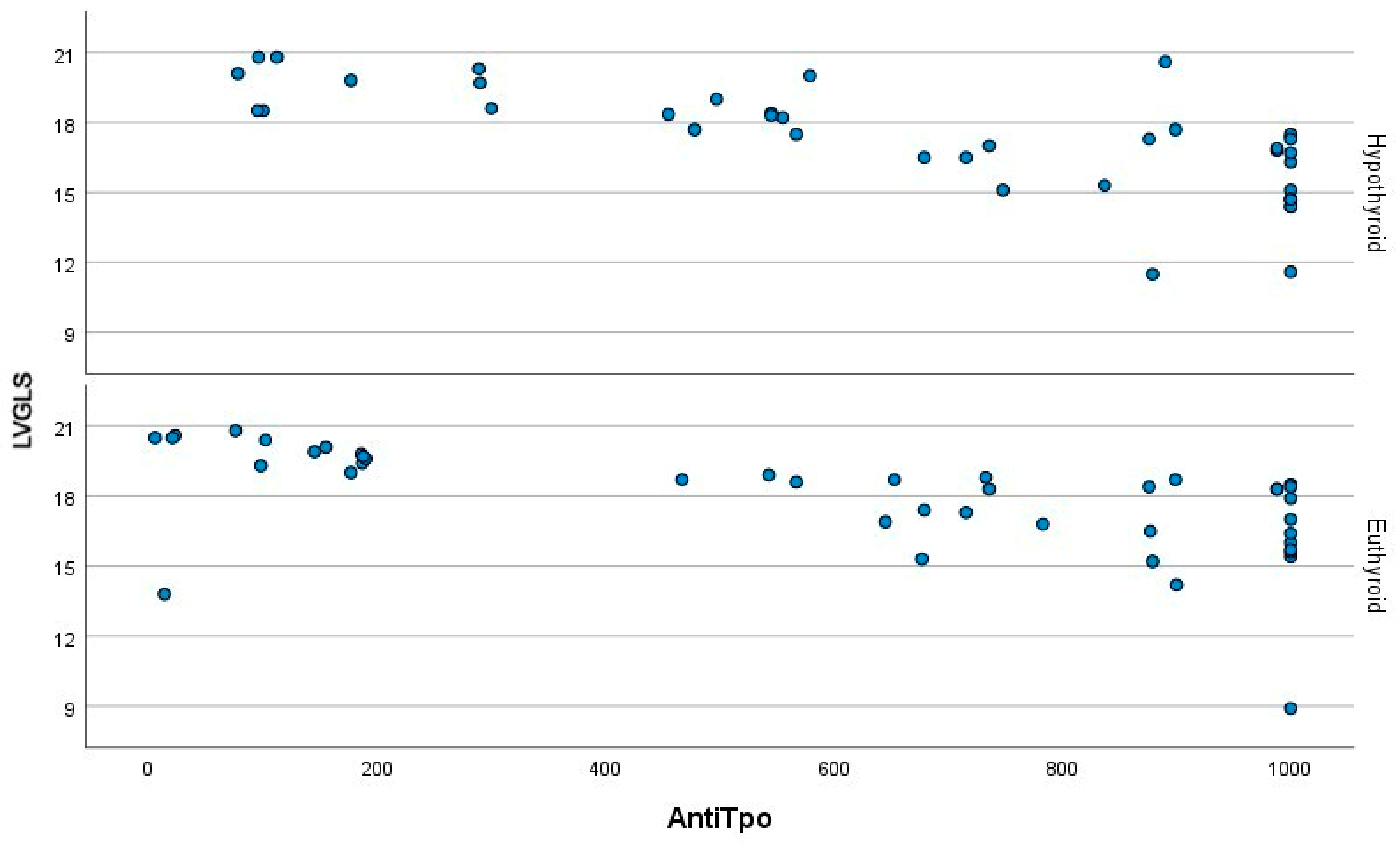
| LVGLS | −20.5 (4.4) | −17.5 (3.0) | −18.4 (3.1) | <0.001 | <0.001 | <0.001 | 0.958 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duzen, I.V.; Tuluce, S.Y.; Ozturk, S.; Savcılıoglu, M.D.; Goksuluk, H.; Altunbas, G.; Kaplan, M.; Vuruskan, E.; Tabur, S.; Sucu, M.; et al. Assessment of Left Ventricular Strain Echocardiography in Individuals with Hashimoto’s Thyroiditis and Its Association with Serum TIMP-1 Concentration. J. Clin. Med. 2025, 14, 1705. https://doi.org/10.3390/jcm14051705
Duzen IV, Tuluce SY, Ozturk S, Savcılıoglu MD, Goksuluk H, Altunbas G, Kaplan M, Vuruskan E, Tabur S, Sucu M, et al. Assessment of Left Ventricular Strain Echocardiography in Individuals with Hashimoto’s Thyroiditis and Its Association with Serum TIMP-1 Concentration. Journal of Clinical Medicine. 2025; 14(5):1705. https://doi.org/10.3390/jcm14051705
Chicago/Turabian StyleDuzen, Irfan V., Selcen Y. Tuluce, Sadettin Ozturk, Mert D. Savcılıoglu, Huseyin Goksuluk, Gokhan Altunbas, Mehmet Kaplan, Ertan Vuruskan, Suzan Tabur, Murat Sucu, and et al. 2025. "Assessment of Left Ventricular Strain Echocardiography in Individuals with Hashimoto’s Thyroiditis and Its Association with Serum TIMP-1 Concentration" Journal of Clinical Medicine 14, no. 5: 1705. https://doi.org/10.3390/jcm14051705
APA StyleDuzen, I. V., Tuluce, S. Y., Ozturk, S., Savcılıoglu, M. D., Goksuluk, H., Altunbas, G., Kaplan, M., Vuruskan, E., Tabur, S., Sucu, M., & Taysi, S. (2025). Assessment of Left Ventricular Strain Echocardiography in Individuals with Hashimoto’s Thyroiditis and Its Association with Serum TIMP-1 Concentration. Journal of Clinical Medicine, 14(5), 1705. https://doi.org/10.3390/jcm14051705






