Sugammadex Safely Reduces Total Intubation Time in the Intensive Care Unit Following Coronary Artery Bypass Grafting (CABG) at a Real-World Community Hospital
Abstract
1. Introduction
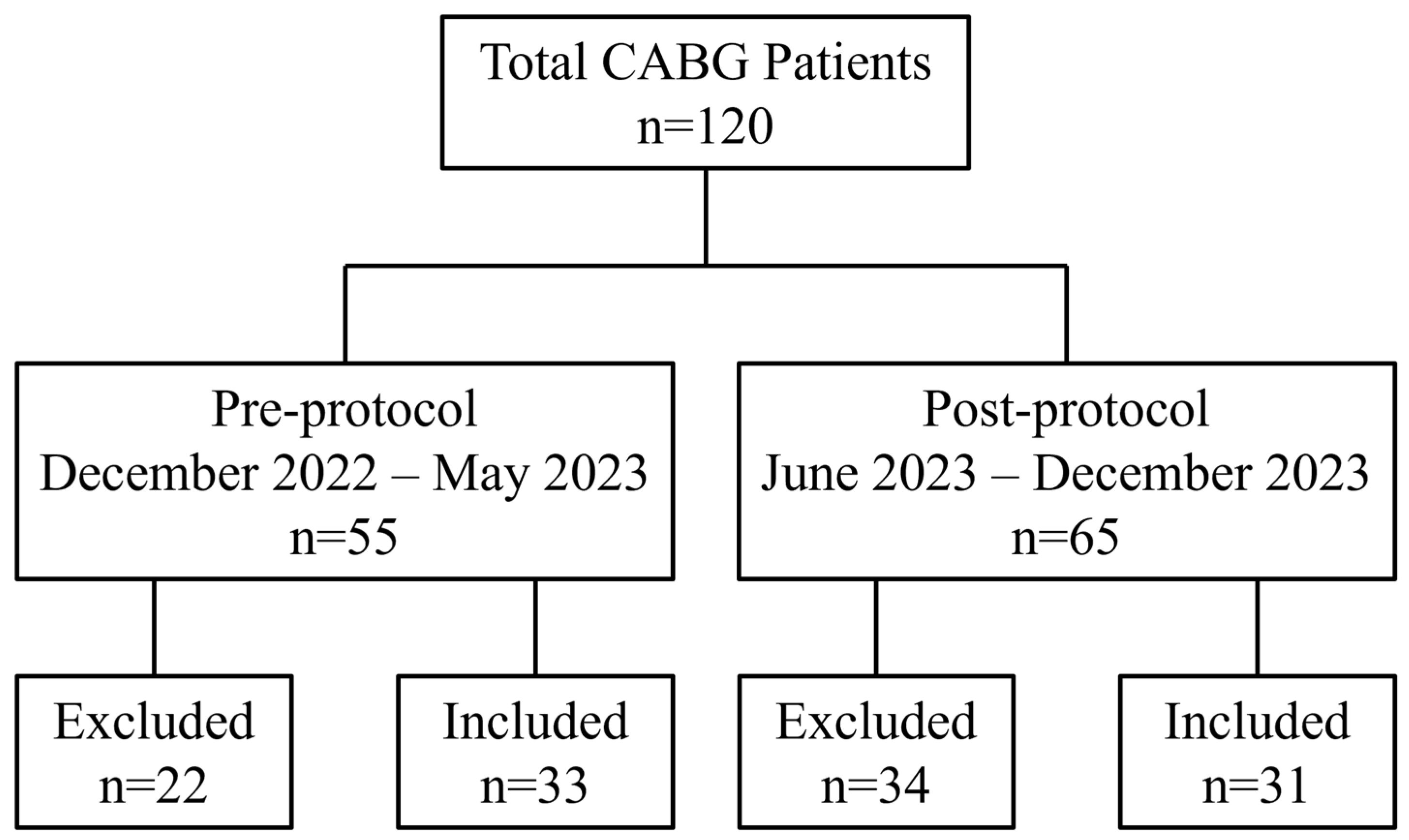
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| 1W-ANOVA | One-way analysis of variance |
| 1W-ANCOVA | One-way analysis of covariance |
| ABG | Arterial blood gas |
| AKI | Acute kidney injury |
| AVR | Aortic valve replacement |
| BMI | Body mass index |
| CABG | Coronary artery bypass grafting |
| COPD | Chronic obstructive pulmonary disease |
| eGFR | Estimated glomerular filtration rate |
| FTE | Fast-track extubation |
| HF | Heart failure |
| ICU | Intensive care unit |
| LOS | Length of stay |
| NMB | Neuromuscular blockade |
| NMBA | Neuromuscular blocking agent |
| PACU | Post-anesthesia care unit |
| PPC | Postoperative pulmonary complication |
| RR | Respiratory rate |
| SaO2 | Arterial oxygen saturation |
| STS | Society of Thoracic Surgeons |
| TOF | Train-of-four |
| TOFR | Train-of-four ratio |
References
- Greenberg, S.B.; Ben-Isvy, N.; Russell, H.; Whitney, H.; Wang, C.; Minhaj, M. A retrospective pilot comparison trial investigating clinical outcomes in cardiac surgical patients who received sugammadex reversal during 2018 to 2021. J. Cardiothorac. Vasc. Anesth. 2023, 37, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- What Is Coronary Artery Bypass Grafting? Available online: https://www.nhlbi.nih.gov/health/coronary-artery-bypass-grafting#:~:text=CABG (accessed on 25 June 2024).
- Lemaire, A.; Soto, C.; Salgueiro, L.; Ikegami, H.; Russo, M.J.; Lee, L.Y. The impact of age on outcomes of coronary artery bypass grafting. J. Cardiothorac. Surg. 2020, 15, 158. [Google Scholar] [CrossRef] [PubMed]
- Alamri, H.M.; Alotaibi, T.O.; Alghatani, A.A.; Alharthy, T.F.; Sufyani, A.M.; Alharthi, A.M.; Mahmoud, A.A.; Almahdi, M.K.; Alama, N.; Al-Ebrahim, K.E. Effect of gender on postoperative outcome and duration of ventilation after coronary artery bypass grafting (CABG). Cureus 2023, 15, e37717. [Google Scholar] [CrossRef]
- Wagner, B.D.; Grunwald, G.K.; Rumsfeld, J.S.; Hill, J.O.; Ho, P.M.; Wyatt, H.R.; Shroyer, A.L.W. Relationship of body mass index with outcomes after coronary artery bypass graft surgery. Ann. Thorac. Surg. 2007, 84, 10–16. [Google Scholar] [CrossRef]
- Laimoud, M.; Alanazi, M.N.; Maghirang, M.J.; Al-Mutlaq, S.M.; Althibait, S.; Ghamry, R.; Qureshi, R.; Alanazi, B.; Alomran, M.; Bakheet, Z.; et al. Impact of chronic kidney Disease on clinical outcomes during hospitalization and five-year follow-up after coronary artery bypass grafting. Crit. Care Res. Pract. 2023, 2023, 9364913. [Google Scholar] [CrossRef]
- Cooper, W.A.; O’Brien, S.M.; Thourani, V.H.; Guyton, R.A.; Bridges, C.R.; Szczech, L.A.; Petersen, R.; Peterson, E.D. Impact of renal dysfunction on outcomes of coronary artery bypass surgery: Results from the Society of Thoracic Surgeons National Adult Cardiac Database. Circulation 2006, 113, 1063–1070. [Google Scholar] [CrossRef]
- Andell, P.; Sjögren, J.; Batra, G.; Szummer, K.; Koul, S. Outcome of patients with chronic obstructive pulmonary disease and severe coronary artery disease who had a coronary artery bypass graft or a percutaneous coronary intervention. Eur. J. Cardiothorac. Surg. 2017, 52, 930–936. [Google Scholar] [CrossRef]
- Al-Sarraf, N.; Thalib, L.; Hughes, A.; Tolan, M.; Young, V.; McGovern, E. Effect of smoking on short-term outcome of patients undergoing coronary artery bypass surgery. Ann. Thorac. Surg. 2008, 86, 517–523. [Google Scholar] [CrossRef]
- Jin, R.; Grunkemeier, G.L.; Furnary, A.P.; Handy, J.R.J. Is obesity a risk factor for mortality in coronary artery bypass surgery? Circulation 2005, 111, 3359–3365. [Google Scholar] [CrossRef]
- Gaudino, M.; Di Franco, A.; Alexander, J.H.; Bakaeen, F.; Egorova, N.; Kurlansky, P.; Boening, A.; Chikwe, J.; Demetres, M.; Devereaux, P.J.; et al. Sex differences in outcomes after coronary artery bypass grafting: A pooled analysis of individual patient data. Eur. Heart J. 2021, 43, 18–28. [Google Scholar] [CrossRef]
- Hravnak, M.; Ibrahim, S.; Kaufer, A.; Sonel, A.; Conigliaro, J. Racial disparities in outcomes following coronary artery bypass grafting. J. Cardiovasc. Nurs. 2006, 21, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.C.; Karski, J.; Peniston, C.; Asokumar, B.; Raveendran, G.; Carroll, J.; Nierenberg, H.; Roger, S.; Mickle, D.; Tong, J.; et al. Morbidity outcome in early versus conventional tracheal extubation after coronary artery bypass grafting: A prospective randomized controlled trial. J. Thorac. Cardiovasc. Surg. 1996, 112, 755–764. [Google Scholar] [CrossRef]
- Guller, U.; Anstrom, K.J.; Holman, W.L.; Allman, R.M.; Sansom, M.; Peterson, E.D. Outcomes of early extubation after bypass surgery in the elderly. Ann. Thorac. Surg. 2004, 77, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Silbert, B.S.; Santamaria, J.D.; O’Brien, J.L.; Blyth, C.M.; Kelly, W.J.; Molnar, R.R.; The Fast Track Cardiac Care Team. Early extubation following coronary artery bypass surgery: A prospective randomized controlled trial. Chest 1998, 113, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Flynn, B.C.; He, J.; Richey, M.; Wirtz, K.; Daon, E. Early extubation without increased adverse events in high-risk cardiac surgical patients. Ann. Thorac. Surg. 2019, 107, 453–459. [Google Scholar] [CrossRef]
- Bardia, A.; Treggiari, M.M.; Dai, F.; Johnson, C.; Singh, M.; Kunze, K.; Tickoo, M.; Tantawy, H.; Giersson, A.; Darr, U.; et al. Efficacy and safety of sugammadex to shorten time-to-extubation following cardiac surgery: A single-center randomized placebo-controlled trial. Crit. Care Explor. 2022, 4, e0821. [Google Scholar] [CrossRef]
- Hayanga, H.K.; Ellison, M.B.; Badhwar, V. Patients should be extubated in the operating room after routine cardiac surgery: An inconvenient truth. JTCVS Tech. 2021, 8, 95–99. [Google Scholar] [CrossRef]
- Motamed, C. Intraoperative monitoring of neuromuscular blockade. Life 2023, 13, 1184. [Google Scholar] [CrossRef]
- Sacan, O.; White, P.F.; Tufanogullari, B.; Klein, K. Sugammadex reversal of rocuronium-induced neuromuscular blockade: A comparison with neostigmine-glycopyrrolate and edrophonium-atropine. Anesth. Analg. 2007, 104, 569–574. [Google Scholar] [CrossRef]
- Kizilay, D.; Dal, D.; Saracoglu, K.T.; Eti, Z.; Gogus, F.Y. Comparison of neostigmine and sugammadex for hemodynamic parameters in cardiac patients undergoing noncardiac surgery. J. Clin. Anesth. 2016, 28, 30–35. [Google Scholar] [CrossRef]
- Dahl, V.; Pendeville, P.E.; Hollmann, M.W.; Heier, T.; Abels, E.A.; Blobner, M. Safety and efficacy of sugammadex for the reversal of rocuronium-induced neuromuscular blockade in cardiac patients undergoing noncardiac surgery. Eur. J. Anaesthesiol. 2009, 26, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Bowdle, T.A.; Haththotuwegama, K.J.; Jelacic, S.; Nguyen, S.T.; Togashi, K.; Michaelsen, K.E. A dose-finding study of sugammadex for reversal of rocuronium in cardiac surgery patients and postoperative monitoring for recurrent paralysis. Anesthesiology 2023, 139, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, L.; Szabó-Maák, Z.; Gajdos, A.; Nemes, R.; Pongrácz, A.; Lengyel, S.; Fülesdi, B.; Tassonyi, E. Reversal of vecuronium-induced neuromuscular blockade with low-dose sugammadex at train-of-four count of four: A randomized controlled trial. Anesthesiology 2017, 127, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Khuenl-Brady, K.S.; Wattwil, M.; Vanacker, B.F.; Lora-Tamayo, J.I.; Rietbergen, H.; Alvarez-Gómez, J.A. Sugammadex provides faster reversal of vecuronium-induced neuromuscular blockade compared with neostigmine: A multicenter, randomized, controlled trial. Anesth. Analg. 2010, 110, 64–73. [Google Scholar] [CrossRef]
- Nag, K.; Singh, D.R.; Shetti, A.N.; Kumar, H.; Sivashanmugam, T.; Parthasarathy, S. Sugammadex: A revolutionary drug in neuromuscular pharmacology. Anesth. Essays Res. 2013, 7, 302–306. [Google Scholar] [CrossRef]
- Ajetunmobi, O.; Wong, D.; Perlas, A.; Rajaleelan, W.; Wang, S.; Huszti, E.; Jackson, T.; Chung, F.; Wong, J. Impact of sugammadex versus neostigmine reversal on postoperative recovery time in patients with obstructive sleep apnea undergoing bariatric surgery: A double-blind, randomized controlled trial. Anesth. Analg. 2024, 140, 568–576. [Google Scholar] [CrossRef]
- Subramani, Y.; Querney, J.; He, S.; Nagappa, M.; Yang, H.; Fayad, A. Efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade in morbidly obese adult patients: A systematic review and meta-analysis. Anesth. Essays Res. 2021, 15, 111–118. [Google Scholar] [CrossRef]
- Thilen, S.R.; Weigel, W.A.; Todd, M.M.; Dutton, R.P.; Lien, C.A.; Grant, S.A.; Szokol, J.W.; Eriksson, L.I.; Yaster, M.; Grant, M.D.; et al. 2023 American Society of Anesthesiologists practice guidelines for monitoring and antagonism of neuromuscular blockade: A report by the American Society of Anesthesiologists Task Force on Neuromuscular Blockade. Anesthesiology 2023, 138, 13–41. [Google Scholar] [CrossRef]
- Bailey, C.R. Sugammadex: When should we be giving it? Anaesthesia 2017, 72, 1170–1175. [Google Scholar] [CrossRef]
- Girard, T.D.; Alhazzani, W.; Kress, J.P.; Ouellette, D.R.; Schmidt, G.A.; Truwit, J.D.; Burns, S.M.; Epstein, S.K.; Esteban, A.; Fan, E.; et al. An official American Thoracic Society/American College of Chest Physicians Clinical Practice Guideline: Liberation from mechanical ventilation in critically ill adults. Rehabilitation protocols, ventilator liberation protocols, and cuff leak tests. Am. J. Respir. Crit. Care Med. 2017, 195, 120–133. [Google Scholar] [CrossRef]
- Cove, M.E.; Ying, C.; Taculod, J.M.; Oon, S.E.; Oh, P.; Kollengode, R.; MacLaren, G.; Tan, C.S. Multidisciplinary extubation protocol in cardiac surgical patients reduces ventilation time and length of stay in the intensive care unit. Ann. Thorac. Surg. 2016, 102, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.F.; Pena, H.; Cadavero, A.; Farrell, D.; Kettle, M.; Kaatz, A.R.; Thomas, T.; Granger, B.; Ghadimi, K. Reducing intubation time in adult cardiothoracic surgery patients with a fast-track extubation protocol. Crit. Care Nurse 2021, 41, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Dubovoy, T.Z.; Saager, L.; Shah, N.J.; Colquhoun, D.A.; Mathis, M.R.; Kapeles, S.; Mentz, G.; Kheterpal, S.; Vaughn, M.T. Utilization patterns of perioperative neuromuscular blockade reversal in the United States: A retrospective observational study from the Multicenter Perioperative Outcomes Group. Anesth. Analg. 2020, 131, 1510–1519. [Google Scholar] [CrossRef]
- Hristovska, A.-M.; Duch, P.; Allingstrup, M.; Afshari, A. Efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade in adults. Cochrane Database Syst. Rev. 2017, 8, CD012763. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Sreedharan, R.; Sonny, A.; Bose, S. Con: Sugammadex should be used routinely for reversal of neuromuscular blockade in patients undergoing thoracic surgery. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1792–1797. [Google Scholar] [CrossRef]
- Keating, G.M. Sugammadex: A review of neuromuscular blockade reversal. Drugs 2016, 76, 1041–1052. [Google Scholar] [CrossRef]
- Kotfis, K.; Szylińska, A.; Listewnik, M.; Lechowicz, K.; Kosiorowska, M.; Drożdżal, S.; Brykczyński, M.; Rotter, I.; Żukowski, M. Balancing intubation time with postoperative risk in cardiac surgery patients—A retrospective cohort analysis. Ther. Clin. Risk Manag. 2018, 14, 2203–2212. [Google Scholar] [CrossRef]
- Kuduvalli, M.; Grayson, A.D.; Oo, A.Y.; Fabri, B.M.; Rashid, A. Risk of morbidity and in-hospital mortality in obese patients undergoing coronary artery bypass surgery. Eur. J. Cardiothorac. Surg. 2002, 22, 787–793. [Google Scholar] [CrossRef]
- Tolpin, D.A.; Collard, C.D.; Lee, V.-V.; Elayda, M.A.; Pan, W. Obesity is associated with increased morbidity after coronary artery bypass graft surgery in patients with renal insufficiency. J. Thorac. Cardiovasc. Surg. 2009, 138, 873–879. [Google Scholar] [CrossRef]
- Murphy, G.S.; Szokol, J.W.; Avram, M.J.; Greenberg, S.B.; Shear, T.D.; Vender, J.S.; Parikh, K.N.; Patel, S.S.; Patel, A. Residual neuromuscular block in the elderly: Incidence and clinical implications. Anesthesiology 2015, 123, 1322–1336. [Google Scholar] [CrossRef]
- Park, J.-Y. Benefits and risks of sugammadex. Korean J. Anesthesiol. 2015, 68, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Vanacker, B.F.; Vermeyen, K.M.; Struys, M.M.R.F.; Rietbergen, H.; Vandermeersch, E.; Saldien, V.; Kalmar, A.F.; Prins, M.E. Reversal of rocuronium-induced neuromuscular block with the novel drug sugammadex is equally effective under maintenance anesthesia with propofol or sevoflurane. Anesth. Analg. 2007, 104, 563–568. [Google Scholar] [CrossRef]
- Duvaldestin, P.; Kuizenga, K.; Saldien, V.; Claudius, C.; Servin, F.; Klein, J.; Debaene, B.; Heeringa, M. A randomized, dose-response study of sugammadex given for the reversal of deep rocuronium- or vecuronium-induced neuromuscular blockade under sevoflurane anesthesia. Anesth. Analg. 2010, 110, 74–82. [Google Scholar] [CrossRef]
- Carron, M.; Bertoncello, F.; Ieppariello, G. Profile of sugammadex for reversal of neuromuscular blockade in the elderly: Current perspectives. Clin. Interv. Aging 2018, 13, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Li, D.-Y.; Wu, J.-X.; Jiang, Q.-L.; Zhu, H.-W.; Xu, M.-Y. Comparison of deep or moderate neuromuscular blockade for thoracoscopic lobectomy: A randomized controlled trial. BMC Anesthesiol. 2018, 18, 195. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-J.; Lee, H.N.; Chung, J.-Y.; Kim, D.; Kim, J.I.; Seo, H. Effect of deep versus moderate neuromuscular blockade on quantitatively assessed postoperative atelectasis using computed tomography in thoracic surgery; a randomized double-blind controlled trial. J. Clin. Med. 2021, 10, 3228. [Google Scholar] [CrossRef]
- Yu, H.; Zuo, Y.; Xu, Z.; Zhao, D.; Yue, J.; Liu, L.; Guo, Y.; Huang, J.; Deng, X.; Liang, P. Comparison effects of two muscle relaxant strategies on postoperative pulmonary complications in transapical transcatheter aortic valve implantation: A propensity score-matched analysis. J. Cardiothorac. Surg. 2023, 18, 50. [Google Scholar] [CrossRef]
- Wang, J.-F.; Zhao, Z.-Z.; Jiang, Z.-Y.; Liu, H.-X.; Deng, X.-M. Influence of sugammadex versus neostigmine for neuromuscular block reversal on the incidence of postoperative pulmonary complications: A meta-analysis of randomized controlled trials. Perioper. Med. 2021, 10, 32. [Google Scholar] [CrossRef]
- Li, G.; Freundlich, R.E.; Gupta, R.K.; Hayhurst, C.J.; Le, C.H.; Martin, B.J.; Shotwell, M.S.; Wanderer, J.P. Postoperative pulmonary complications’ association with sugammadex versus neostigmine: A retrospective registry analysis. Anesthesiology 2021, 134, 862–873. [Google Scholar] [CrossRef]
- Cho, H.C.; Lee, J.H.; Lee, S.C.; Park, S.Y.; Rim, J.C.; Choi, S.R. Use of sugammadex in lung cancer patients undergoing video-assisted thoracoscopic lobectomy. Korean J. Anesthesiol. 2017, 70, 420–425. [Google Scholar] [CrossRef]
- Moon, T.S.; Reznik, S.; Pak, T.; Jan, K.; Pruszynski, J.; Kim, A.; Smith, K.M.; Lu, R.; Chen, J.; Gasanova, I.; et al. Sugammadex versus neostigmine for reversal of rocuronium-induced neuromuscular blockade: A randomized, double-blinded study of thoracic surgical patients evaluating hypoxic episodes in the early postoperative period. J. Clin. Anesth. 2020, 64, 109804. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.S.; Avram, M.J.; Greenberg, S.B.; Bilimoria, S.; Benson, J.; Maher, C.E.; Teister, K.J.; Szokol, J.W. Neuromuscular and clinical recovery in thoracic surgical patients reversed with neostigmine or sugammadex. Anesth. Analg. 2021, 133, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Kheterpal, S.; Vaughn, M.T.; Dubovoy, T.Z.; Shah, N.J.; Bash, L.D.; Colquhoun, D.A.; Shanks, A.M.; Mathis, M.R.; Soto, R.G.; Bardia, A.; et al. Sugammadex versus neostigmine for reversal of neuromuscular blockade and postoperative pulmonary complications (STRONGER): A multicenter matched cohort analysis. Anesthesiology 2020, 132, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Ashabi, G.; Khalaj, L.; Khodagholi, F.; Goudarzvand, M.; Sarkaki, A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab. Brain Dis. 2015, 30, 747–754. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, H.; Bao, Q.; Zhang, T.; Chen, B.; Ding, J. Sugammadex versus neostigmine for neuromuscular block reversal and postoperative pulmonary complications in patients undergoing resection of lung cancer. J. Cardiothorac. Vasc. Anesth. 2022, 36, 3626–3633. [Google Scholar] [CrossRef]
- Muedra, V.; Rodilla, V.; Llansola, M.; Agustí, A.; Pla, C.; Canto, A.; Hernández-Rabaza, V. Potential neuroprotective role of sugammadex: A clinical study on cognitive function assessment in an enhanced recovery after cardiac surgery approach and an experimental study. Front. Cell. Neurosci. 2022, 16, 789796. [Google Scholar] [CrossRef]
- Rössler, J.; Abramczyk, E.; Paredes, S.; Anusic, N.; Pu, X.; Maheshwari, K.; Turan, A.; Ruetzler, K. Association of intravenous neostigmine and anticholinergics or sugammadex with postoperative delirium: A retrospective cohort study. Anesth. Analg. 2025, 140, 110–118. [Google Scholar] [CrossRef]
- Blobner, M.; Hollmann, M.W.; Luedi, M.M.; Johnson, K.B. Pro-con debate: Do we need quantitative neuromuscular monitoring in the era of sugammadex? Anesth. Analg. 2022, 135, 39–48. [Google Scholar] [CrossRef]
- Mao, X.; Zhang, R.; Liang, X.; Liu, F.; Dai, Y.; Wang, M.; Huang, H.; Fu, G. A pharmacovigilance study of FDA adverse events for sugammadex. J. Clin. Anesth. 2024, 97, 111509. [Google Scholar] [CrossRef]
- Teng, I.-C.; Chang, Y.-J.; Lin, Y.-T.; Chu, C.-C.; Chen, J.-Y.; Wu, Z.-F. Sugammadex induced bradycardia and hypotension: A case report and literature review. Medicine 2021, 100, e26796. [Google Scholar] [CrossRef]
- Ruetzler, K.; Li, K.; Chhabada, S.; Maheshwari, K.; Chahar, P.; Khanna, S.; Schmidt, M.T.; Yang, D.; Turan, A.; Sessler, D.I. Sugammadex versus neostigmine for reversal of residual neuromuscular blocks after surgery: A retrospective cohort analysis of postoperative side effects. Anesth. Analg. 2022, 134, 1043–1053. [Google Scholar] [CrossRef]
- Craig, R.G.; Hunter, J.M. Neuromuscular blocking drugs and their antagonists in patients with organ disease. Anaesthesia 2009, 64 (Suppl. S1), 55–65. [Google Scholar] [CrossRef] [PubMed]
- Orihara, M.; Takazawa, T.; Horiuchi, T.; Sakamoto, S.; Nagumo, K.; Tomita, Y.; Tomioka, A.; Yoshida, N.; Yokohama, A.; Saito, S. Comparison of incidence of anaphylaxis between sugammadex and neostigmine: A retrospective multicentre observational study. Br. J. Anaesth. 2020, 124, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Sunaga, H.; Kida, K.; Hobo, S.; Inoue, N.; Muto, M.; Uezono, S. Incidence of anaphylaxis associated with sugammadex. Anesth. Analg. 2018, 126, 1505–1508. [Google Scholar] [CrossRef]
- McKenzie, A.J. Neostigmine anaphlaxis: A rare and missed diagnosis. Anaesth. Intensive Care 2020, 48, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Seed, M.J.; Ewan, P.W. Anaphylaxis caused by neostigmine. Anaesthesia 2000, 55, 574–575. [Google Scholar] [CrossRef]
- Hermite, L.; Louvier, N.; Hilaire, P.; Orry, D.; Seltzer, S.; Collet, E. Neostigmine induced anaphylaxis in the wake of surgery. Anaesthesia Crit. Care Pain Med. 2015, 34, 109–111. [Google Scholar] [CrossRef]
- Zwiers, A.; van den Heuvel, M.; Smeets, J.; Rutherford, S. Assessment of the potential for displacement interactions with sugammadex: A pharmacokinetic-pharmacodynamic modelling approach. Clin. Drug Investig. 2011, 31, 101–111. [Google Scholar] [CrossRef]
- Devoy, T.; Smith, N. Sugammadex and oral contraceptives. Curr. Opin. Anaesthesiol. 2024, 37, 338–343. [Google Scholar] [CrossRef]
- Kirmeier, E.; Eriksson, L.I.; Lewald, H.; Jonsson Fagerlund, M.; Hoeft, A.; Hollmann, M.; Meistelman, C.; Hunter, J.M.; Ulm, K.; Blobner, M. Post-anaesthesia pulmonary complications after use of muscle relaxants (POPULAR): A multicentre, prospective observational study. Lancet. Respir. Med. 2019, 7, 129–140. [Google Scholar] [CrossRef]
- Carron, M.; Zarantonello, F.; Tellaroli, P.; Ori, C. Efficacy and safety of sugammadex compared to neostigmine for reversal of neuromuscular blockade: A meta-analysis of randomized controlled trials. J. Clin. Anesth. 2016, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kopman, A.F. Neostigmine versus sugammadex: Which, when, and how much? Anesthesiology 2010, 113, 1010–1011. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Song, J.; Gao, J.; Cheng, J.; Xie, H.; Zhang, L.; Wang, Y.H.; Gao, Z.; Wang, Y.; Wang, X.; et al. Adipocyte-derived kynurenine promotes obesity and insulin resistance by activating the AhR/STAT3/IL-6 signaling. Nat. Commun. 2022, 13, 3489. [Google Scholar] [CrossRef] [PubMed]
- Hurford, W.E.; Welge, J.A.; Eckman, M.H. Sugammadex versus neostigmine for routine reversal of rocuronium block in adult patients: A cost analysis. J. Clin. Anesth. 2020, 67, 110027. [Google Scholar] [CrossRef]
- Jiang, Y.; Bash, L.D.; Saager, L. A clinical and budgetary impact analysis of introducing sugammadex for routine reversal of neuromuscular blockade in a hypothetical cohort in the US. Adv. Ther. 2021, 38, 2689–2708. [Google Scholar] [CrossRef]

| Variable | Pre-Protocol (n = 33) | Post-Protocol (n = 31) | p-Value | |
|---|---|---|---|---|
| Age, years | 69.36 ± 1.80 | 67.19 ± 2.24 | 0.450 | |
| Age ≥ 65, n (%) | 25 (75.76%) | 19 (61.29%) | 0.283 | |
| Male sex, n (%) | 24 (72.73) | 20 (64.52) | 0.592 | |
| White race, n (%) | 26 (78.79) | 29 (93.55) | 0.150 | |
| Body mass index (BMI), kg/m2 | 30.05 ± 0.96 | 31.31 ± 1.23 | 0.422 | |
| Obese BMI, n (%) | 17 (51.52%) | 15 (48.39) | >0.999 | |
| eGFR, mL/min/1.73 m2 | 56.88 ± 1.17 | 57.21 ± 1.31 | 0.850 | |
| eGFR ≥ 60 mL/min/1.73 m2, n (%) | 25 (75.76) | 25 (80.65) | 0.765 | |
| Duration of CABG procedure, hours | 5.14 ± 0.25 | 5.72 ± 0.20 | 0.080 | |
| Number of coronary vessels grafted, n (%) | 1 | 5 (15.15) | 1 (3.23) | 0.090 |
| 2 | 3 (9.09) | 0 (0.00) | ||
| 3 | 7 (21.21) | 5 (16.13) | ||
| 4 | 14 (42.42) | 14 (45.16) | ||
| 5 | 4 (12.12) | 10 (32.26) | ||
| 6 | 0 (0.00) | 1 (3.23) | ||
| NMBA amount administered, mg | Rocuronium | 68.48 ± 9.10 | 69.03 ± 7.64 | 0.964 |
| Vecuronium | 13.09 ± 1.42 | 10.26 ± 0.97 | 0.109 | |
| Sugammadex amount administered, mg | 0.00 (0.00) | 184.71 ± 8.60 | <0.001 * | |
| Endpoint Data | Pre-Protocol (n = 33) | Post-Protocol (n = 31) | Cohen’s d | p-Value |
|---|---|---|---|---|
| Primary outcomes | ||||
| ICU intubation time, hours (SD) | 11.78 ± 1.23 (7.07) | 7.78 ± 1.13 (6.31) | 0.596 | 0.020 * |
| ICU intubation time >12 h, n (%) | 14 (42.42) | 4 (12.90) | 0.328 | 0.012 * |
| Primary safety outcomes | ||||
| Anaphylaxis, n (%) | 0 (0.00) | 0 (0.00) | 0.178 | 0.567 |
| Acute kidney injury, n (%) | 6 (18.18) | 2 (6.45) | ||
| New onset of dysrhythmia, n (%) | 4 (12.12) | 4 (12.90) | ||
| Postoperative heart failure, n (%) | 1 (3.03) | 1 (3.23) | ||
| No adverse events, n (%) | 22 (66.67) | 24 (77.42) | ||
| Secondary outcomes | ||||
| ABG pH (SD) | 7.37 ± 0.01 (0.05) | 7.35 ± 0.01 (0.06) | 0.484 | 0.057 |
| SaO2, % (SD) | 97.15 ± 0.33 (1.91) | 97.10 ± 0.53 (2.94) | 0.022 | 0.929 |
| Respiratory rate, breaths/min (SD) | 18.06 ± 0.77 (4.44) | 16.90 ± 0.56 (3.13) | 0.299 | 0.236 |
| ICU LOS post-CABG, days (SD) | 6.18 ± 0.45 (2.60) | 5.71 ± 0.29 (1.62) | 0.216 | 0.390 |
| Total hospital LOS, days (SD) | 10.70 ± 1.06 (6.07) | 9.84 ± 0.72 (4.00) | 0.166 | 0.510 |
| Statistical Test | Covariates | Main Variables | Point Estimates | Parameter Estimates | Cohen’s d | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| Independent | Dependent | 95% Confidence Interval | ||||||
| Lower Bound | Upper Bound | |||||||
| 1-W ANOVA | None | Protocol category | ICU intubation time | 4.002 | 0.644 | 7.360 | 0.084 | 0.020 * |
| 1-W ANCOVA | Age category, sex category, race category, body mass index category | Protocol category | ICU intubation time | 3.927 | 0.316 | 7.538 | 0.076 | 0.034 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, K.; Jackson, J.; Bourgeois, C.; Delgado, E.; Burmeister, M.A. Sugammadex Safely Reduces Total Intubation Time in the Intensive Care Unit Following Coronary Artery Bypass Grafting (CABG) at a Real-World Community Hospital. J. Clin. Med. 2025, 14, 1660. https://doi.org/10.3390/jcm14051660
Lam K, Jackson J, Bourgeois C, Delgado E, Burmeister MA. Sugammadex Safely Reduces Total Intubation Time in the Intensive Care Unit Following Coronary Artery Bypass Grafting (CABG) at a Real-World Community Hospital. Journal of Clinical Medicine. 2025; 14(5):1660. https://doi.org/10.3390/jcm14051660
Chicago/Turabian StyleLam, Kimberly, Julia Jackson, Chelsey Bourgeois, Elina Delgado, and Melissa A. Burmeister. 2025. "Sugammadex Safely Reduces Total Intubation Time in the Intensive Care Unit Following Coronary Artery Bypass Grafting (CABG) at a Real-World Community Hospital" Journal of Clinical Medicine 14, no. 5: 1660. https://doi.org/10.3390/jcm14051660
APA StyleLam, K., Jackson, J., Bourgeois, C., Delgado, E., & Burmeister, M. A. (2025). Sugammadex Safely Reduces Total Intubation Time in the Intensive Care Unit Following Coronary Artery Bypass Grafting (CABG) at a Real-World Community Hospital. Journal of Clinical Medicine, 14(5), 1660. https://doi.org/10.3390/jcm14051660





