A Meta-Analysis of the Impact of Intranasal Dexmedetomidine on Emergence Delirium and Agitation in Children and Adolescents Undergoing Tonsillectomy and/or Adenoidectomy
Abstract
1. Introduction
2. Methods
2.1. Study Registration
2.2. Literature Search and Study Selection
2.3. Eligibility Criteria
- Population: children and adolescents (2–18) years old undergoing tonsillectomy and/or adenoidectomy surgery.
- Intervention: intranasal DEX in a dose of 1 or 2 µg/kg.
- Comparator: Placebo/no intervention.
- At least one of the following outcomes: emergence agitation/emergence delirium, extubation time (min), time to discharge from PACU, Pediatric Anesthesia Emergency Delirium (PAED) Scale score, and adverse events.
- Study design: randomized controlled trials (RCTs). We excluded observational studies to avoid bias related to them and provide more robust evidence from RCTs.
2.4. Data Extraction
2.5. Quality Assessment
2.6. Outcome Data Measurement
2.7. Statistical Analysis, Sensitivity Analysis, and Trial Sequence Analysis
3. Results
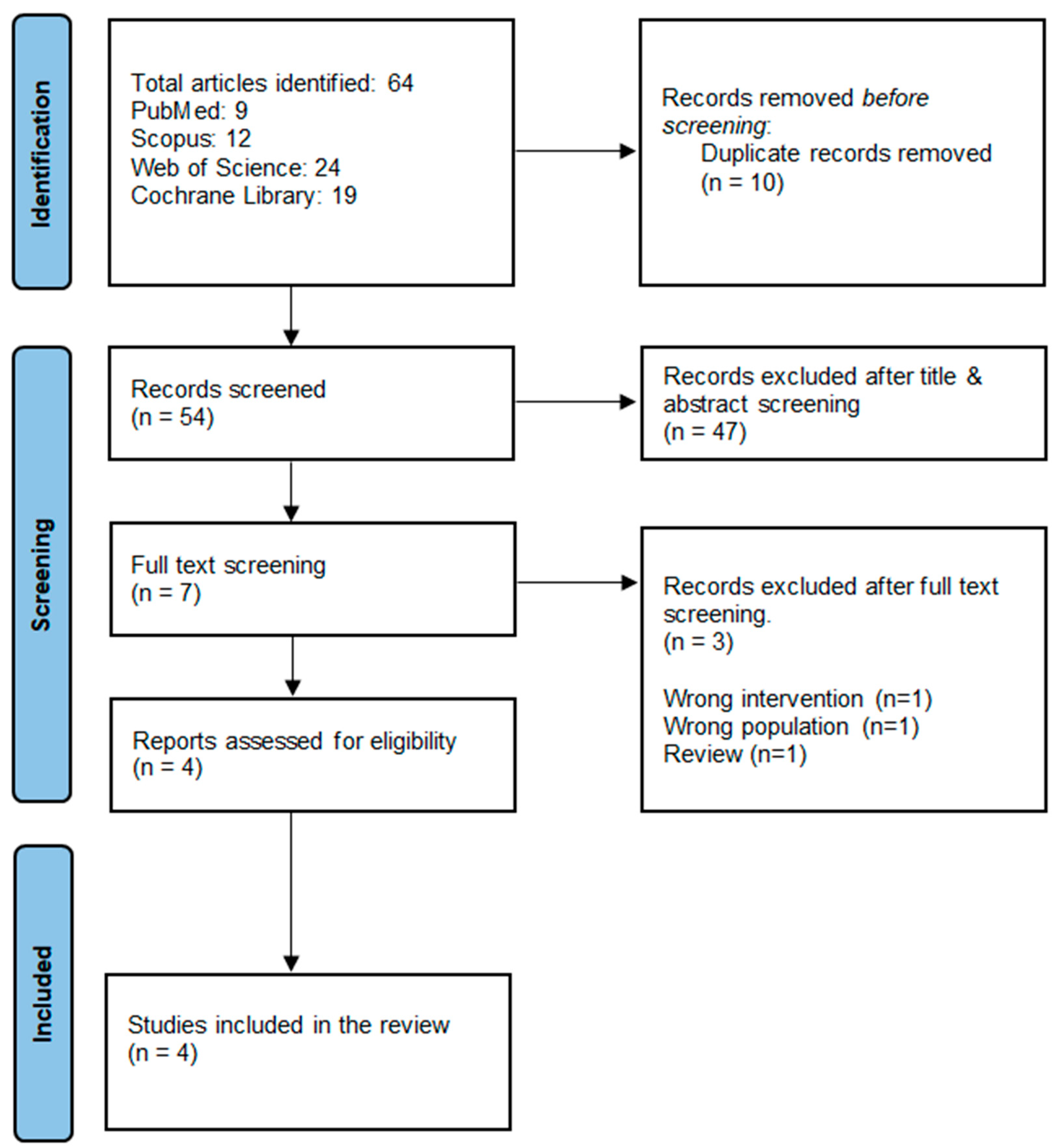
3.1. Literature Search
3.2. Study Characteristics
3.3. Quality Assessment
3.3.1. Primary Efficacy Outcomes: Emergence Agitation and Delirium
3.3.2. Secondary Efficacy Outcomes
3.3.3. Safety Outcomes: Adverse Events
3.3.4. Sensitivity Analysis
3.3.5. TSA of Emergence Agitation
3.3.6. TSA of Intervention’s Effect on Delirium
3.4. Quality of Evidence
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watson, A.T.; Visram, A. Children’s preoperative anxiety and postoperative behaviour. Paediatr. Anaesth. 2003, 13, 188–204. [Google Scholar] [CrossRef] [PubMed]
- ZKain, N.; Mayes, L.C.; O’Connor, T.Z.; Cicchetti, D.V. Preoperative anxiety in children: Predictors and outcomes. Arch. Pediatr. Adolesc. Med. 1996, 150, 1238–1245. [Google Scholar]
- Kain, Z.N.; Mayes, L.C.; Caldwell-Andrews, A.A.; Karas, D.E.; McClain, B.C. Preoperative anxiety, postoperative pain, and behavioral recovery in young children undergoing surgery. Pediatrics 2006, 118, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Mountain, B.W.; Smithson, L.; Cramolini, M.; Wyatt, T.H.; Newman, M. Dexmedetomidine as a pediatric anesthetic premedication to reduce anxiety and to deter emergence delirium. AANA J. 2011, 79, 219–224. [Google Scholar]
- Kuratani, N.; Oi, Y. Greater incidence of emergence agitation in children after sevoflurane anesthesia as compared with halothane: A meta-analysis of randomized controlled trials. Anesthesiology 2008, 109, 225–232. [Google Scholar] [CrossRef]
- Xiao, Y.; Jin, X.; Zhang, Y.; Huang, T.; Zhou, L.; Gao, J. Efficacy of propofol for the prevention of emergence agitation after sevoflurane anaesthesia in children: A meta-analysis. Front. Surg. 2022, 9, 1031010. [Google Scholar] [CrossRef]
- Tan, Y.; Shi, Y.; Ding, H.; Kong, X.; Zhou, H.; Tian, J. μ-Opioid agonists for preventing emergence agitation under sevoflurane anesthesia in children: A meta-analysis of randomized controlled trials. Pediatr. Anesth. 2016, 26, 139–150. [Google Scholar] [CrossRef]
- Monaco, F.; D’Andria Ursoleo, J.; Lerose, C.C.; Barucco, G.; Licheri, M.; Della Bella, P.E.; Fioravanti, F.; Gulletta, S. Anaesthetic management of paediatric patients undergoing electrophysiology study and ablation for supraventricular tachycardia: A focused narrative review. J. Clin. Anesth. 2024, 93, 111361. [Google Scholar] [CrossRef] [PubMed]
- Hebbar, C.; Reddy, A.; Luthra, A.; Chauhan, R.; Meena, S.C.; Tripathi, M. Comparison of the efficacy of intranasal atomised dexmedetomidine versus intranasal atomised ketamine as a premedication for sedation and anxiolysis in children undergoing spinal dysraphism surgery: A randomized controlled trial. Eur. J. Anaesthesiol. EJA 2024, 41, 288–295. [Google Scholar] [CrossRef]
- Qian, B.; Zheng, W.; Shi, J.; Chen, Z.; Guo, Y.; Yao, Y. Ketamine enhances intranasal dexmedetomidine-induced sedation in children: A randomized, double-blind trial. Drug Des. Devel. Ther. 2020, 14, 3559–3565. [Google Scholar] [CrossRef]
- Shen, F.; Zhang, Q.; Xu, Y.; Wang, X.; Xia, J.; Chen, C.; Liu, H.; Zhang, Y. Effect of Intranasal Dexmedetomidine or Midazolam for Premedication on the Occurrence of Respiratory Adverse Events in Children Undergoing Tonsillectomy and Adenoidectomy: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2225473. [Google Scholar] [CrossRef]
- Liao, Y.; Xie, S.; Zhuo, Y.; Chen, S.; Luo, Y.; Wei, Y.; Yao, Y. Intranasal Dexmedetomidine-Esketamine Combination Premedication versus Monotherapy for Reducing Emergence Delirium and Postoperative Behavioral Changes in Pediatric Tonsillectomy and/or Adenoidectomy: A Randomized Controlled Trial. Drug Des. Dev. Ther. 2024, 18, 4693–4703. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaye, A.D.; Urman, R.D.; Rappaport, Y.; Siddaiah, H.; Cornett, E.M.; Belani, K.; Salinas, O.J.; Fox, C.J. Multimodal analgesia as an essential part of enhanced recovery protocols in the ambulatory settings. J. Anaesthesiol. Clin. Pharmacol. 2019, 35 (Suppl. S1), S40–S45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sottas, C.E.; Anderson, B.J. Dexmedetomidine: The new all-in-one drug in paediatric anaesthesia? Curr. Opin. Anesthesiol. 2017, 30, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.; Bailey, C.R. Intranasal dexmedetomidine for sedation in children; a review. J. Perioper. Pract. 2020, 30, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Aki, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Schmidt, L.; Shokraneh, F.; Steinhausen, K.; Adams, C.E. Introducing RAPTOR: RevMan Parsing Tool for Reviewers. Syst. Rev. 2019, 8, 151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El-Hamid, A.M.A.; Yassin, H.M. Effect of intranasal dexmedetomidine on emergence agitation after sevoflurane anesthesia in children undergoing tonsillectomy and/or adenoidectomy. Saudi J. Anaesth. 2017, 11, 137–143. [Google Scholar]
- Li, L.-Q.; Wang, C.; Xu, H.-Y.; Lu, H.-L.; Zhang, H.-Z. Effects of different doses of intranasal dexmedetomidine on preoperative sedation and postoperative agitation in pediatric with total intravenous anesthesia undergoing adenoidectomy with or without tonsillectomy. Medicine 2018, 97, e12140. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Gong, H.; Zhao, X.; Peng, Q.; Zhao, H.; Yu, S. Parental presence and intranasal dexmedetomidine for the prevention of anxiety during anesthesia induction in children undergoing tonsillectomy and/or adenoidectomy surgery: A randomized controlled trial. Front. Pharmacol. 2022, 13, 1015357. [Google Scholar] [CrossRef] [PubMed]
- Akin, A.; Bayram, A.; Esmaoglu, A.; Tosun, Z.; Aksu, R.; Altuntas, R.; Boyaci, A. Dexmedetomidine vs midazolam for premedication of pediatric patients undergoing anesthesia. Pediatr. Anesth. 2012, 22, 871–876. [Google Scholar] [CrossRef]
- Hu, W.; Wang, M.; Sun, F. Effects of different doses of intranasal dexmedetomidine on related complications and parents’ satisfaction in anesthetized children: A systematic review. BMC Pediatr. 2024, 24, 377. [Google Scholar] [CrossRef]
- Lv, H.; Li, Y.; Cheng, Q.; Chen, J.; Chen, W. Neuroprotective effects against cerebral ischemic Injury exerted by Dexmedetomidine via the HDAC5/NPAS4/MDM2/PSD-95 Axis. Mol. Neurobiol. 2021, 58, 1990–2004. [Google Scholar] [CrossRef]
- Weerink, M.A.; Struys, M.M.; Hannivoort, L.N.; Barends, C.R.; Absalom, A.R.; Colin, P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin. Pharmacokinet. 2017, 56, 893–913. [Google Scholar] [CrossRef]
- Najafi, N.; Veyckemans, F.; Van de Velde, A.; Poelaert, J. Usability of dexmedetomidine for deep sedation in infants and small children with respiratory morbidities. Acta Anaesthesiol. Scand. 2016, 60, 865–873. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, M.Z.; Sun, Y.; Wu, C.; Xu, W.Y.; Bai, J.; Cai, M.H.; Lin, L. The sedative effects and the attenuation of cardiovascular and arousal responses during anesthesia induction and intubation in pediatric patients: A randomized comparison between two different doses of preoperative intranasal dexmedetomidine. Pediatr. Anesth. 2014, 24, 275–281. [Google Scholar] [CrossRef]
- Sheta, S.A.; Al-Sarheed, M.A.; Abdelhalim, A.A. Intranasal dexmedetomidine vs midazolam for premedication in children undergoing complete dental rehabilitation: A double-blinded randomized controlled trial. Pediatr. Anesth. 2014, 24, 181–189. [Google Scholar] [CrossRef]
- Han, J.I.; Lee, H.; Kim, C.H.; Lee, G.Y. The frequency of fentanyl-induced cough in children and its effects on tracheal intubation. J. Clin. Anesth. 2010, 22, 3–6. [Google Scholar] [CrossRef]
- Pieri, M.; D’Andria Ursoleo, J.; Di Prima, A.L.; Bugo, S.; Barucco, G.; Licheri, M.; Losiggio, R.; Frau, G.; Monaco, F. on behalf of Collaborators. Remimazolam for anesthesia and sedation in pediatric patients: A scoping review. J. Anesth. 2024, 38, 692–710. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Q.; Ihmsen, H.; Hu, Z.Y.; Sun, W.; Fang, Y.B.; Wang, Z.; Schüttler, J.; Jeleazcov, C.; Liu, H.C. Pharmacokinetics of remimazolam after intravenous infusion in anaesthetised children. Br. J. Anaesth. 2023, 131, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Simonini, A.; Brogi, E.; Cascella, M.; Vittori, A. Advantages of ketamine in pediatric anesthesia. Open Med. 2022, 17, 1134–1147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Biricik, E.; Karacaer, F.; Tunay, D.L.; Ilgınel, M.; Küçükbingöz, Ç. The Effect of Different Propofol-Ketamine Combinations on Emergence Delirium in Children Undergoing Adenoidectomy and Tonsillectomy Surgery. J. PeriAnesthesia Nurs. 2024, 39, 1012–1018. [Google Scholar] [CrossRef] [PubMed]


| Study | Country | Study Type | Intervention | Sample Size | Age(y) * | Males, n (%) | Weight (kg), Mean ± SD | Type of Surgery | Duration of Surgery(min) | Duration of Anesthesia (min) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenoidectomy | Adenoidectomy and Tonsillectomy | Tonsillectomy | ||||||||||
| Li et al. 2018 [21] | China | Double-blind randomized controlled trial | dexmedetomidine (1.0 μg/kg) | 30 | 4.47 ± 1.17 | 16 (53.3) | 19.82 ± 5.51 | 13 | 17 | - | 36.87 ± 20.06 | 45.87 ± 20.29 |
| Dexmedetomidine (2.0 μg/kg) | 30 | 4.53 ± 1.55 | 14 (46.7%) | 20.05 ± 5.79 | 9 | 21 | - | 40.03 ± 17.33 | 48.60 ± 17.35 | |||
| 0.9% saline | 30 | 4.37 ± 1.30 | 20 (66.7%) | 18.67 ± 4.10 | 12 | 18 | - | 34.10 ± 15.65 | 43.77 ± 16.11 | |||
| Shen et al. 2022 [11] | China | Double-blind randomized controlled trial | Midazolam (0.1 mg/kg) | 124 | 0–12 * | 75 (60.5) | - | 21 | 101 | 2 | 40 ± 15 | 47.9 ± 21.6 |
| Dexmedetomidine (2.0 μg/kg) | 124 | 0–12 * | 74 (60) | - | 28 | 95 | 1 | 35 ± 15 | 40 ± 15 | |||
| 0.9% saline | 125 | 0–12 * | 72 (58.1) | - | 25 | 96 | 4 | 38.3 ± 18.75 | 45 ± 18.75 | |||
| Yao et al.2022 [22] | China | Double-blind randomized controlled trial | dexmedetomidine (1.0 μg/kg) | 30 | 4.4 ± 1.2 | 18 (60) | 18.4 ± 4.9 | - | 14 | 16 | 36.00 ± 7.29 | NA |
| parental presence intervention and intranasal dexmedetomidine (1.0 μg/kg) | 30 | 4.6 ± 1.4 | 16 (53.3) | 19.7 ± 5.3 | - | 19 | 11 | 37.77 ± 8.89 | NA | |||
| parental presence intervention only | 30 | 4.6 ± 1.2 | 18 (60) | 20.9 ± 4.5 | - | 15 | 15 | 36.40 ± 7.20 | NA | |||
| Control | 30 | 4.3 ± 1.1 | 20 (66.7) | 19.9 ± 4.5 | - | 19 | 11 | 34.87 ± 1.03 | NA | |||
| Abd el-Hamid et al. 2017 [20] | Egypt | Double-blind randomized controlled trial | dexmedetomidine (1.0 μg/kg) | 43 | 4.4 ± 1.3 | 25 (58.1) | 17.4 ± 3.4 | 2 | 31 | 10 | 22.4 ± 5.2 | 33.6 ± 6.5 |
| 0.9% saline | 43 | 4.2 ± 0.93 | 19 (44.2) | 18.6 ± 4.1 | 3 | 28 | 12 | 24.1 ± 4.8 | 35.1 ± 5.9 | |||
| Certainty Assessment | Study Event Rates (%) | Effect | Certainty * | n of Studies | Study Design | RoB | Inconsistency | Indirectness | Imprecision | Others |
|---|---|---|---|---|---|---|---|---|---|---|
| Emergence Agitation | Dexmedetomidine vs. Control | RR 0.39 (0.16 to 0.92) | ⨁⨁⨁◯ Moderate | 2 | RCTs | not serious | serious | not serious | not serious | none |
| Emergence Delirium | Dexmedetomidine vs. Control | RR 0.45 (0.24 to 0.84) | ⨁⨁⨁◯ Moderate | 1 | RCTs | not serious | serious | not serious | not serious | none |
| Emergence Agitation and Delirium | Dexmedetomidine vs. Control | RR 0.42 (0.24 to 0.75) | ⨁⨁⨁◯ Moderate | 3 | RCTs | not serious | serious | not serious | not serious | none |
| PAED Scale Scores | Dexmedetomidine vs. Control | MD −2.11 (−3.77 to −0.44) | ⨁⨁⨁◯ Moderate | 3 | RCTs | not serious | very serious | not serious | serious | none |
| Time to Discharge from PACU | Dexmedetomidine vs. Control | MD −0.93 (−3.80 to 1.94) | ⨁⨁◯◯ Low | 2 | RCTs | not serious | serious | not serious | serious | none |
| Extubation Time | Dexmedetomidine vs. Control | MD 0.13 (−2.16 to 2.42) | ⨁⨁◯◯ Low | 2 | RCTs | not serious | serious | not serious | very serious | none |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Mutair, A.; Alabbasi, Y.; Alshammari, B.; Alrasheeday, A.M.; Alharbi, H.F.; Aleid, A.M. A Meta-Analysis of the Impact of Intranasal Dexmedetomidine on Emergence Delirium and Agitation in Children and Adolescents Undergoing Tonsillectomy and/or Adenoidectomy. J. Clin. Med. 2025, 14, 1586. https://doi.org/10.3390/jcm14051586
Al Mutair A, Alabbasi Y, Alshammari B, Alrasheeday AM, Alharbi HF, Aleid AM. A Meta-Analysis of the Impact of Intranasal Dexmedetomidine on Emergence Delirium and Agitation in Children and Adolescents Undergoing Tonsillectomy and/or Adenoidectomy. Journal of Clinical Medicine. 2025; 14(5):1586. https://doi.org/10.3390/jcm14051586
Chicago/Turabian StyleAl Mutair, Abbas, Yasmine Alabbasi, Bushra Alshammari, Awatif M. Alrasheeday, Hanan F. Alharbi, and Abdulsalam M. Aleid. 2025. "A Meta-Analysis of the Impact of Intranasal Dexmedetomidine on Emergence Delirium and Agitation in Children and Adolescents Undergoing Tonsillectomy and/or Adenoidectomy" Journal of Clinical Medicine 14, no. 5: 1586. https://doi.org/10.3390/jcm14051586
APA StyleAl Mutair, A., Alabbasi, Y., Alshammari, B., Alrasheeday, A. M., Alharbi, H. F., & Aleid, A. M. (2025). A Meta-Analysis of the Impact of Intranasal Dexmedetomidine on Emergence Delirium and Agitation in Children and Adolescents Undergoing Tonsillectomy and/or Adenoidectomy. Journal of Clinical Medicine, 14(5), 1586. https://doi.org/10.3390/jcm14051586







