Extended Postoperative Analgesia via Caudal Catheters for Major Surgery in Neonates—A 6-Year Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
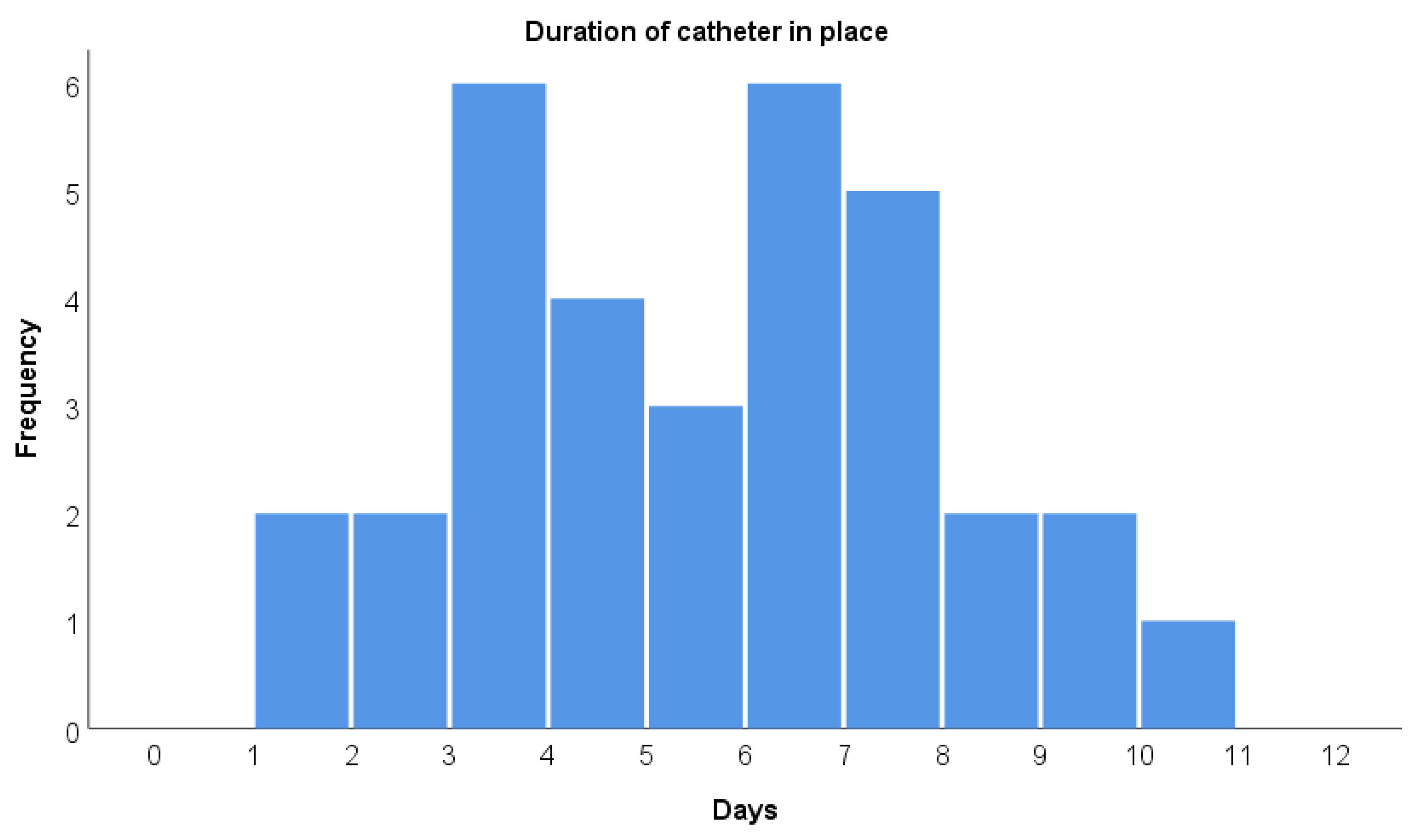
3. Results
3.1. Patient Characteristics
3.2. Postoperative Epidural Morphine
3.3. Additional Intravenous Opioid
3.4. Type of Surgery
3.5. Microbiology
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ecoffey, C. Safety in Pediatric Regional Anesthesia. Pediatr. Anesth. 2012, 22, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Menzies, R.; Congreve, K.; Herodes, V.; Berg, S.; Mason, D.G. A Survey of Pediatric Caudal Extradural Anesthesia Practice. Paediatr. Anaesth. 2009, 19, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Jöhr, M.; Berger, T.M. Caudal Blocks. Paediatr. Anaesth. 2012, 22, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Krane, E.J.; Tyler, D.C.; Jacobson, L.E. The Dose Response of Caudal Morphine in Children. Anesthesiology 1989, 71, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Ecoffey, C.; Lacroix, F.; Giaufré, E.; Orliaguet, G.; Courrèges, P. Epidemiology and Morbidity of Regional Anesthesia in Children: A Follow-up One-Year Prospective Survey of the French-Language Society of Paediatric Anaesthesiologists (ADARPEF). Paediatr. Anaesth. 2010, 20, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Tsui, B.C.H.; Wagner, A.; Cave, D.; Kearney, R. Thoracic and Lumbar Epidural Analgesia via the Caudal Approach Using Electrical Stimulation Guidance in Pediatric Patients: A Review of 289 Patients. Anesthesiology 2004, 100, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Valairucha, S.; Seefelder, C.; Houck, C.S. Thoracic Epidural Catheters Placed by the Caudal Route in Infants: The Importance of Radiographic Confirmation. Paediatr. Anaesth. 2002, 12, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Büttner, W.; Finke, W.; Hilleke, M.; Reckert, S.; Vsianska, L.; Brambrink, A. Entwicklung eines Fremdbeobachtungsbogens zur Beurteilung des postoperativen Schmerzes bei Säuglingen TT-Development of an Observational Scale for the Assessment of Postoperartive Pain in Infants. Anästhesiologie Intensivmed. Notfallmedizin Schmerzther. 1998, 33, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Ecoffey, C.; Bosenberg, A.; Lonnqvist, P.A.; De Oliveira, G.S.; De Leon Casasola, O.; De Andrés, J.; Ivani, G. The European Society of Regional Anaesthesia and Pain Therapy/American Society of Regional Anesthesia and Pain Medicine Recommendations on Local Anesthetics and Adjuvants Dosage in Pediatric Regional Anesthesia. Reg. Anesth. Pain. Med. 2018, 43, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Wiegele, M.; Marhofer, P.; Lönnqvist, P.A. Caudal Epidural Blocks in Paediatric Patients: A Review and Practical Considerations. Br. J. Anaesth. 2019, 122, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Ponde, V.C.; Bedekar, V.V.; Desai, A.P.; Puranik, K.A. Does Ultrasound Guidance Add Accuracy to Continuous Caudal-Epidural Catheter Placements in Neonates and Infants? Pediatr. Anesth. 2017, 27, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Bachman, S.A.; Taenzer, A.H. Thoracic Caudal Epidural Catheter Localization Using Ultrasound Guidance. Paediatr. Anaesth. 2019, 30, 194–195. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.A.; Galvez, I. Ultrasound Assessment of Caudal Catheter Position in Infants. Paediatr. Anaesth. 2005, 15, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Bromage, P.R.; Camporesi, E.M.; Durant, P.A.; Nielsen, C.H. Rostral Spread of Epidural Morphine. Anesthesiology 1982, 56, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Krane, E.J.; Jacobson, L.E.; Lynn, A.M.; Parrot, C.; Tyler, D.C. Caudal Morphine for Postoperative Analgesia in Children: A Comparison with Caudal Bupivacaine and Intravenous Morphine. Anesth. Analg. 1987, 66, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Rasch, D.K.; Webster, D.E.; Pollard, T.G.; Gurkowski, M.A. Lumbar and Thoracic Epidural Analgesia via the Caudal Approach for Postoperative Pain Relief in Infants and Children. Can. J. Anaesth. 1990, 37, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Nagappa, S.; Kalappa, S.; Vijayakumar, H.; Nethra, H. Comparison of the Effectiveness of Intravenous Fentanyl versus Caudal Epidural in Neonates Undergoing Tracheoesophageal Fistula Surgeries. Saudi J. Anaesth. 2022, 16, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Long, J.B.; Sathyamoorthy, M.; Birstler, J.; Wolf, C.; Bosenberg, A.T.; Flack, S.H.; Krane, E.J.; Sethna, N.F.; Suresh, S.; et al. Complications in Pediatric Regional Anesthesia: An Analysis of More than 100,000 Blocks from the Pediatric Regional Anesthesia Network. Anesthesiology 2018, 129, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Kost-Byerly, S.; Tobin, J.R.; Greenberg, R.S.; Billett, C.; Zahurak, M.; Yaster, M. Bacterial Colonization and Infection Rate of Continuous Catheters in Children. Anesth. Analg. 1998, 86, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Bubeck, J.; Boos, K.; Krause, H.; Thies, K.-C. Subcutaneous Tunneling of Caudal Catheters Reduces the Rate of Bacterial Colonization to That of Lumbar Epidural Catheters. Anesth. Analg. 2004, 99, 689–693. [Google Scholar] [CrossRef] [PubMed]
| Age [days] | 2 (1–8) {0; 27} |
| Gestational age at time of operation [days] | 260 (247–277) {222; 297} |
| Weight [g] | 2700 (2100–2900) {1400; 4200} |
| Male [n] | 17 (52%) |
| Thoracotomy [n] | 5 (15%) |
| Laparotomy [n] | 28 (85%) |
| Duration of surgery [minutes] | 107 (67–162) |
| Epidural morphine postoperatively [n] | 26 (79%) |
| Postoperative Epidural Morphine n = 26 | No Postoperative Epidural Morphine n = 7 | |
|---|---|---|
| Age [days] (median, IQR) | 3 (1–8) | 2 (0–6) |
| Gestational age [days] (mean, SD) | 261 (19) | 259 (17) |
| Weight [g] (mean, SD) | 2600 (700) | 2800 (800) |
| Male [n] | 14 (54%) | 3 (43%) |
| Duration of surgery [minutes] (mean, SD) | 102 (56) | 166 (62) |
| Duration of catheter [hours] (mean, SD) | 140 (59) | 123 (52) |
| Type of surgery | ||
| Laparotomy [n] | 23 (89%) | 5 (71%) |
| Thoracotomy [n] | 3 (11%) | 2 (29%) |
| i.v. opioid [n] | 22 (85%) | 5 (71%) |
| Duration of i.v. opioid [hours] (median, IQR) | 29 (16–70) | 25 (0–120) |
| Total i.v. morphine [mg/kg] (median, IQR) | 0.2 | 1.0 |
| Postop ventilation [n] | 16 (62%) | 5 (71%) |
| Duration of ventilation [hours] (mean, SD) | 71 (52) | 71 (32) |
| Time to first stool [hours] (mean, SD) | 41 (33) | 35 (19) |
| Time to enteral nutrition [hours] (median, IQR) | 30 (21–73) | 29 (25–46) |
| PICU LOS [hours] (median, IQR) | 671 (478–739) | 420 (287–1640) |
| No i.v. Opioid n = 6 | i.v. Opioid n = 27 | |
|---|---|---|
| Age [days] (median, IQR) | 4 (1–12) | 2 (1–8) |
| Gestational age [days] (mean, SD) | 273 (18) | 257 (17) |
| Weight [g] (mean, SD) | 3200 (500) | 2500 (700) |
| Male [n] | 2 (33%) | 15 (56%) |
| Type of surgery | ||
| Laparotomy [n] | 6 (100%) | 22 (82%) |
| Thoracotomy [n] | 0 (0%) | 5 (18%) |
| Duration of surgery [minutes] (mean, SD) | 111 (62) | 116 (63) |
| Duration of catheter [hours] (mean, SD) | 98 (49) | 145 (56) |
| Epidural morphine [n] | 4 (67%) | 22 (82%) |
| Duration of epidural morphine [hours] (median, IQR) | 3 (0–43) | 38 (18–105) |
| Postop ventilation [n] | 2 (33%) | 19 (70%) |
| Duration of ventilation [hours] (mean, SD) | 39 (31) | 74 (48) |
| Time to first stool [hours] (mean, SD) | 23 (25) | 44 (30) |
| Time to enteral nutrition [hours] (median, IQR) | 24 (13–66) | 30 (24–72) |
| PICU LOS [hours] (median, IQR) | 341 (182–530) | 674 (505–1058) |
| Laparotomy n = 28 | Thoracotomy n = 5 | |
|---|---|---|
| Age [days] (median, IQR) | 4 (1–8) | 1 (1–2) |
| Gestational age [days] (mean, SD) | 261 (19) | 260 (16) |
| Weight [g] (mean, SD) | 2500 (600) | 3300 (1000) |
| Male [n] | 13 (46%) | 4 (80%) |
| Duration of surgery [minutes] (mean, SD) | 101 (55) | 194 (43) |
| Duration of catheter [hours] (mean, SD) | 141 (57) | 110 (59) |
| Epidural morphine [n] | 23 (82%) | 3 (60%) |
| Duration of epidural morphine [hours] (median, IQR) | 35 (7–100) | 27 (0–87) |
| i.v. opioid [n] | 22 (82%) | 5 (100%) |
| Duration of i.v. opioid (median, IQR) | 26 (9–72) | 66 (30–95) |
| Postop ventilation [n] | 17 (61%) | 4 (80%) |
| Duration of ventilation [hours] (mean, SD) | 72 (48) | 63 (50) |
| Time to first stool [hours] (mean, SD) | 39 (32) | 41 (1) |
| Time to enteral nutrition [hours] (median, IQR) | 30 (23–74) | 26 (21–58) |
| PICU LOS [hours] (median, IQR) | 649 (465–742) | 406 (296–1298) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heschl, S.; Messerer, B.; Binder-Heschl, C.; Schörghuber, M.; Vittinghoff, M. Extended Postoperative Analgesia via Caudal Catheters for Major Surgery in Neonates—A 6-Year Retrospective Study. J. Clin. Med. 2025, 14, 2651. https://doi.org/10.3390/jcm14082651
Heschl S, Messerer B, Binder-Heschl C, Schörghuber M, Vittinghoff M. Extended Postoperative Analgesia via Caudal Catheters for Major Surgery in Neonates—A 6-Year Retrospective Study. Journal of Clinical Medicine. 2025; 14(8):2651. https://doi.org/10.3390/jcm14082651
Chicago/Turabian StyleHeschl, Stefan, Brigitte Messerer, Corinna Binder-Heschl, Michael Schörghuber, and Maria Vittinghoff. 2025. "Extended Postoperative Analgesia via Caudal Catheters for Major Surgery in Neonates—A 6-Year Retrospective Study" Journal of Clinical Medicine 14, no. 8: 2651. https://doi.org/10.3390/jcm14082651
APA StyleHeschl, S., Messerer, B., Binder-Heschl, C., Schörghuber, M., & Vittinghoff, M. (2025). Extended Postoperative Analgesia via Caudal Catheters for Major Surgery in Neonates—A 6-Year Retrospective Study. Journal of Clinical Medicine, 14(8), 2651. https://doi.org/10.3390/jcm14082651






