Assessment of Factors Related to Sarcopenia in Patients with Systemic Sclerosis
Abstract
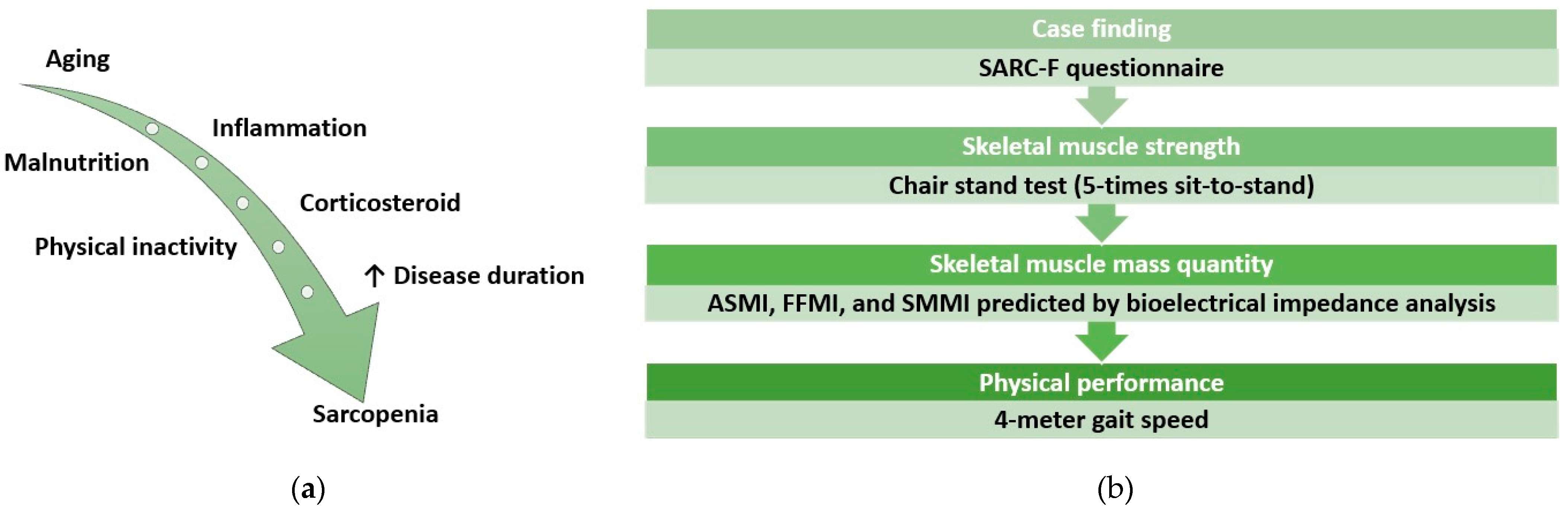
1. Introduction
2. Method
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhong, L.; Pope, M.; Shen, Y.; Hernandez, J.J.; Wu, L. Prevalence and incidence of systemic sclerosis: A systematic review and meta-analysis. Int. J. Rheum. Dis. 2019, 22, 2096–2107. [Google Scholar] [CrossRef] [PubMed]
- Siegert, E.; March, C.; Otten, L.; Makowka, A.; Preis, E.; Buttgereit, F.; Riemekasten, G.; Müller-Werdan, U.; Norman, K. Prevalence of sarcopenia in systemic sclerosis: Assessing body composition and functional disability in patients with systemic sclerosis. Nutrition 2018, 55–56, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, G.; Santoriello, C.; Polverino, F.; Ruocco, L.; Valentini, G.; Polverino, M. Impaired exercise performance in systemic sclerosis and its clinical correlations. Scand. J. Rheumatol. 2010, 39, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Corallo, C.; Fioravanti, A.; Tenti, S.; Pecetti, G.; Nuti, R.; Giordano, N. Sarcopenia in systemic sclerosis: The impact of nutritional, clinical, and laboratory features. Rheumatol. Int. 2019, 39, 1767–1775. [Google Scholar] [CrossRef]
- Tu, X.; Lin, T.; Ju, Y.; Shu, X.; Jiang, T.; Ge, N.; Yue, J. Sarcopenia in systemic sclerosis: Prevalence and impact—A systematic review and meta-analysis. BMJ Open 2024, 14, e078034. [Google Scholar] [CrossRef]
- Sari, A.; Esme, M.; Aycicek, G.S.; Armagan, B.; Kilic, L.; Ertenli, A.I.; Halil, M.G.; Akdogan, A. Evaluating skeletal muscle mass with ultrasound in patients with systemic sclerosis. Nutrition 2021, 84, 110999. [Google Scholar] [CrossRef]
- Van Den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef]
- Clements, P.; Lachenbruch, P.; Siebold, J.; White, B.; Weiner, S.; Martin, R.; Weinstein, A.; Weisman, M.; Mayes, M.; Collier, D. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J. Rheumatol. 1995, 22, 1281–1285. [Google Scholar]
- LeRoy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A., Jr.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar] [PubMed]
- Medsger, T.A.; Bombardieri, S.; Czirják, L.; Scorza, R.; Rossa, A.D.; Bencivelli, W. Assessment of disease severity and prognosis. Clin. Exp. Rheumatol. 2003, 21, S42–S46. [Google Scholar] [PubMed]
- Deurenberg, P.; Pietrobelli, A.; Wang, Z.; Heymsfield, S. Prediction of total body skeletal muscle mass from fat-free mass or intra-cellular water. Int. J. Body Compos. Res. 2004, 2, 107–114. [Google Scholar]
- Bahat, G.; Tufan, A.; Kilic, C.; Öztürk, S.; Akpinar, T.S.; Kose, M.; Erten, N.; Karan, M.A.; Cruz-Jentoft, A.J. Cut-off points for weight and body mass index adjusted bioimpedance analysis measurements of muscle mass. Aging Clin. Exp. Res. 2019, 31, 935–942. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.; et al. Diagnostic criteria for malnutrition–an ESPEN consensus statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef]
- Saglam, M.; Arikan, H.; Savci, S.; Inal-Ince, D.; Bosnak-Guclu, M.; Karabulut, E.; Tokgozoglu, L. International physical activity questionnaire: Reliability and validity of the Turkish version. Percept. Mot. Ski. 2010, 111, 278–284. [Google Scholar] [CrossRef]
- Maggio, M.; Ceda, G.P.; Ticinesi, A.; De Vita, F.; Gelmini, G.; Costantino, C.; Meschi, T.; Kressig, R.W.; Cesari, M.; Fabi, M.; et al. Instrumental and non-instrumental evaluation of 4-meter walking speed in older individuals. PLoS ONE 2016, 11, e0153583. [Google Scholar] [CrossRef]
- Cesari, M.; Kritchevsky, S.B.; Newman, A.B.; Simonsick, E.M.; Harris, T.B.; Penninx, B.W.; Brach, J.S.; Tylavsky, F.A.; Satterfield, S.; Bauer, D.C. Added value of physical performance measures in predicting adverse health-related events: Results from the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2009, 57, 251–259. [Google Scholar] [CrossRef]
- Sato, R.; Vatic, M.; Peixoto da Fonseca, G.W.; Anker, S.D.; von Haehling, S. Biological basis and treatment of frailty and sarcopenia. Cardiovasc. Res. 2024, 120, 982–998. [Google Scholar] [CrossRef]
- Sangaroon, A.; Foocharoen, C.; Theerakulpisut, D.; Srichompoo, K.; Mahakkanukrauh, A.; Suwannaroj, S.; Seerasaporn, P.; Pongchaiyakul, C. Prevalence and clinical association of sarcopenia among Thai patients with systemic sclerosis. Sci. Rep. 2022, 12, 18198. [Google Scholar] [CrossRef] [PubMed]
- Masanés, F.; Rojano i Luque, X.; Salva, A.; Serra-Rexach, J.; Artaza, I.; Formiga, F.; Cuesta, F.; Lopez Soto, A.; Ruiz, D.; Cruz-Jentoft, A.J. Cut-off points for muscle mass—Not grip strength or gait speed—Determine variations in sarcopenia prevalence. J. Nutr. Health Aging 2017, 21, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Ngeuleu, A.; Allali, F.; Medrare, L.; Madhi, A.; Rkain, H.; Hajjaj-Hassouni, N. Sarcopenia in rheumatoid arthritis: Prevalence, influence of disease activity and associated factors. Rheumatol. Int. 2017, 37, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef]
- Marighela, T.F.; Genaro, P.d.S.; Pinheiro, M.M.; Szejnfeld, V.L.; Kayser, C. Risk factors for body composition abnormalities in systemic sclerosis. Clin. Rheumatol. 2013, 32, 1037–1044. [Google Scholar] [CrossRef]
- Rosato, E.; Gigante, A.; Iacolare, A.; Villa, A.; Gasperini, M.L.; Muscaritoli, M. Reduction of fat free mass index and phase angle is a risk factor for development digital ulcers in systemic sclerosis patients. Clin. Rheumatol. 2020, 39, 3693–3700. [Google Scholar] [CrossRef]
- Caimmi, C.; Caramaschi, P.; Venturini, A.; Bertoldo, E.; Vantaggiato, E.; Viapiana, O.; Ferrari, M.; Lippi, G.; Frulloni, L.; Rossini, M. Malnutrition and sarcopenia in a large cohort of patients with systemic sclerosis. Clin. Rheumatol. 2018, 37, 987–997. [Google Scholar] [CrossRef]
- Rosato, E.; Gigante, A.; Pellicano, C.; Villa, A.; Iannazzo, F.; Alunni Fegatelli, D.; Muscaritoli, M. Symptoms related to gastrointestinal tract involvement and low muscularity in systemic sclerosis. Clin. Rheumatol. 2022, 41, 1687–1696. [Google Scholar] [CrossRef]
- Hongkanjanapong, S.; Pongkulkiat, P.; Mahakkanukrauh, A.; Suwannaroj, S.; Foocharoen, C. Clinical outcomes and associated factors with mortality in systemic sclerosis patients with sarcopenia. Am. J. Med. Sci. 2025, 369, 35–43. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef]
| Variable | n (%) |
|---|---|
| Gender, female | 73 (91.3) |
| Age, years * | 56.5 (45–65) |
| Smoker | 24 (30) |
| SSc subtype | |
| dcSSc | 23 (28.8) |
| lcSSc | 56 (70) |
| Sine SSc | 1 (1.3) |
| Overlap | 29 (36.3) |
| Antinuclear antibody positive | |
| Anti-centromere antibody | 27 (33.8) |
| Anti Scl-70 antibody | 32 (40) |
| Presence of comorbidity | 57 (71.3) |
| Diagnosis duration, months * | 90 (47.25–151) |
| Body mass index (kg/m2) * | 26.5 (23.75–31.88) |
| modified Rodnan skin score * | 4 (1.25–10) |
| Medsger disease severity scale * | 6 (4–7) |
| Pulmonary arterial hypertension | 5 (6.3) |
| Interstitial lung disease | 27 (33.8) |
| International Physical Activity Questionnaire | |
| Inactive | 33 (41.3) |
| Moderate | 37 (46.3) |
| Active | 10 (12.5) |
| Serum albumin (g/dL) * | 4.24 (4.0–4.36) |
| Total protein (g/dL) * | 7.23 (6.99–7.57) |
| C-reactive protein (mg/L) * | 3.7 (1.5–6.4) |
| Erythrocyte sedimentation rate (mm/h) * | 18 (10.25–35) |
| Creatinin kinase (U/L) * | 74 (54–108) |
| Steroid use | 47 (58.8) |
| Immunosuppressive treatment | 40 (50) |
| SARC-F score ≥4 | 16 (20) |
| Chair stand test, seconds * | 11.8 (9.8–14.7) |
| 4 m gait speed test, seconds * | 3.87 (3.4–4.6) |
| ≥0.8 m/s | 71 (88.8) |
| Fat-free muscle mass index (kg/m2) | |
| Female | 17.8 (16.7–19.8) |
| Male | 18.4 (16.8–21.2) |
| Skeletal muscle mass index (%) | |
| Female | 37.2 (34.2–40.2) |
| Male | 41.8 (37.6–43.5) |
| Variable | SARC-F < 4 (n = 64) | SARCF ≥ 4 (n = 16) | p |
|---|---|---|---|
| Gender, female | 58 (90.6) | 15 (93.8) | 0.670 |
| Age, years* | 51 (43.3–62.8) | 65 (61–71.3) | 0.001 |
| Smoker | 20 (31.3) | 4 (25) | 0.625 |
| SSc subtype | |||
| dcSSc | 20 (31.3) | 3 (18.8) | 0.363 |
| lcSSc | 43 (67.2) | 13 (81.3) | |
| Overlap | 20 (31.3) | 9 (56.3) | 0.090 |
| Antinuclear antibody positive | |||
| Anti-centromere antibody | 20 (31.3) | 7 (43.8) | 0.459 |
| Anti Scl-70 antibody | 26 (40.6) | 6 (37.5) | |
| Presence of comorbidity | 41 (64.1) | 16 (100) | 0.001 |
| Diagnosis duration, months * | 89 (47.3–138) | 106 (39.8–189) | 0.470 |
| Body mass index (kg/m2) * | 26.3 (23.8–31.7) | 29.7 (23.7–35.2) | 0.500 |
| modified Rodnan skin score * | 5 (1.25–10) | 4 (0.5–10) | 0.979 |
| Medsger disease severity scale * | 5.5 (3.25–7) | 7 (4.25–9.75) | 0.124 |
| Pulmonary arterial hypertension | 2 (3.1) | 3 (18.8) | 0.149 |
| Interstitial lung disease | 24 (37.5) | 3 (18.8) | 0.123 |
| International Physical Activity Questionnaire | |||
| Inactive | 20 (31.3) | 13 (81.3) | 0.002 |
| Moderate | 35 (54.7) | 2 (12.5) | |
| Active | 9 (14.1) | 1 (6.3) | |
| Serum albumin (g/dL) * | 4.26 (4.06–4.37) | 4.13 (3.86–4.32) | 0.096 |
| Total protein (g/dL) * | 7.2 (7.0–7.6) | 7.3 (6.7–7.4) | 0.868 |
| C-reactive protein (mg/L) * | 3.1 (1.4–6) | 4 (2.5–9.3) | 0.141 |
| Erythrocyte sedimentation rate (mm/h) * | 18 (8.5–31.3) | 19.5 (12–44.8) | 0.197 |
| Creatinin kinase (U/L) * | 74 (55–108) | 68 (39.5–119.8) | 0.893 |
| Steroid use | 37 (57.5) | 10 (62.5) | 0.740 |
| Immunosuppressive treatment | 33 (51.6) | 7 (43.8) | 0.589 |
| Chair-stand test, seconds * | 11.5 (9.7–13.5) | 15.6 (12.6–20.7) | 0.025 |
| 4 m gait speed test, seconds * | 3.7 (3.3–4.4) | 4.2 (3.9–6.2) | 0.045 |
| Experiencing falls within the past year | 14 (21.9) | 8 (50) | 0.057 |
| ASMI (kg/m2) | 7.3 (6.8–8.2) | 7.8 (6.7–8.5) | 0.395 |
| Fat-free muscle mass index (kg/m2) | 17.6 (16.7–19.4) | 18.8 (16.2–20.5) | 0.525 |
| Skeletal muscle mass index, % | 37.6 (34.7–40.3) | 36.8 (33.7–40.6) | 0.688 |
| Variable | <60 Years, n = 40 n (%) | ≥60 Years, n = 40 n (%) | p |
| Gender, female | 36 (90) | 37 (92.5) | 0.697 |
| Age, years * | 45 (38.5–50) | 65 (61–69) | 0.001 |
| Smoker | 15 (37.5) | 9 (22.5) | 0.147 |
| SSc subtype | |||
| dcSSc | 12 (30) | 11 (27.5) | 1.000 |
| lcSSc | 27 (67.5) | 29 (72.5) | |
| Antinuclear antibody positive | |||
| Anti-centromere antibody | 13 (32.5) | 14 (35) | 0.406 |
| Anti Scl-70 antibody | 15 (37.5) | 17 (42.5) | |
| Presence of comorbidity | 23 (57.5) | 34 (85) | 0.006 |
| Diagnosis duration, months * | 85 (33.5–138) | 91.5 (48–166.7) | 0.176 |
| Body mass index (kg/m2) * | 25.9 (23.6–28.8) | 29.8 (24.1–33.9) | 0.116 |
| modified Rodnan skin score * | 3 (0.25–10) | 5 (2–10.7) | 0.747 |
| Medsger disease severity scale * | 6 (3.2–7.7) | 5.5 (4–7) | 0.777 |
| International Physical Activity Questionnaire | |||
| Inactive | 10 (25) | 23 (57.5) | 0.013 |
| Moderate | 24 (60) | 13 (32.5) | |
| Active | 6 (15) | 4 (10) | |
| Serum albumin (g/dL) * | 4.27 (4.05–4.37) | 4.15 (3.96–4.36) | 0.136 |
| Total protein (g/dL) * | 7.22 (7.0–7.63) | 7.24 (6.93–7.39) | 0.170 |
| C-reactive protein (mg/L) * | 2.55 (0.9–4.65) | 4.4 (2.6–9.3) | 0.015 |
| Erythrocyte sedimentation rate (mm/h) * | 15.5 (7.25–24.5) | 20 (12.2–39.5) | 0.054 |
| Creatinin kinase (U/L) * | 73.5 (56–103.2) | 74 (52–120.5) | 0.239 |
| SARC-F score ≥4 | 2 (5) | 14 (35) | 0.001 |
| Chair stand test, seconds * | 10.9 (9–13) | 13.5 (11.1–16.6) | 0.008 |
| 4 m gait speed test, seconds * | 3.5 (3.2–3.9) | 4.3 (3.8–5) | 0.001 |
| Experiencing falls within the past year | 7 (17.5) | 15 (37.5) | 0.039 |
| ASMI (kg/m2) * | 7.0 (6.7–7.6) | 7.7 (6.8–8.6) | 0.154 |
| Fat-free muscle mass index (kg/m2) * | 17.2 (16.6–18.7) | 18.8 (16.8–20.4) | 0.338 |
| Skeletal muscle mass index (%) * | 38.4 (36.1–40.7) | 36.4 (33.2–39.9) | 0.109 |
| Chair-Stand Test (Seconds) | Gaid Speed (Seconds) | SARC-F | CRP (mg/L) | Albumin (g/dL) | SSMI (%) | |
|---|---|---|---|---|---|---|
| Age (years) | 0.429 | 0.617 | 0.407 | 0.351 | −0.211 | −0.316 |
| 0.001 | 0.001 | 0.001 | 0.001 | 0.060 | 0.004 | |
| Chair-stand test (seconds) | 1.000 | 0.541 | 0.502 | 0.156 | −0.211 | −0.281 |
| 0.001 | 0.001 | 0.170 | 0.060 | 0.012 | ||
| Gaid speed (seconds) | 0.541 | 1.000 | 0.447 | 0.244 | −0.222 | −0.416 |
| 0.001 | 0.001 | 0.030 | 0.047 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuce Inel, T.; Dervis Hakim, G.; Birlik, M. Assessment of Factors Related to Sarcopenia in Patients with Systemic Sclerosis. J. Clin. Med. 2025, 14, 1573. https://doi.org/10.3390/jcm14051573
Yuce Inel T, Dervis Hakim G, Birlik M. Assessment of Factors Related to Sarcopenia in Patients with Systemic Sclerosis. Journal of Clinical Medicine. 2025; 14(5):1573. https://doi.org/10.3390/jcm14051573
Chicago/Turabian StyleYuce Inel, Tuba, Gozde Dervis Hakim, and Merih Birlik. 2025. "Assessment of Factors Related to Sarcopenia in Patients with Systemic Sclerosis" Journal of Clinical Medicine 14, no. 5: 1573. https://doi.org/10.3390/jcm14051573
APA StyleYuce Inel, T., Dervis Hakim, G., & Birlik, M. (2025). Assessment of Factors Related to Sarcopenia in Patients with Systemic Sclerosis. Journal of Clinical Medicine, 14(5), 1573. https://doi.org/10.3390/jcm14051573






