Management of Bleeding, Thrombotic and Pregnancy-Related Complications in Women with Myeloproliferative Neoplasms: A Case-Based Review Focusing on Sex-Specific Challenges
Abstract
1. Introduction
2. Bleeding in Women with MPNs
2.1. The Challenge of Assessment of Bleeding in MPNs
2.2. Risk Factors for MPN Bleeding
2.3. Mechanisms for Bleeding
2.4. Management of Bleeding
3. Thrombosis in Women with MPNs
4. MPNs and Pregnancy
4.1. MPN and Fertility
4.2. Pregnancy Outcomes and Their Prediction in MPN
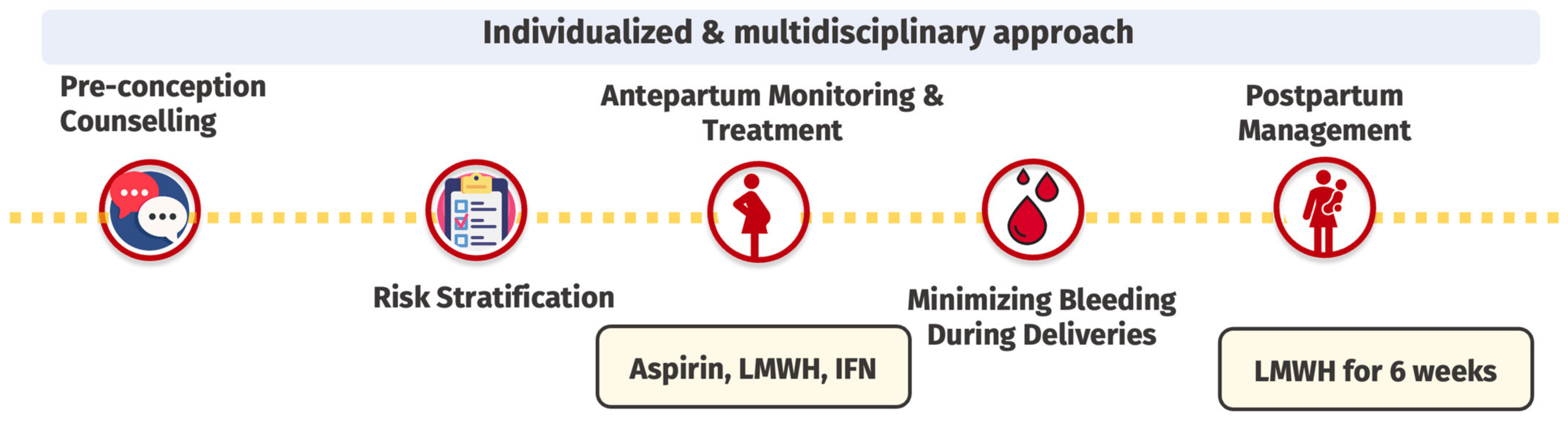
4.3. Management of MPN in Pregnancy
4.3.1. Preconception Counseling
4.3.2. Risk Stratification
4.3.3. Antepartum Monitoring and Treatment
4.3.4. Prevention of Peripartum Bleeding
4.3.5. Postpartum Management
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Spivak, J.L. Myeloproliferative Neoplasms. N. Engl. J. Med. 2017, 376, 2168–2181. [Google Scholar] [CrossRef] [PubMed]
- Dores, G.M.; Curtis, R.E.; Linet, M.S.; Morton, L.M. Cause-Specific Mortality Following Polycythemia Vera, Essential Thrombocythemia, and Primary Myelofibrosis in the US Population, 2001–2017. Am. J. Hematol. 2021, 96, E451–E454. [Google Scholar] [CrossRef] [PubMed]
- Rungjirajittranon, T.; Owattanapanich, W.; Ungprasert, P.; Siritanaratkul, N.; Ruchutrakool, T. A Systematic Review and Meta-Analysis of the Prevalence of Thrombosis and Bleeding at Diagnosis of Philadelphia-Negative Myeloproliferative Neoplasms. BMC Cancer 2019, 19, 184. [Google Scholar] [CrossRef]
- Tefferi, A.; Vannucchi, A.M.; Barbui, T. Essential Thrombocythemia: 2024 Update on Diagnosis, Risk Stratification, and Management. Am. J. Hematol. 2024, 99, 697–718. [Google Scholar] [CrossRef]
- Tremblay, D.; Kremyanskaya, M.; Mascarenhas, J.; Hoffman, R. Diagnosis and Treatment of Polycythemia Vera: A Review. JAMA 2024, 333, 153–160. [Google Scholar] [CrossRef]
- Robinson, S.; Ragheb, M.; Harrison, C. How I Treat Myeloproliferative Neoplasms in Pregnancy. Blood 2024, 143, 777–785. [Google Scholar] [CrossRef]
- Accurso, V.; Santoro, M.; Mancuso, S.; Napolitano, M.; Carlisi, M.; Mattana, M.; Russo, C.; Stefano, A.D.; Sirocchi, D.; Siragusa, S. The Essential Thrombocythemia in 2020: What We Know and Where We Still Have to Dig Deep. Clin. Med. Insights Blood Disord. 2020, 13, 2634853520978210. [Google Scholar] [CrossRef]
- Appelmann, I.; Kreher, S.; Parmentier, S.; Wolf, H.-H.; Bisping, G.; Kirschner, M.; Bergmann, F.; Schilling, K.; Brümmendorf, T.H.; Petrides, P.E.; et al. Diagnosis, Prevention, and Management of Bleeding Episodes in Philadelphia-Negative Myeloproliferative Neoplasms: Recommendations by the Hemostasis Working Party of the German Society of Hematology and Medical Oncology (DGHO) and the Society of Thrombosis and Hemostasis Research (GTH). Ann. Hematol. 2016, 95, 707–718. [Google Scholar] [CrossRef]
- Stein, B.L.; Martin, K. From Budd-Chiari Syndrome to Acquired von Willebrand Syndrome: Thrombosis and Bleeding Complications in the Myeloproliferative Neoplasms. Hematology 2019, 2019, 397–406. [Google Scholar] [CrossRef]
- Karantanos, T.; Chaturvedi, S.; Braunstein, E.M.; Spivak, J.; Resar, L.; Karanika, S.; Williams, D.M.; Rogers, O.; Gocke, C.D.; Moliterno, A.R. Sex Determines the Presentation and Outcomes in MPN and Is Related to Sex-Specific Differences in the Mutational Burden. Blood Adv. 2020, 4, 2567–2576. [Google Scholar] [CrossRef] [PubMed]
- Geyer, H.L.; Kosiorek, H.; Dueck, A.C.; Scherber, R.; Slot, S.; Zweegman, S.; Boekhorst, P.A.T.; Senyak, Z.; Schouten, H.C.; Sackmann, F.; et al. Associations between Gender, Disease Features and Symptom Burden in Patients with Myeloproliferative Neoplasms: An Analysis by the MPN QOL International Working Group. Haematologica 2017, 102, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Stein, B.L.; Williams, D.M.; Wang, N.Y.; Rogers, O.; Isaacs, M.A.; Pemmaraju, N.; Spivak, J.L.; Moliterno, A.R. Sex Differences in the JAK2 V617F Allele Burden in Chronic Myeloproliferative Disorders. Haematologica 2010, 95, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Spivak, J.L.; Considine, M.; Williams, D.M.; Talbot, C.C., Jr.; Rogers, O.; Moliterno, A.R.; Jie, C.; Ochs, M.F. Two Clinical Phenotypes in Polycythemia Vera. N. Engl. J. Med. 2014, 371, 808–817. [Google Scholar] [CrossRef]
- Stein, B.L.; Rademaker, A.; Spivak, J.L.; Moliterno, A.R. Gender and Vascular Complications in the JAK2 V617F-Positive Myeloproliferative Neoplasms. Thrombosis 2011, 2011, 874146. [Google Scholar] [CrossRef]
- Lancellotti, S.; Dragani, A.; Ranalli, P.; Petrucci, G.; Basso, M.; Tartaglione, R.; Rocca, B.; Cristofaro, R.D. Qualitative and Quantitative Modifications of von Willebrand Factor in Patients with Essential Thrombocythemia and Controlled Platelet Count. J. Thromb. Haemost. 2015, 13, 1226–1237. [Google Scholar] [CrossRef]
- Faro, V.L.; Johansson, T.; Johansson, Å. The Risk of Venous Thromboembolism in Oral Contraceptive Users: The Role of Genetic Factors—A Prospective Cohort Study of 240,000 Women in the UK Biobank. Am. J. Obstet. Gynecol. 2024, 230, 360.e1–360.e13. [Google Scholar] [CrossRef]
- Shallis, R.M.; Zeidan, A.M.; Wang, R.; Podoltsev, N.A. Epidemiology of the Philadelphia Chromosome-Negative Classical Myeloproliferative Neoplasms. Hematol. Oncol. Clin. N. Am. 2021, 35, 177–189. [Google Scholar] [CrossRef]
- Hultcrantz, M.; Landtblom, A.R.; Andreasson, B.; Samuelsson, J.; Dickman, P.W.; Kristinsson, S.Y.; Bjorkholm, M.; Andersson, T.M. Incidence of Myeloproliferative Neoplasms—Trends by Subgroup and Age in a Population-Based Study in Sweden. J. Intern. Med. 2020, 287, 448–454. [Google Scholar] [CrossRef]
- Nicol, C.; Lacut, K.; Pan-Petesch, B.; Lippert, E.; Ianotto, J.-C. Hemorrhage in Essential Thrombocythemia or Polycythemia Vera: Epidemiology, Location, Risk Factors, and Lessons Learned from the Literature. Thromb. Haemost. 2020, 121, 553–564. [Google Scholar] [CrossRef]
- Finazzi, G.; Carobbio, A.; Thiele, J.; Passamonti, F.; Rumi, E.; Ruggeri, M.; Rodeghiero, F.; Randi, M.L.; Bertozzi, I.; Vannucchi, A.M.; et al. Incidence and Risk Factors for Bleeding in 1104 Patients with Essential Thrombocythemia or Prefibrotic Myelofibrosis Diagnosed According to the 2008 WHO Criteria. Leukemia 2012, 26, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Rumi, E.; Finazzi, G.; Gisslinger, H.; Vannucchi, A.M.; Rodeghiero, F.; Randi, M.L.; Vaidya, R.; Cazzola, M.; Rambaldi, A.; et al. Survival and Prognosis among 1545 Patients with Contemporary Polycythemia Vera: An International Study. Leukemia 2013, 27, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Landtblom, A.R.; Andersson, T.M.; Johansson, A.L.V.; Wendel, S.B.; Lundberg, F.E.; Samuelsson, J.; Bjorkholm, M.; Hultcrantz, M. Pregnancy and Childbirth Outcomes in Women with Myeloproliferative Neoplasms-a Nationwide Population-Based Study of 342 Pregnancies in Sweden. Leukemia 2022, 36, 2461–2467. [Google Scholar] [CrossRef] [PubMed]
- Venkat, R.K.; Redd, R.A.; Harris, A.C.; Aryee, M.J.; Marneth, A.E.; Kamaz, B.; Kim, C.J.; Wazir, M.; Weeks, L.D.; Stahl, M.; et al. Risk of Bleeding in Patients with Essential Thrombocythemia and Extreme Thrombocytosis. Blood Adv. 2024, 8, 6043–6054. [Google Scholar] [CrossRef]
- Arya, S.; Wilton, P.; Page, D.; Boma-Fischer, L.; Floros, G.; Winikoff, R.; Teitel, J.; Dainty, K.; Sholzberg, M. “They Don’t Really Take My Bleeds Seriously”: Barriers to Care for Women with Inherited Bleeding Disorders. J. Thromb. Haemost. 2021, 19, 1506–1514. [Google Scholar] [CrossRef]
- Campbell, P.J.; MacLean, C.; Beer, P.A.; Buck, G.; Wheatley, K.; Kiladjian, J.-J.; Forsyth, C.; Harrison, C.N.; Green, A.R. Correlation of Blood Counts with Vascular Complications in Essential Thrombocythemia: Analysis of the Prospective PT1 Cohort. Blood 2012, 120, 1409–1411. [Google Scholar] [CrossRef]
- Bertozzi, I.; Bogoni, G.; Biagetti, G.; Duner, E.; Lombardi, A.M.; Fabris, F.; Randi, M.L. Thromboses and Hemorrhages Are Common in MPN Patients with High JAK2V617F Allele Burden. Ann. Hematol. 2017, 96, 1297–1302. [Google Scholar] [CrossRef]
- Borowczyk, M.; Wojtaszewska, M.; Lewandowski, K.; Gil, L.; Lewandowska, M.; Lehmann-Kopydłowska, A.; Kroll-Balcerzak, R.; Balcerzak, A.; Iwoła, M.; Michalak, M.; et al. The JAK2 V617F Mutational Status and Allele Burden May Be Related with the Risk of Venous Thromboembolic Events in Patients with Philadelphia-Negative Myeloproliferative Neoplasms. Thromb. Res. 2015, 135, 272–280. [Google Scholar] [CrossRef]
- Kander, E.M.; Raza, S.; Zhou, Z.; Gao, J.; Zakarija, A.; McMahon, B.J.; Stein, B.L. Bleeding Complications in BCR-ABL Negative Myeloproliferative Neoplasms: Prevalence, Type, and Risk Factors in a Single-Center Cohort. Int. J. Hematol. 2015, 102, 587–593. [Google Scholar] [CrossRef]
- Tosoni, L.; Liberi, M.; Morelli, G.; Zannier, M.E.; Lazzarotto, D.; Filì, C.; Simeone, E.; Battaglia, G.; Callegari, C.; Fanin, M.; et al. Correlation between IPSET-t Risk at Diagnosis and Subsequent Hemorrhage in Patients with Essential Thrombocythemia; a Single Institution Experience. Ann. Hematol. 2024, 103, 443–448. [Google Scholar] [CrossRef]
- Stuckey, R.; Ianotto, J.-C.; Santoro, M.; Czyż, A.; Encinas, M.M.P.; Gómez-Casares, M.T.; Pereira, M.S.N.; de Nałęcz, A.K.; Gołos, A.; Lewandowski, K.; et al. Prediction of Major Bleeding Events in 1381 Patients with Essential Thrombocythemia. Int. J. Hematol. 2023, 118, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, M. Aspirin in Essential Thrombocythemia. For Whom? What Formulation? What Regimen? Haematologica 2023, 108, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.I.; Manoharan, A. Platelet Function in Myeloproliferative Disorders: Characterization and Sequential Studies Show Multiple Platelet Abnormalities, and Change with Time. Eur. J. Haematol. 1988, 40, 267–272. [Google Scholar] [CrossRef]
- Mital, A.; Prejzner, W.; Bieniaszewska, M.; Hellmann, A. Prevalence of Acquired von Willebrand Syndrome during Essential Thrombocythemia: A Retrospective Analysis of 170 Consecutive Patients. Pol. Arch. Intern. Med. 2015, 125, 914–920. [Google Scholar] [CrossRef]
- Rottenstreich, A.; Kleinstern, G.; Krichevsky, S.; Varon, D.; Lavie, D.; Kalish, Y. Factors Related to the Development of Acquired von Willebrand Syndrome in Patients with Essential Thrombocythemia and Polycythemia Vera. Eur. J. Intern. Med. 2017, 41, 49–54. [Google Scholar] [CrossRef]
- Kubo, M.; Sakai, K.; Hayakawa, M.; Kashiwagi, H.; Yagi, H.; Seki, Y.; Hasegawa, A.; Tanaka, H.; Amano, I.; Tomiyama, Y.; et al. Increased Cleavage of von Willebrand Factor by ADAMTS13 May Contribute Strongly to Acquired von Willebrand Syndrome Development in Patients with Essential Thrombocythemia. J. Thromb. Haemost. 2022, 20, 1589–1598. [Google Scholar] [CrossRef]
- Song, I.-C.; Kang, S.; Lee, M.-W.; Ryu, H.; Lee, H.-J.; Yun, H.-J.; Jo, D.-Y. Acquired von Willebrand Syndrome in Patients with Philadelphia-Negative Myeloproliferative Neoplasm. Blood Res. 2023, 58, 42–50. [Google Scholar] [CrossRef]
- Elliott, M.A.; Tefferi, A. Thrombosis and Haemorrhage in Polycythaemia Vera and Essential Thrombocythaemia. Br. J. Haematol. 2005, 128, 275–290. [Google Scholar] [CrossRef]
- Budde, U.; Genderen, P.V. Acquired von Willebrand Disease in Patients with High Platelet Counts. Semin. Thromb. Hemost. 1997, 23, 425–431. [Google Scholar] [CrossRef]
- Tefferi, A.; Smock, K.J.; Divgi, A.B. Polycythemia Vera-associated Acquired von Willebrand Syndrome despite Near-normal Platelet Count. Am. J. Hematol. 2010, 85, 545–548. [Google Scholar] [CrossRef]
- Janjetovic, S.; Rolling, C.C.; Budde, U.; Schneppenhem, S.; Schafhausen, P.; Peters, M.C.; Bokemeyer, C.; Holstein, K.; Langer, F. Evaluation of Different Diagnostic Tools for Detection of Acquired von Willebrand Syndrome in Patients with Polycythemia Vera or Essential Thrombocythemia. Thromb. Res. 2022, 218, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Scharf, R.E. Platelets in Thrombotic and Non-Thrombotic Disorders, Pathophysiology, Pharmacology and Therapeutics: An Update; Springer: Cham, Switzerland, 2017; pp. 951–973. [Google Scholar] [CrossRef]
- Lussana, F.; Femia, E.A.; Pugliano, M.; Podda, G.; Razzari, C.; Maugeri, N.; Lecchi, A.; Caberlon, S.; Gerli, G.; Cattaneo, M. Evaluation of Platelet Function in Essential Thrombocythemia under Different Analytical Conditions. Platelets 2020, 31, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Repsold, L.; Joubert, A.M. Platelet Function, Role in Thrombosis, Inflammation, and Consequences in Chronic Myeloproliferative Disorders. Cells 2021, 10, 3034. [Google Scholar] [CrossRef]
- Thomas, S.; Krishnan, A. Platelet Heterogeneity in Myeloproliferative Neoplasms. Arter. Thromb. Vasc. Biol. 2021, 41, 2661–2670. [Google Scholar] [CrossRef]
- Gordon, N.; Thom, J.; Cole, C.; Baker, R. Rapid Detection of Hereditary and Acquired Platelet Storage Pool Deficiency by Flow Cytometry. Br. J. Haematol. 1995, 89, 117–123. [Google Scholar] [CrossRef]
- Kelliher, S.; Gamba, S.; Weiss, L.; Shen, Z.; Marchetti, M.; Schieppati, F.; Scaife, C.; Madden, S.; Bennett, K.; Fortune, A.; et al. Platelet Proteo-Transcriptomic Profiling Validates Mediators of Thrombosis and Proteostasis in Patients with Myeloproliferative Neoplasms. bioRxiv 2023. [Google Scholar] [CrossRef]
- Manoharan, A.; Gemmell, R.; Brighton, T.; Dunkley, S.; Lopez, K.; Kyle, P. Thrombosis and Bleeding in Myeloproliferative Disorders: Identification of At-risk Patients with Whole Blood Platelet Aggregation Studies. Br. J. Haematol. 1999, 105, 618–625. [Google Scholar] [CrossRef]
- Pedersen, O.H.; Larsen, M.L.; Grove, E.L.; Niekerk, P.B.v.K.; Bønløkke, S.; Nissen, P.H.; Kristensen, S.D.; Hvas, A. Platelet Characteristics in Patients with Essential Thrombocytosis. Cytom. Part B Clin. Cytom. 2018, 94, 918–927. [Google Scholar] [CrossRef]
- Ross, D.M.; Liang, H.P.H.; Iqra, Z.; Whittaker, S.; Tan, C.W.; Dale, B.J.; Chen, V.M. Platelets from Patients with Myeloproliferative Neoplasms Have Increased Numbers of Mitochondria That Are Hypersensitive to Depolarization by Thrombin. Sci. Rep. 2023, 13, 9172. [Google Scholar] [CrossRef]
- Shen, Z.; Du, W.; Perkins, C.; Fechter, L.; Natu, V.; Maecker, H.; Rowley, J.; Gotlib, J.; Zehnder, J.; Krishnan, A. Platelet Transcriptome Identifies Progressive Markers and Potential Therapeutic Targets in Chronic Myeloproliferative Neoplasms. Cell Rep. Med. 2021, 2, 100425. [Google Scholar] [CrossRef]
- Manoharan, A.; Gemmell, R.; Cavanaugh, L.; Shadood, N. Thrombosis in Myeloproliferative Neoplasms: A Single Center Experience of Using Whole Blood Platelet Aggregation Studies for Risk Assessment and Thromboprophylaxis. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221117482. [Google Scholar] [CrossRef] [PubMed]
- Szuber, N.; Toliopoulos, P.; Busque, L.; Cerquozzi, S.; Foltz, L.; Gupta, V.; Tefferi, A.; Vannucchi, A.M.; Hillis, C.; Leber, B.; et al. Perioperative Management of Myeloproliferative Neoplasms: A Pan-Canadian Physician Survey and International Expert Opinion. Am. J. Hematol. 2022, 97, E466–E469. [Google Scholar] [CrossRef] [PubMed]
- Szuber, N.; Dagenais-Bellefeuille, S.; Tanguay, M.; Shehabeldeen, A.; Ahmed, S.; Harnois, M.; Prchal, J.F.; Olney, H.J.; Busque, L.; Sirhan, S. Perioperative Outcomes and Management in Patients with Myeloproliferative Neoplasms: A Multicentric Retrospective Analysis of 354 Surgical Interventions. Blood 2023, 142, 3183. [Google Scholar] [CrossRef]
- Hultcrantz, M.; Bjorkholm, M.; Dickman, P.W.; Landgren, O.; Derolf, A.R.; Kristinsson, S.Y.; Andersson, T.M.L. Risk for Arterial and Venous Thrombosis in Patients with Myeloproliferative Neoplasms: A Population-Based Cohort Study. Ann. Intern. Med. 2018, 168, 317–325. [Google Scholar] [CrossRef]
- Hultcrantz, M.; Wilkes, S.R.; Kristinsson, S.Y.; Andersson, T.M.; Derolf, A.R.; Eloranta, S.; Samuelsson, J.; Landgren, O.; Dickman, P.W.; Lambert, P.C.; et al. Risk and Cause of Death in Patients Diagnosed with Myeloproliferative Neoplasms in Sweden Between 1973 and 2005: A Population-Based Study. J. Clin. Oncol. 2015, 33, 2288–2295. [Google Scholar] [CrossRef]
- Todor, S.B.; Ichim, C.; Boicean, A.; Mihaila, R.G. Cardiovascular Risk in Philadelphia-Negative Myeloproliferative Neoplasms: Mechanisms and Implications—A Narrative Review. Curr. Issues Mol. Biol. 2024, 46, 8407–8423. [Google Scholar] [CrossRef]
- Wautier, M.-P.; Nemer, W.E.; Gane, P.; Rain, J.-D.; Cartron, J.-P.; Colin, Y.; Kim, C.L.V.; Wautier, J.-L. Increased Adhesion to Endothelial Cells of Erythrocytes from Patients with Polycythemia Vera Is Mediated by Laminin A5 Chain and Lu/BCAM. Blood 2007, 110, 894–901. [Google Scholar] [CrossRef]
- Barraco, D.; Mora, B.; Guglielmelli, P.; Rumi, E.; Maffioli, M.; Rambaldi, A.; Caramella, M.; Komrokji, R.; Gotlib, J.; Kiladjian, J.J.; et al. Gender Effect on Phenotype and Genotype in Patients with Post-Polycythemia Vera and Post-Essential Thrombocythemia Myelofibrosis: Results from the MYSEC Project. Blood Cancer J. 2018, 8, 89. [Google Scholar] [CrossRef]
- Landolfi, R.; Gennaro, L.D.; Nicolazzi, M.A.; Giarretta, I.; Marfisi, R.; Marchioli, R. Polycythemia Vera: Gender-Related Phenotypic Differences. Intern. Emerg. Med. 2012, 7, 509–515. [Google Scholar] [CrossRef]
- Tremblay, D.; Vogel, A.S.; Moshier, E.; Hoffman, R.; Kremyanskaya, M.; Zhou, S.; Schiano, T.; Mascarenhas, J. Outcomes of Splanchnic Vein Thrombosis in Patients with Myeloproliferative Neoplasms in a Single Center Experience. Eur. J. Haematol. 2020, 104, 72–73. [Google Scholar] [CrossRef]
- How, J.; Trinkaus, K.M.; Oh, S.T. Distinct Clinical, Laboratory and Molecular Features of Myeloproliferative Neoplasm Patients with Splanchnic Vein Thrombosis. Br. J. Haematol. 2018, 183, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Sant’Antonio, E.; Guglielmelli, P.; Pieri, L.; Primignani, M.; Randi, M.L.; Santarossa, C.; Rumi, E.; Cervantes, F.; Delaini, F.; Carobbio, A.; et al. Splanchnic Vein Thromboses Associated with Myeloproliferative Neoplasms: An International, Retrospective Study on 518 Cases. Am. J. Hematol. 2020, 95, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Smalberg, J.H.; Arends, L.R.; Valla, D.C.; Kiladjian, J.J.; Janssen, H.L.; Leebeek, F.W. Myeloproliferative Neoplasms in Budd-Chiari Syndrome and Portal Vein Thrombosis: A Meta-Analysis. Blood 2012, 120, 4921–4928. [Google Scholar] [CrossRef] [PubMed]
- Barnum, K.J.; Patell, R.; Berry, J.; Bauer, K.A. Splanchnic Vein Thrombosis: Management for the Thrombosis Specialist. J. Thromb. Haemost. 2024, 23, 404–416. [Google Scholar] [CrossRef]
- Finazzi, G.; Stefano, V.D.; Barbui, T. Splanchnic Vein Thrombosis in Myeloproliferative Neoplasms: Treatment Algorithm 2018. Blood Cancer J. 2018, 8, 64. [Google Scholar] [CrossRef]
- Chrysafi, P.; Barnum, K.; Gerhard, G.M.; Chiasakul, T.; Narang, A.; McNichol, M.; Riva, N.; Semmler, G.; Scheiner, B.; Acosta, S.; et al. Anticoagulation for Splanchnic Vein Thrombosis in Myeloproliferative Neoplasms: A Systematic Review and Meta-Analysis. J. Thromb. Haemost. 2024, 22, 3479–3489. [Google Scholar] [CrossRef]
- Ageno, W.; Westendorf, J.B.; Contino, L.; Bucherini, E.; Sartori, M.T.; Senzolo, M.; Grandone, E.; Santoro, R.; Carrier, M.; Delluc, A.; et al. Rivaroxaban for the Treatment of Noncirrhotic Splanchnic Vein Thrombosis: An Interventional Prospective Cohort Study. Blood Adv. 2022, 6, 3569–3578. [Google Scholar] [CrossRef]
- Ramanathan, G.; Hoover, B.M.; Fleischman, A.G. Impact of Host, Lifestyle and Environmental Factors in the Pathogenesis of MPN. Cancers 2020, 12, 2038. [Google Scholar] [CrossRef]
- Gangat, N.; Wolanskyj, A.P.; Schwager, S.M.; Mesa, R.A.; Tefferi, A. Estrogen-Based Hormone Therapy and Thrombosis Risk in Women with Essential Thrombocythemia. Cancer 2006, 106, 2406–2411. [Google Scholar] [CrossRef]
- Alimam, S.; Bewley, S.; Chappell, L.C.; Knight, M.; Seed, P.; Gray, G.; Harrison, C.; Robinson, S. Pregnancy Outcomes in Myeloproliferative Neoplasms: UK Prospective Cohort Study. Br. J. Haematol. 2016, 175, 31–36. [Google Scholar] [CrossRef]
- Landtblom, A.R.; Andersson, T.M.; Johansson, A.L.V.; Lundberg, F.E.; Samuelsson, J.; Bjorkholm, M.; Hultcrantz, M. Childbirth Rates in Women with Myeloproliferative Neoplasms. Leukemia 2024, 38, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Maze, D.; Kazi, S.; Gupta, V.; Malinowski, A.K.; Fazelzad, R.; Shah, P.S.; Shehata, N. Association of Treatments for Myeloproliferative Neoplasms During Pregnancy with Birth Rates and Maternal Outcomes: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2019, 2, e1912666. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.J.; Curtin, S.C.; Abma, J.C.; Henshaw, S.K. Estimated Pregnancy Rates and Rates of Pregnancy Outcomes for the United States, 1990–2008. Natl. Vital. Stat. Rep. 2012, 60, 1–21. [Google Scholar] [PubMed]
- Skeith, L.; Carrier, M.; Robinson, S.E.; Alimam, S.; Rodger, M.A. Risk of Venous Thromboembolism in Pregnant Women with Essential Thrombocythemia: A Systematic Review and Meta-Analysis. Blood 2017, 129, 934–939. [Google Scholar] [CrossRef]
- Wille, K.; Brouka, M.; Bernhardt, J.; Rufer, A.; Niculescu-Mizil, E.; Gotic, M.; Isfort, S.; Koschmieder, S.; Barbui, T.; Sadjadian, P.; et al. Outcome of 129 Pregnancies in Polycythemia Vera Patients: A Report of the European LeukemiaNET. HemaSphere 2023, 7, e882. [Google Scholar] [CrossRef]
- Gangat, N.; Singh, A.; Ilyas, R.; Loscocco, G.G.; Elliott, M.; Begna, K.; Pardanani, A.; Tefferi, A. Aspirin Therapy Is Associated with a Lower Risk of Pregnancy Loss in Both JAK2- and CALR-Mutated Essential Thrombocythemia—A Mayo Clinic Study of 200 Pregnancies. Am. J. Hematol. 2024, 99, 1862–1869. [Google Scholar] [CrossRef]
- How, J.; Leiva, O.; Bogue, T.; Fell, G.G.; Bustoros, M.W.; Connell, N.T.; Connors, J.M.; Ghobrial, I.M.; Kuter, D.J.; Mullally, A.; et al. Pregnancy Outcomes, Risk Factors, and Cell Count Trends in Pregnant Women with Essential Thrombocythemia. Leuk. Res. 2020, 98, 106459. [Google Scholar] [CrossRef]
- Rumi, E.; Bertozzi, I.; Casetti, I.C.; Roncoroni, E.; Cavalloni, C.; Bellini, M.; Sant’Antonio, E.; Gotti, M.; Ferretti, V.V.; Milanesi, C.; et al. Impact of Mutational Status on Pregnancy Outcome in Patients with Essential Thrombocytemia. Haematologica 2015, 100, e443–e445. [Google Scholar] [CrossRef]
- Passamonti, F.; Randi, M.L.; Rumi, E.; Pungolino, E.; Elena, C.; Pietra, D.; Scapin, M.; Arcaini, L.; Tezza, F.; Moratti, R.; et al. Increased Risk of Pregnancy Complications in Patients with Essential Thrombocythemia Carrying the JAK2 (617V>F) Mutation. Blood 2007, 110, 485–489. [Google Scholar] [CrossRef]
- Beauverd, Y.; Radia, D.; Cargo, C.; Knapper, S.; Drummond, M.; Pillai, A.; Harrison, C.; Robinson, S. Pegylated Interferon Alpha-2a for Essential Thrombocythemia during Pregnancy: Outcome and Safety. A Case Series. Haematologica 2016, 101, e182–e184. [Google Scholar] [CrossRef]
- Quenby, S.; Booth, K.; Hiller, L.; Coomarasamy, A.; de Jong, P.G.; Hamulyak, E.N.; Scheres, L.J.; van Haaps, T.F.; Ewington, L.; Tewary, S.; et al. Heparin for Women with Recurrent Miscarriage and Inherited Thrombophilia (ALIFE2): An International Open-Label, Randomised Controlled Trial. Lancet 2023, 402, 54–61. [Google Scholar] [CrossRef]


| Female patients |
|
| Male patients |
|
| High-risk MPN |
|
| History of pregnancy morbidity |
|
| Challenges | Management Approaches | |
|---|---|---|
| Bleeding |
|
|
| Thrombosis |
|
|
| Fertility |
|
|
| Pregnancy and postpartum |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiasakul, T.; Baker, R.I. Management of Bleeding, Thrombotic and Pregnancy-Related Complications in Women with Myeloproliferative Neoplasms: A Case-Based Review Focusing on Sex-Specific Challenges. J. Clin. Med. 2025, 14, 1537. https://doi.org/10.3390/jcm14051537
Chiasakul T, Baker RI. Management of Bleeding, Thrombotic and Pregnancy-Related Complications in Women with Myeloproliferative Neoplasms: A Case-Based Review Focusing on Sex-Specific Challenges. Journal of Clinical Medicine. 2025; 14(5):1537. https://doi.org/10.3390/jcm14051537
Chicago/Turabian StyleChiasakul, Thita, and Ross I. Baker. 2025. "Management of Bleeding, Thrombotic and Pregnancy-Related Complications in Women with Myeloproliferative Neoplasms: A Case-Based Review Focusing on Sex-Specific Challenges" Journal of Clinical Medicine 14, no. 5: 1537. https://doi.org/10.3390/jcm14051537
APA StyleChiasakul, T., & Baker, R. I. (2025). Management of Bleeding, Thrombotic and Pregnancy-Related Complications in Women with Myeloproliferative Neoplasms: A Case-Based Review Focusing on Sex-Specific Challenges. Journal of Clinical Medicine, 14(5), 1537. https://doi.org/10.3390/jcm14051537






