Evaluation of Subclinical Left Ventricular Dysfunction in HIV Patients Receiving Abacavir, Dolutegravir, and Lamivudine Therapy with Novel Tissue Doppler Imaging Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Population and Sample
2.3. Data Collection and Echocardiographic Assessment
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Left Ventricular Echocardiographic Findings
3.3. Right Ventricular Echocardiographic Findings
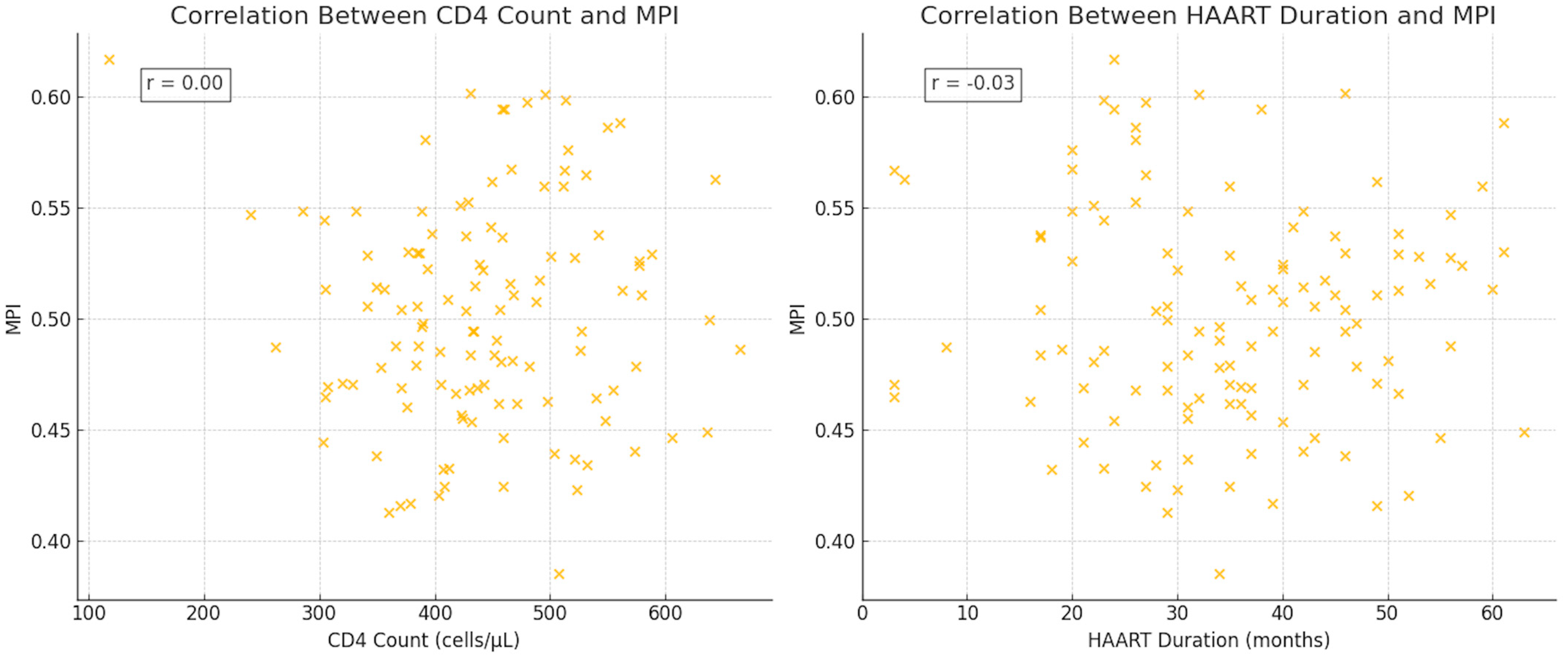
3.4. Correlation Analysis
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grandominico, J.M.; Fichtenbaum, C.J. Short-term effect of HAART on blood pressure in HIV-infected individuals. HIV Clin. Trials 2008, 9, 52–60. [Google Scholar] [CrossRef]
- Savvoulidis, P.; Butler, J.; Kalogeropoulos, A. Cardiomyopathy and Heart Failure in Patients With HIV Infection. Can. J. Cardiol. 2019, 35, 299–309. [Google Scholar] [CrossRef]
- Sani, M.U. Myocardial disease in human immunodeficiency virus (HIV) infection: A review. Wien. Klin. Wochenschr. 2008, 120, 77–87. [Google Scholar] [CrossRef]
- Walmsley, S.L.; Antela, A.A.; Clumeck, N.; Duiculescu, D.; Eberhard, A.A.; Gutiérrez, F.; Hocqueloux, L.L.; Maggiolo, F.F.; Sandkovsky, U.U.; Granier, C.C.; et al. SINGLE Investigators. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N. Engl. J. Med. 2013, 369, 1807–1818. [Google Scholar] [CrossRef] [PubMed]
- Nan, C.; Shaefer, M.; Urbaityte, R.; Oyee, J.; Hopking, J.; Ragone, L.; Perger, T.; Win, B.; Vangerow, H.; McCoig, C.; et al. Abacavir Use and Risk for Myocardial Infarction and Cardiovascular Events: Pooled Analysis of Data From Clinical Trials. Open Forum Infect. Dis. 2018, 5, ofy086. [Google Scholar] [CrossRef] [PubMed]
- Depairon, M.; Chessex, S.; Sudre, P.; Rodondi, N.; Doser, N.; Chave, J.-P.; Riesen, W.; Nicod, P.; Darioli, R.; Telenti, A.; et al. Premature atherosclerosis in HIV-infected individuals: Focus on protease inhibitor therapy. AIDS 2001, 15, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Sharma, A.M.; Pellicelli, A.M.; Grisorio, B.; Barbarini, G.; Barbaro, G. Epicardial adipose tissue is related to carotid intima-media thickness and visceral adiposity in HIV-infected patients with highly active antiretroviral therapy-associated metabolic syndrome. Curr. HIV Res. 2007, 5, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Schuster, I.; Thöni, G.J.; Edérhy, S.; Walther, G.; Nottin, S.; Vinet, A.; Boccara, F.; Khireddine, M.; Girard, P.-M.; Mauboussin, J.-M.; et al. Subclinical cardiac abnormalities in human immunodeficiency virus-infected men receiving antiretroviral therapy. Am. J. Cardiol. 2008, 101, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Donovan, C.L.; Armstrong, W.F.; Bach, D.S. Quantitative Doppler tissue imaging of the left ventricular myocardium: Validation in normal subjects. Am. Heart J. 1995, 130, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Tei, C.; Nishimura, R.A.; Seward, J.B.; Tajik, A.J. Noninvasive Doppler-derived myocardial performance index: Correlation with simultaneous measurements of cardiac catheterization measurements. J. Am. Soc. Echocardiogr. 1997, 10, 169–178. [Google Scholar] [CrossRef]
- Quiñones, M.A.; Otto, C.M.; Stoddard, M.; Waggoner, A.; Zoghbi, W.A.; Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. Recommendations for quantification of Doppler echocardiography: A report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2002, 15, 167–184. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, R.; Nasution, S.A.; Parlindungan, F.; Dalimunthe, N.N.; Alvianto, S.; Widjanarko, N.D.; Kultsum, U.; Efendi, C.; Gotama, Y. Myocardial Performance Index to assess cardiac function in autoimmune connective tissue disease: A systematic review and meta-analysis. Lupus Sci. Med. 2024, 11, e001272. [Google Scholar] [CrossRef]
- D:A:D Study Group; Sabin, C.A.; Worm, S.W.; Weber, R.; Reiss, P.; El-Sadr, W.; Dabis, F.; De Wit, S.; Law, M.; D’Arminio Monforte, A.; et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: A multi-cohort collaboration. Lancet 2008, 371, 1417–1426. [Google Scholar]
- Frerichs, F.C.; Dingemans, K.P.; Brinkman, K. Cardiomyopathy with mitochondrial damage associated with nucleoside reverse-transcriptase inhibitors. N. Engl. J. Med. 2002, 347, 1895–1896. [Google Scholar] [CrossRef] [PubMed]
- Louw, S.; Mayne, E.S.; Jacobson, B.F.; Mayne, A.L. Selected inflammatory and coagulation biomarkers pre-viral suppression in people with human immunodeficiency virus (HIV) infection without overt cardiovascular disease: Is there a need to redefine reference indices? Cytokine 2023, 165, 156174. [Google Scholar] [CrossRef] [PubMed]
- Sims, A.; Frank, L.; Cross, R.; Clauss, S.; Dimock, D.; Purdy, J.; Mikhail, I.; Hazra, R.; Hadigan, C.; Sable, C. Abnormal cardiac strain in children and young adults with HIV acquired in early life. J. Am. Soc. Echocardiogr. 2012, 25, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Reinsch, N.; Kahlert, P.; Esser, S.; Sundermeyer, A.; Neuhaus, K.; Brockmeyer, N.; Potthoff, A.; Erbel, R.; Buck, T.; Neumann, T. Echocardiographic findings and abnormalities in HIV-infected patients: Results from a large, prospective, multicenter HIV-heart study. Am. J. Cardiovasc. Dis. 2011, 1, 176–184. [Google Scholar] [PubMed]
- Onur, I.; Ikitimur, B.; Oz, F.; Ekmekci, A.; Elitok, A.; Cagatay, A.A.; Adalet, K.; Bilge, A.K.; Kaya, M.G. Evaluation of human immunodeficiency virus infection-related left ventricular systolic dysfunction by tissue Doppler strain echocardiography. Echocardiography 2014, 31, 1199–1204. [Google Scholar] [CrossRef]
- Sitbon, O.; Lascoux-Combe, C.; Delfraissy, J.F.; Yeni, P.; Simonneau, G. Pulmonary arterial hypertension in HIV-infected patients. N. Engl. J. Med. 1998, 339, 930–931. [Google Scholar]
- Zola, C.E.; Duncan, M.S.; So-Armah, K.; Crothers, K.A.; Butt, A.A.; Gibert, C.L.; Kim, J.W.W.; Lim, J.K.; Re, V.L., 3rd; Tindle, H.A.; et al. HIV- and HCV-specific markers and echocardiographic pulmonary artery systolic pressure among United States veterans. Sci. Rep. 2020, 10, 18729. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, G.; Di Lorenzo, G.; Grisorio, B.; Barbarini, G. Pulmonary arterial hypertension in HIV infection: A prospective echocardiographic study. AIDS. 2003, 17, 174–175. [Google Scholar]
- Baba, M.M.; Buba, F.; Talle, M.A.; Umar, H.; Garbati, M.; Abdul, H. Relationship between CD4 Cell Count, Viral Load and Left Ventricular Function among HIV-1 Infected Patients Asymptomatic for Cardiac Disease on HAART. West. Afr. J. Med. 2021, 38, 571–577. [Google Scholar]
- Hu, X.; Zhang, Y.; Zhang, T.; Li, W.; Han, J.; Zhang, X.; Meng, F. Echocardiographic assessment of left cardiac structure and function in antiretroviral therapy (ART)-naïve people living with HIV/AIDS. Immun. Inflamm. Dis. 2023, 11, e799. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, G.; Carerj, S.; Di Bella, G.; Manganaro, R.; Pizzino, F.; Restelli, D.; Pelaggi, G.; Lofrumento, F.; Licordari, R.; Taverna, G.; et al. Clinical Applications of Myocardial Work in Echocardiography: A Comprehensive Review. J. Cardiovasc. Echogr. 2024, 34, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, G.; Pizzino, F.; Paradossi, U.; Gueli, I.A.; Palazzini, M.; Gentile, P.; Di Spigno, F.; Ammirati, E.; Garascia, A.; Tedeschi, A.; et al. Charting the Unseen: How Non-Invasive Imaging Could Redefine Cardiovascular Prevention. J. Cardiovasc. Dev. Dis. 2024, 11, 245. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | PLWH (n = 118) | Controls (n = 80) | p-Values |
|---|---|---|---|
| Age (years) | 44.10 ± 12.67 | 43.97 ± 7.20 | 0.852 |
| Male sex, n (%) | 78 (66.1) | 45 (56.2) | 0.280 |
| BMI (kg/m2) | 24.32 ± 3.10 | 25.44 ± 3.55 | 0.156 |
| Vital Signs | |||
| DBP (mmHg) | 78.26 ± 3.90 | 80.12 ± 5.54 | 0.324 |
| SBP (mmHg) | 120.20 ± 8.47 | 124.12 ± 7.94 | 0.098 |
| Heart rate (bpm) | 82.15 ± 5.23 | 78.12 ± 8.58 | 0.142 |
| Comorbidities, n (%) | |||
| Current smoking | 65 (55.0) | 28 (35.0) | 0.043 * |
| Diabetes mellitus | 22 (18.6) | 12 (15.0) | 0.307 |
| Hypertension | 17 (14.4) | 13 (16.2) | 0.462 |
| Hyperlipidemia | 33 (27.9) | 18 (22.5) | 0.064 |
| HIV-specific Parameters | |||
| Viral load (copies/mL) | 162.35 ± 79.4 | - | - |
| CD4 count (cells/µL) | 443.59 ± 90.62 | - | - |
| HAART duration (months) | 35.09 ± 13.34 | - | - |
| Parameters | PLWH (n = 118) | Controls (n = 80) | p-Values |
|---|---|---|---|
| Conventional Parameters | |||
| LVED (mm) | 47.41 ± 5.30 | 45.96 ± 3.90 | 0.198 |
| LVES (mm) | 26.63 ± 4.10 | 25.23 ± 3.70 | 0.582 |
| IVS (mm) | 10.72 ± 1.54 | 9.12 ± 1.50 | 0.135 |
| PW (mm) | 9.78 ± 1.43 | 9.63 ± 1.34 | 0.326 |
| LVEF (%) | 61.25 ± 5.32 | 63.08 ± 5.05 | 0.098 |
| LA (mm) | 34.54 ± 4.60 | 32.70 ± 3.40 | 0.068 |
| Doppler Parameters | |||
| E velocity (cm/s) | 7.23 ± 2.87 | 8.68 ± 3.01 | 0.048 * |
| A velocity (cm/s) | 11.33 ± 2.80 | 10.24 ± 3.00 | 0.235 |
| E/A ratio | 0.86 ± 0.42 | 0.77 ± 0.40 | 0.030 * |
| Tissue Doppler Parameters | |||
| Sa velocity (cm/s) | 9.40 ± 2.30 | 10.10 ± 2.60 | 0.343 |
| IVV (cm/s) | 9.30 ± 3.40 | 11.27 ± 3.30 | 0.403 |
| AT (ms) | 30.41 ± 5.10 | 26.97 ± 4.30 | 0.030 * |
| IVA (m/s2) | 3.70 ± 1.60 | 4.30 ± 1.80 | 0.004 * |
| IVRT (ms) | 76.12 ± 13.54 | 67.25 ± 12.00 | 0.098 |
| IVCT (ms) | 60.64 ± 10.40 | 56.32 ± 10.66 | 0.104 |
| ET (ms) | 214.31 ± 30.40 | 225.75 ± 32.10 | 0.027 * |
| MPI | 0.54 ± 0.08 | 0.43 ± 0.10 | 0.001 * |
| Parameters | PLWH (n = 118) | Controls (n = 80) | p-Values |
|---|---|---|---|
| Conventional Parameters | |||
| TAPSE (mm) | 27.73 ± 3.50 | 27.11 ± 3.10 | 0.742 |
| Mean PAP (mmHg) | 18.51 ± 6.00 | 13.69 ± 5.40 | 0.012 * |
| Doppler Parameters | |||
| E velocity (cm/s) | 9.12 ± 3.20 | 8.58 ± 3.00 | 0.405 |
| A velocity (cm/s) | 12.53 ± 3.50 | 11.49 ± 3.10 | 0.340 |
| E/A ratio | 0.70 ± 0.35 | 0.80 ± 0.33 | 0.098 |
| Tissue Doppler Parameters | |||
| Sa velocity (cm/s) | 14.91 ± 2.60 | 15.54 ± 2.40 | 0.604 |
| IVV (cm/s) | 9.68 ± 4.20 | 10.14 ± 4.10 | 0.124 |
| AT (ms) | 35.40 ± 7.00 | 29.35 ± 6.50 | <0.001 † |
| IVA (m/s2) | 1.83 ± 0.95 | 2.49 ± 1.00 | <0.001 † |
| IVCT (ms) | 69.00 ± 11.60 | 63.72 ± 11.00 | 0.054 |
| IVRT (ms) | 67.20 ± 17.30 | 68.00 ± 13.20 | 0.560 |
| ET (ms) | 174.65 ± 27.20 | 237.50 ± 23.50 | 0.006 * |
| MPI | 0.64 ± 0.09 | 0.47 ± 0.08 | 0.010 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oksen, D.; Aslan, M.; Serin, E.; Gecit, M.H.; Yavuz, Y.E.; Zerdali, E.Y.; Oktay, V. Evaluation of Subclinical Left Ventricular Dysfunction in HIV Patients Receiving Abacavir, Dolutegravir, and Lamivudine Therapy with Novel Tissue Doppler Imaging Techniques. J. Clin. Med. 2025, 14, 1534. https://doi.org/10.3390/jcm14051534
Oksen D, Aslan M, Serin E, Gecit MH, Yavuz YE, Zerdali EY, Oktay V. Evaluation of Subclinical Left Ventricular Dysfunction in HIV Patients Receiving Abacavir, Dolutegravir, and Lamivudine Therapy with Novel Tissue Doppler Imaging Techniques. Journal of Clinical Medicine. 2025; 14(5):1534. https://doi.org/10.3390/jcm14051534
Chicago/Turabian StyleOksen, Dogac, Muzaffer Aslan, Ebru Serin, Muhammed Heja Gecit, Yunus Emre Yavuz, Esra Yerlikaya Zerdali, and Veysel Oktay. 2025. "Evaluation of Subclinical Left Ventricular Dysfunction in HIV Patients Receiving Abacavir, Dolutegravir, and Lamivudine Therapy with Novel Tissue Doppler Imaging Techniques" Journal of Clinical Medicine 14, no. 5: 1534. https://doi.org/10.3390/jcm14051534
APA StyleOksen, D., Aslan, M., Serin, E., Gecit, M. H., Yavuz, Y. E., Zerdali, E. Y., & Oktay, V. (2025). Evaluation of Subclinical Left Ventricular Dysfunction in HIV Patients Receiving Abacavir, Dolutegravir, and Lamivudine Therapy with Novel Tissue Doppler Imaging Techniques. Journal of Clinical Medicine, 14(5), 1534. https://doi.org/10.3390/jcm14051534





